Exploring the sublethal genotoxic effects of class II organophosphorus insecticide quinalphos on freshwater fish Cyprinus carpio
Devan HEMALATHA , Bojan NATARAJ , Basuvannan RANGASAMY ,Kannan MAHARAJAN , Mathan RAMESH ,*
1 Unit of Toxicology, Department of Zoology, School oflife Sciences, Bharathiar University, Coimbatore-641046, Tamil Nadu,India
2 Department of Zoology, PSG College of Arts & Science, Coimbatore-641014, Tamil Nadu, India
Abstract The genotoxic eff ects of quinalphos (QP) were evaluated in the erythrocytes of Cyprinus carpio using the comet, micronucleus, and nuclear abnormality assays. The 96 h LC50 value of quinalphos, estimated by static renewal system, was 10.9 μL/L. On the basis of the LC50 value, two sublethal concentrations(1.09 and 2.18 μL/L) of the compound were used together with cyclophosphamide (5 mg/L) as the positive control. The fish were exposed for 35 d, and sampling was done at the end of 7, 14, 21, 28, and 35 d. Our results reveal a significant dose-dependent increase in the frequencies of DNA strand breaks, micronucleus,and nuclear abnormalities. Similar responses were also observed in fish exposed to the positive genotoxin cyclophosphamide. The collective findings demonstrated that quinalphos exerts a genotoxic eff ect on C.carpio. The results further confirmed that the comet, micronucleus, and nuclear abnormality assays are useful tools in determining the potential genotoxicity of pesticides towards aquatic organisms.
Keyword: quinalphos (QP); micronucleus; nuclear abnormalities; cyclophosphamide; Cyprinus carpio
1 INTRODUCTION
Quinalphos (O,O-diethyl O-quinoxalin-2-yl phosphorothioate) is a synthetic organophosphate insecticide widely used in Indian agriculture to control a broad range ofinsect pests attacking crops such as cotton, groundnut, cereals, tea, coff ee, sugarcane and vegetables (Hayes and Laws, 1991; Kokilavani et al.,2014). Common formulations of quinalphos (QP) are emulsion concentrates, dust and granules (Pant and Srivastava, 2003; http://agriculture.indiabizclub.com/info/organic cultivation/ crop management). In India,farmers use a 4% solution of commercial-grade QP for spraying on the crops (Sharma and Mathur, 1972).QP is classified as number 2 pesticide (moderately hazardous) by the World Health Organization (WHO,2002). In addition, the European Commission has included this insecticide in risk classification as hazardous to the environment. Like other organophosphates, the insecticidal activity of QP is caused by the inhibition of the acetylcholinesterases(AChE) in the central nervous system (CNS) and peripheral nervous system (PNS). Inhibition of AChE results in the accumulation of acetylcholine (ACh) in the cholinergic synapses present at the neuromuscular junctions, leading to an excessive transmission of nerve impulses and causing mortality in the target pest (Ecobichon, 1991; Amitai et al., 1998; Li et al.,2012).
The half-life of QP in the soil is in the range of 10-16 d and in water it is >24 h (Racke, 1993). The actual half-life of QP mostly depends on environmental variables such as soil type, temperature and moisture.Upon degradation, QP produces various metabolites at diff erent time intervals through hydrolysis,oxidation, de-alkylation and isomerization processes.These metabolites include quinalphosoxan, O-ethyl-O-quinoxalin-2-yl phosphoric acid, quinoxaline-2-thiol and 2-hydroxy quinoxaline, which are further broken down to carbon-di-oxide. The metabolite 2-hydroxy quinoxaline and the parent compound persist in the environment for a longer period and are more toxic than the parent compound (Gupta et al.,2012). Extensive use of QP ultimately finds its way into aquatic environments, where it poses considerable toxicological risks to the resident organisms (Scott et al., 1990; Hemalatha et al., 2016). Generally, the toxic eff ect of the chemicals depends on the exposure period and also the size and age of the exposed animal.Hence, evaluating the toxicity of a chemical using a wide range of animal species is routinely employed in toxicological research (Oruc et al., 2004). Numerous studies have been conducted on the toxic eff ect of QP on plants and soil microorganisms (Vig et al., 2006),mammals (Rupa et al., 1991b; Vasilić et al., 1992;Subramaneyaan et al., 2012) and aquatic organisms(Sastry et al., 1982; Durairaj and Selvarajan, 1992;Das and Mukherjee, 2000; Kavitha and Venkateswara Rao, 2008).
Quinalphos has been demonstrated to induce endocrine disruption, genotoxicity, reproductive toxicity and developmental toxicity in both male and female rats (Rupa et al., 1991a; Ray et al., 1992;Srivastava et al., 1992). In a recent investigation,Subramaneyaan et al. (2012) have reported QPinduced oxidative stress and histological alterations in adult male albino rats. Dwivedi et al. (1998) have also documented the induction of hepatic antioxidant enzymes and hepatic P450 content in mice treated with QP. Moreover, QP can produce adverse eff ects on aquatic organisms (Sadiqul et al., 2016). In this line, among the aquatic fauna, fish is considered as a useful genetic model for the evaluation of pollution in aquatic ecosystems owing to its ability to effi ciently metabolize and accumulate the pollutants (Park et al.,1989; Mitchell et al., 1992).
Fish models are widely used in toxicological research to assess the teratogenic and carcinogenic eff ects of chemicals on human beings (Harshbarger and Clark, 1990; Raisuddin and Jha, 2004;Matsumoto et al., 2006). The toxic eff ects of QP on fish have been assessed in several behavioral,respiratory (Chebbi and Davis, 2010a), biochemical(Das and Mukherjee, 2000), neurological (Chebbi and David, 2009), histological (Chamarthi et al.,2014), nucleic acid content and enzymatic studies(Venkata Rathnamma et al., 2013a, b). Nonetheless,information pertaining to the long-term genotoxic eff ect of QP on fish is scarce.
Among the diverse genotoxicity tests, the comet assay (CA) or single cell gel electrophoresis (SCGE)has become the most important tool for monitoring the genotoxicity in aquatic environments, particularly in fish. The technique is rapid and sensitive in detecting and quantifying DNA damage, such as single and double-strand breaks and alkali-labile sites. Such damage can be induced directly by the contaminants or indirectly via repair processes (Tice et al., 2000). In toxicological research, SCGE/CA is often employed to ascertain the genotoxic eff ects of chemical compounds (Pandey et al., 2006; Ali et al.,2008; de Campos Ventura et al., 2008; Kumar et al.,2010). The micronucleus (MN) test in fish is also widely applied for estimating the genotoxicity in aquatic environments because of the ease of sampling and high sensitivity. Furthermore, water quality and the health condition of the aquatic species can be detected by these assays (Talapatra and Banerjee,2007). Thus, the combined use of comet and micronucleus assays is preferred in aquatic toxicity research due to its sensitivity in detecting DNA damage and cytogenetic eff ects after acute and chronic exposure to various genotoxic agents. Along with the MN test, nuclear abnormalities (NA) have also been widely used in fish toxicology as indicators of genotoxicity (Ergene et al., 2007; Kumar, 2012).The test furnishes additional information for the scoring of micronuclei in genotoxicity surveys(Ayllon and Garcia-Vazquez, 2000; Muranli and Güner, 2011).
In such a context, we evaluated the mutagenic and genotoxic eff ects of QP using C. carpio as a laboratory animal model by means of comet assay, micronucleus and nuclear abnormalities tests. To our knowledge,this study presents the first in vivo evidence for the genotoxicity exerted by QP on C. carpio at sublethal concentrations. Cyprinus carpio is a widely cultivable and edible fish species in India. Moreover, it is a suitable animal model for ecotoxicologial studies.
2 MATERIAL AND METHOD
2.1 Experimental animal and Chemicals
Fingerlings of the common carp C. carpio with average weight (5.0±1 g) and length (6.0±1 cm) were collected from the Aliyar Fish Farm, Tamil Nadu,India. Before commencing the experiments, the organisms were acclimated to laboratory conditions for three weeks in a large tank (1 000-L capacity)containing aerated dechlorinated tap water of the following characteristics: temperature 27.0±1 °C; pH 7.0±1; dissolved oxygen 6.4±0 mg/L; total hardness 18.4±2 mg/L and total alkalinity 18.3±2.0 mg/L. The photoperiod was 12 h꞉12 h. The fishes were fed once a day with commercial fish pellets and water renewal was performed once a day corresponding to 85% of the tank water to minimize contamination from metabolic wastes. The healthy organisms were randomly allocated to various treatment groups.Quinalphos (25% EC) was obtained from the Tudiyalur Cooperative Agricultural Services Limited,Tamil Nadu, India. Cyclophosphamide (CAS No. 50-18-0, purity: >99%) and other chemicals were purchased from Sigma-Aldrich, USA.
2.2 Experimental design
Acute toxicity test was conducted in a static renewal system to determine the 96-h LC50value(APHA-AWWA-WEF, 2005). The stock solutions of QP were prepared in acetone and the range-findings test was carried out by exposing the fishes to five diff erent concentrations (10.3, 10.5, 10.7, 10.9,11.0 μL/L) of QP for 96 h. Control groups were also maintained. The experiments were repeated twice and the median lethal concentration (96 h LC50) was determined by using the probit analysis described by Finney (1952). The 96 h LC50value of QP in C. carpio was found to be 10.9 μL/L. During the acute toxicity experiment, the fishes were not fed and the dead ones were removed from the aquaria. Based on the 96 h LC50value, 1/10th(1.09 μL/L) and 1/5th(2.18 μL/L) of the QP concentrations were taken as the sublethal concentration.A group consisting of 25 fishes was exposed to each of the sublethal concentrations of QP (1.09 and 2.18 μL/L) separately for 35 d. Samples were withdrawn at intervals of 7, 14, 21, 28, and 35 d at the rate of five fishes per sampling. Additional sets were separately maintained for negative control (tap water),solvent control (acetone) and positive control(cyclophosphamide: 5 mg/L; concentration selection based on a previous investigation (Muranli and Güner,2011)). In addition, two replicates for each concentration were also included for the two sublethal concentrations and the control groups.
2.3 Comet assay
For the SCGE/CA, the method of Singh et al.(1988) was adopted with slight changes. To an aliquot of 30 μL diluted blood, 100 μL oflow melting agarose(LMA) (0.65%) in PBS was added, mixed well, and pipetted on the slides (precoated with 0.65% normal agarose). Subsequently, the contents were incubated for 10 min for solidification. The slides were then dipped in cold lysing solution (2.5 mol/L NaCl,100 mol/L EDTA-2Na, 10 mmol/L Tris, 10% DMSO,1% sodium sarcosinate, 1% Triton-X, pH 10.0) and refrigerated for 4 h. The slides were transferred to an electrophoresis unit (15 cm×10 cm) containing denaturation buff er (300 mmol/L NaOH, 1 mmol/L EDTA, pH 13.0). Electrophoresis was done for 20 min at 0.6 V/cm and 300 mA. The buff er volume was adjusted to cover the slides. The slides were neutralized with Tris buff er (pH 7.5) for 2 min and stained with ethidium bromide (20 μg/mL) for microscopic examination. Approximately 100 cells per slide were scored randomly, examined under a florescent microscope, and the comet images were assayed using the Comet Assay Software Project(CASP). The DNA damage was quantified as % DNA tails, which indicate cellular damage (Imanikia et al.,2016).
2.4 Analysis of micronuclei (MN) and other nuclear abnormalities (NA)
The MN test was done according to Çavaş and Ergene-Gözükara (2003b). Peripheral blood samples were obtained from the caudal vein of the specimens and smeared onto pre-cleaned slides and dried overnight. The slides were then fixed with methanol for 10 min and stained with Giemsa (5%). All slides were coded and scored blind. Three slides were prepared for each fish, and a total of 1 000 erythrocytes per fish were examined under an Olympus optical microscope (1 000× magnifications).
2.5 Scoring criteria for micronuclei (MN) and other nuclear abnormalities (NA)
The MN test was carried out according to the protocol of Carrasco et al. (1990). The MN frequency was calculated as follows:

The NAs were classified according to Carrasco et al. (1990) into four categories: binucleated cells (BN),lobed nuclei (LB), blebbed nuclei (BL) and notched nuclei (NT). The NAs in the peripheral blood cells of C. carpio and their frequency was calculated in a manner similar to that of MN.
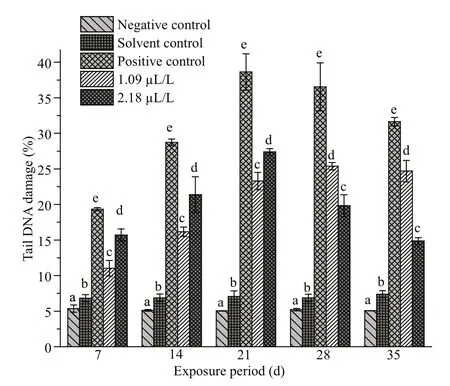
Fig.1 Percentage of tail DNA in erythrocytes of C. carpio exposed to QP
Fig.2 Comet assay in C. carpio erythrocytes
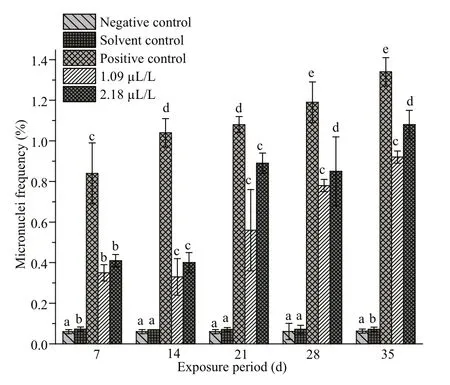
Fig.3 Frequencies of micronuclei (MN) in erythrocytes of C. carpio exposed to QP
2.6 Statistical analysis
The data were subjected to one way analysis of variance (ANOVA) and Duncan’s multiple range test(DMRT) at P <0.05 level. All analyses were performed with SPSS 20.0 statistical software package, and the values were expressed as mean±S.E.
3 RESULT
The fishes exposed to the two diff erent concentrations(1.09 and 2.18 μL/L) of QP exhibited higher DNA damage ( P <0.05) than the control groups (Fig.1).Prominent DNA damage was noticed in 2.18 μL/L treatment on days 7, 14, and 21, and then a decline was noted at days 28 and 35. The DNA strand breaks in C.carpio after exposure to QP are depicted in Fig.2.
The MN frequencies in the peripheral erythrocytes of C. carpio exposed to QP as well as those in the parallel positive, solvent and negative controls are summarized in Fig.3. The induction was significantly higher in the treatment groups than in the control groups at all sampling intervals. Furthermore, the induction of MN was found to be dose dependent as its highest frequency was recorded at the concentration of 2.18 μL/L on the 35thday. In the positive control too, a concentration and duration-dependent induction of MN was noticed. In the negative and solvent control groups, the MN frequency remained almost stable and did not vary significantly from one sampling interval to the other. Cells containing normal nuclei and micronuclei are illustrated in Supplementary Fig.S1a & b.
The frequencies of NAs in the erythrocytes of fishes exposed to QP and their respective negative,solvent and positive control groups are summarized in Figs.4-7. Similar to MN, the frequencies of NAs were also significantly higher in the fishes treated with QP. The frequency of NAs in the erythrocytes of fishes exposed to QP was also concentration and duration dependent. The induction of NA was significantly ( P <0.05) higher in the fishes exposed to positive control when compared with the negative controls. No significant increases were observed in fishes belonging to the solvent and negative control groups. The BN, LB, BL, and NT cells are represented in Supplementary Fig.S1c-f.
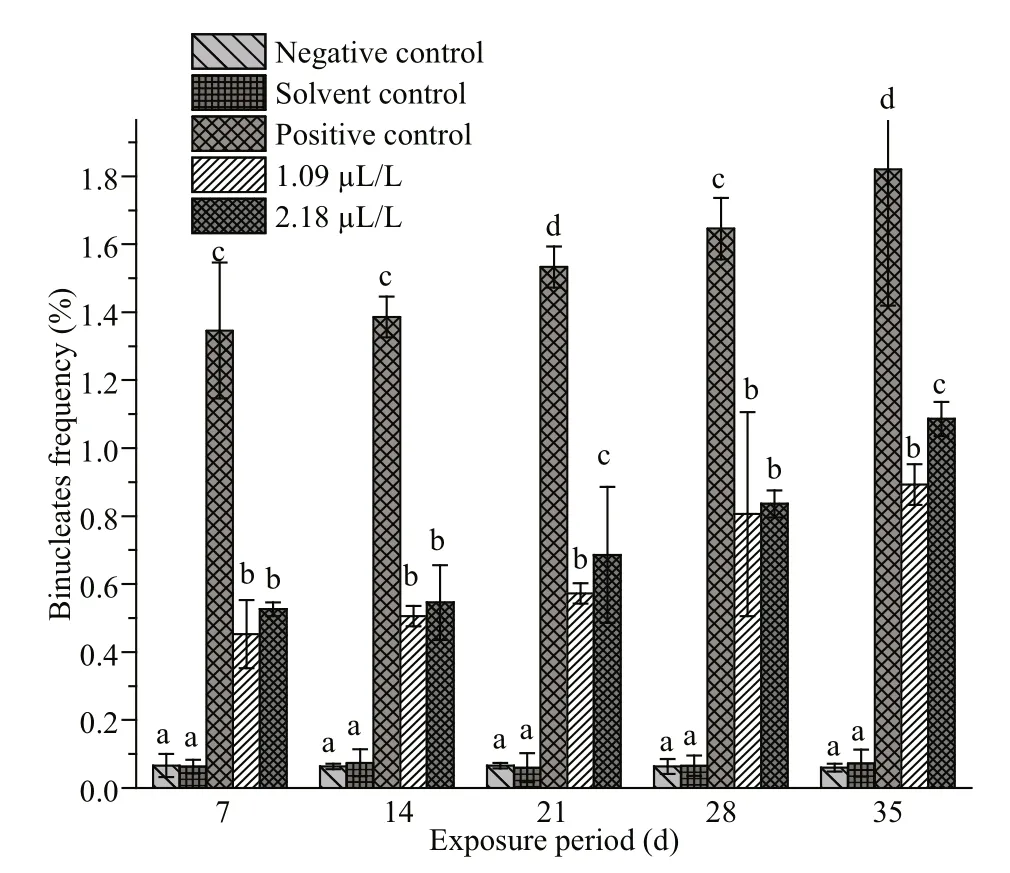
Fig.4 Frequencies of binucleates in erythrocytes of C.carpio exposed to QP
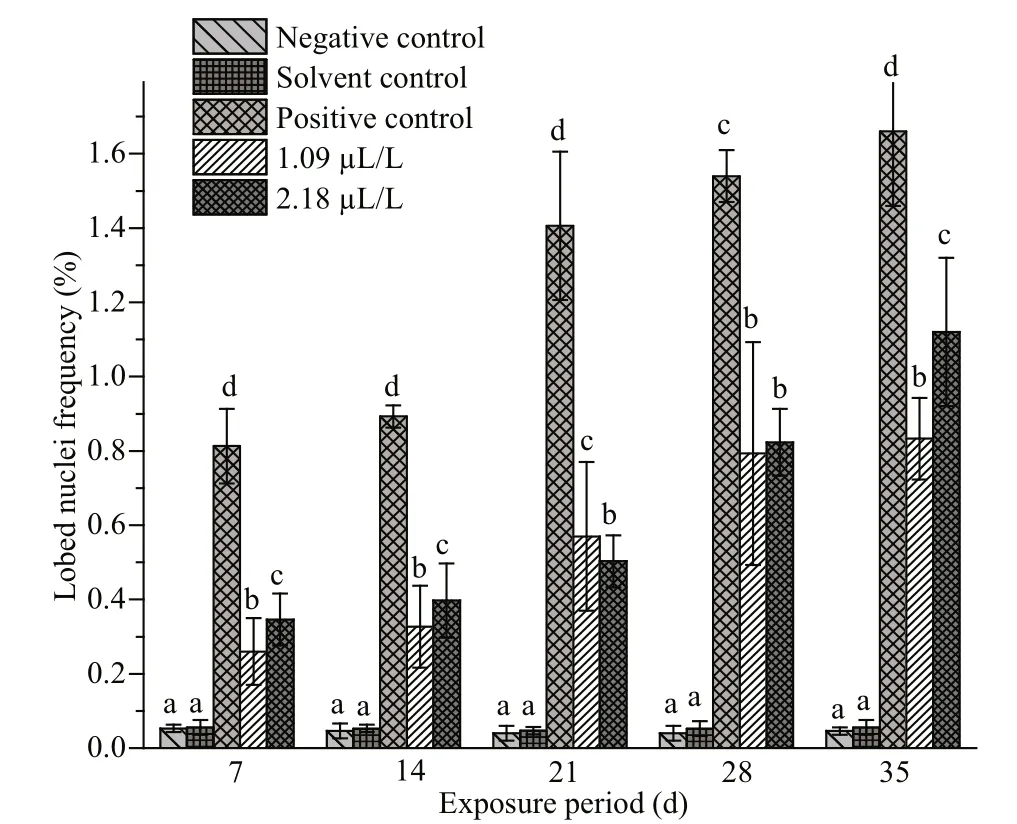
Fig.5 Frequencies oflobed nuclei in erythrocytes of C.carpio exposed to QP
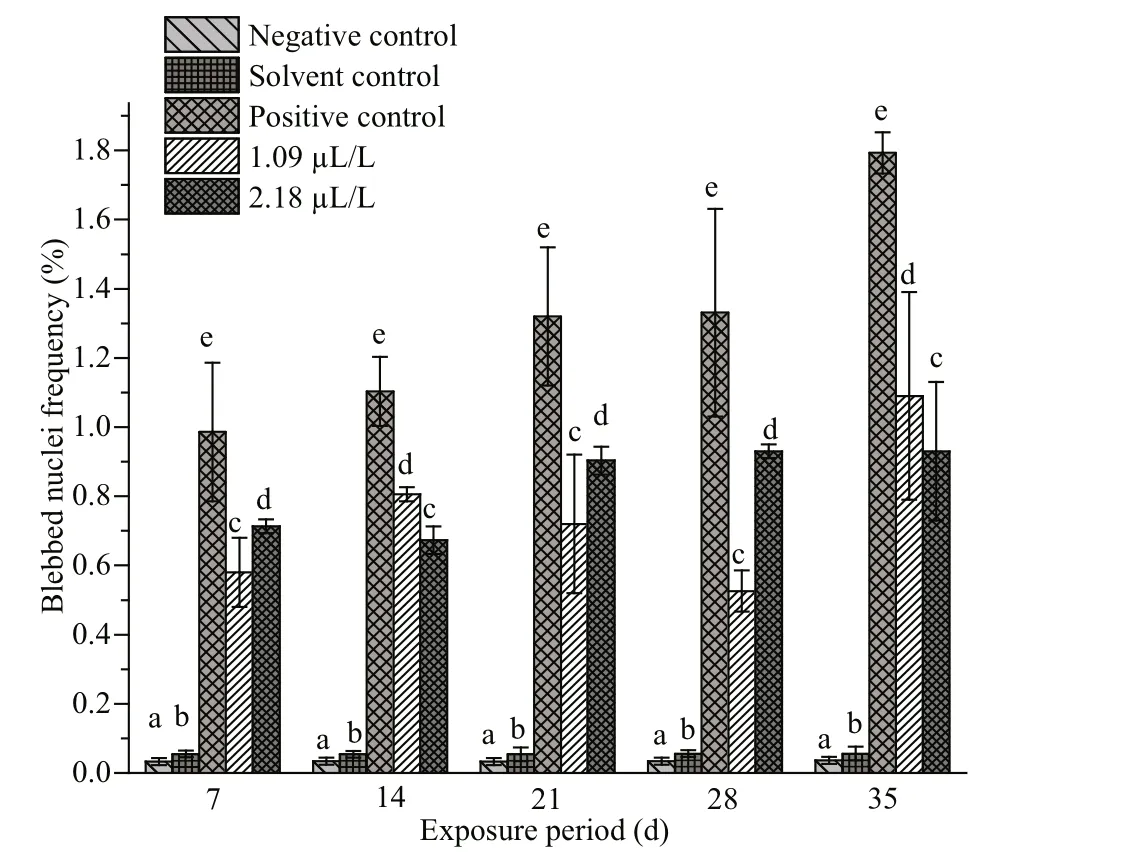
Fig.6 Frequencies of blebbed nuclei in erythrocytes of C.carpio exposed to QP
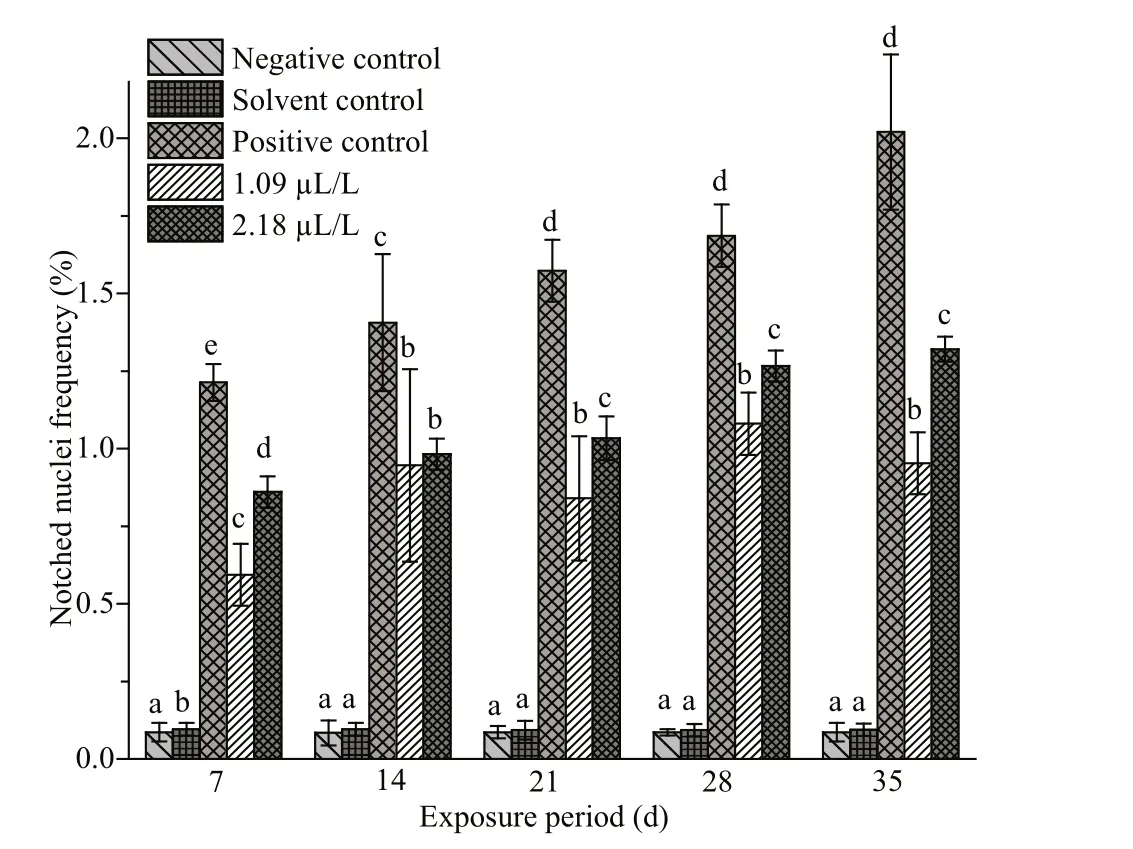
Fig.7 Frequencies of notched nuclei in erythrocytes of C.carpio exposed to QP
4 DISCUSSION
In general, organophosphorus insecticides have a faster rate of degradation and lower environmental persistence than the organochlorine compounds.However, the widespread use and application of QP in agriculture has resulted in the frequent detection ofits residues in water, soil, sediments and aquatic biota.Fish is often used as the sentinel organism for investigating the carcinogenic and mutagenic potential of contaminants present in aquatic environments. This choice is based on the various roles played by these organisms in the food web, such as bio-accumulation of environmental pollutants,biotransformation of xenobiotics, and response to mutagens at low concentrations (Çavaş and Ergene-Gözükara, 2005). In addition, they react to toxicants in a manner similar to that of higher vertebrates,which allows the measurement of substances that are potentially harmful to humans (de Lemos et al., 2007).
Acute toxicity data have been used to derive water quality guidelines for regulatory purposes (Sundaram et al., 1994). For the present investigation, the 96 h LC50value (10.9 μL/L) of QP was taken based on our previous research (Hemalatha et al., 2016). The results reveal that QP is toxic to the fishes; In addition,the findings indicate that the toxicity of the pesticide is both time and dose dependent. The 96 h LC50reported in the present study for QP is slightly higher than the value (7.5 μL/L) reported by Chebbi and David (2010b). Muttappa et al. (2014) have also reported a lower 96 h LC50value (2.75×10-6) for the same species. The differences in the LC50value observed within a single species for the same toxicant may depend on size, age and health of the organisms(Sprague, 1969). Furthermore, other environmental factors, such as temperature, pH, total hardness and dissolved oxygen content of the water, as well the formulation of the chemical and its exposure; greatly influence the results (Gupta et al., 1981).
The CA/SCGE is a widely used tool to detect genotoxicity in aquatic environments (Frenzilli et al.,2009). The assay has the advantage of determining the DNA damage in individual cells (Lee and Steinert,2003; Buschini et al., 2004). The data obtained in the present study demonstrate that the frequency of %tail DNA damage for all concentrations of QP is significantly higher ( P <0.05) than that of the control.The percentage of DNA tail damage was found to be concentration and time dependent, which point to the genotoxicity of QP towards C. carpio. A similar observation has also been made in Prochilodus lineatus exposed to the pesticide Roundup(Cavalcante et al., 2008). In addition, time-dependent responses have been witnessed in Channa punctuatus exposed to chlorpyrifos and malathion (Ali et al.,2008; Kumar et al., 2010). Lee and Steinert (2003)have reported that the interaction between the DNA molecules and the contaminants may occur in diff erent ways, such as DNA damage by reactive oxygen species, inhibition of DNA repair, action of the compound directly on the DNA, interaction of the metabolites with DNA, and DNA damage through a multilateral mechanism. In the present study, the possible reason for the observed DNA strand breaks could not be elucidated due to the lack of any direct evidence. However, it has already been reported that QP can induce oxidative stress in fishes by producing reactive oxygen species (ROS) (Hemalatha et al.,2016). At supranormal levels, ROS has been shown to cause cellular and DNA lesions (Ayllon and Garcia-Vazquez, 2000; Cadet et al., 2003). Thus, it is possible that the increased DNA damage in the erythrocytes of C. carpio after QP exposure might have resulted from the production of ROS during the metabolism of the toxicant. The metabolite might have interacted with the DNA and produced the lesions detected by the assay.
The MN frequency in fish erythrocytes is used to assess the toxicity of aquatic pollutants exhibiting aneugenic and clastogenic properties (Ferraro et al.,2004; Udroiu, 2006). In this work, QP induced significantly higher number of MNs than the control groups, and the frequency escalated as the concentration and the time of exposure surged. A similar observation has been made in diff erent fish species by using the assay to determine the genotoxic eff ect of xenobiotics (Nwani et al., 2011; Palanikumar et al., 2012). A dose-dependent increase in MN frequency has been reported in the peripheral blood of Barbonymus gonionotus in response to QP containing insecticides (convoy) (Sadiqul et al., 2016). The trend has also been observed in Channa punctatus in response to carbosulfan (Nwani et al., 2010),chlorpyrifos (Ali et al., 2008), PCP and 2,4-D (Farah et al., 2003). Time responses have also been documented in Oreochromis niloticus treated with petroleum and chromium processing plant effl uents(Çavaş and Ergene-Gözükara, 2005). Our result provides the first evidence for the sublethal genotoxic eff ects of QP on C. carpio under laboratory conditions.Hence, the induction of micro-nucleated erythrocytes following exposure to QP asserts the potential clastogenic eff ects of the compound in fishes.
Studies have already reported the clastogenic potential of QP in rats (Rupa et al., 1991a; Ray et al.,1992; Srivastava et al., 1992). Furthermore, the findings from previous studies suggest that organophosphorus insecticides, such as dimethoate(Ali et al., 2014) profenofos (Pandey et al., 2011) and diclorvos (Shukla et al., 2010), induce genotoxic eff ects in fish erythrocytes mainly via oxidative damage. Similarly, QP may also cause oxidative stress. Certain chemicals are capable of causing oxidative stress through the generation of ROS, which interact with macromolecules such as DNA and result in molecular and clastogenic damages (Raisuddin and Jha, 2004; Jha, 2008). Thus, the findings of this research suggest that the observed genotoxic damage may be due to the increased oxidative stress resulting from exposure to QP.
In addition to MN, other NAs have also been reported in fish erythrocytes (Monteiro et al., 2011).The formation of NAs was first described by Carrasco et al. (1990), but the mechanism was not fully understood. Pacheco and Santos (1997) uncovered that the changes in nuclear morphology originated from a genotoxic event as a result of exposure to xenobiotic contaminants. To investigate the genotoxic eff ects of chemicals, NAs in fishes such as LB, BL,NT, and BN are widely used (Çavaş and Ergene-Gözükara, 2003a; Kilemade et al., 2004; da Silva Souza and Fontanetti, 2006; Ergene et al., 2007). In the present study, the formation of the abnormalities indicates the damaging eff ect of QP. These defects may aff ect gene amplification and also cause deformed nuclei (Bolognesi et al., 2006; Cavalcante et al.,2008). Nuclear alterations such as BL and LB could also be related to the failure of tubulin polymerization(Vardavas et al., 2016). Additionally, de Campos Ventura et al. (2008) have reported that BN and NT arise from diffi culties in the formation of mitotic fuse caused by the aneugenic action of the chemicals. Our findings on erythrocyte NAs are in agreement with the previous studies that have recorded increased NA frequencies in fishes following exposure to QP containing insecticides (convoy) (Sadiqul et al.,2016), deltamethrin (Ansari et al., 2009), formalin(Mert et al., 2015), carbosulfan, glyphosate and atrazine (Nwani et al., 2014). In the present study, the significant increase in NAs noted throughout the study period implies that they are better biomarkers for QP exposure than the MN alone. Therefore, we conclude that the formation of NAs in the exposed fishes clearly indicates the genotoxicity of QP. The NA and MN assays could together serve as suitable biomarkers for assessing the genotoxicity of chemicals in the aquatic environment.
5 CONCLUSION
The results demonstrate that QP is genotoxic to fishes since it induces DNA strand breaks and the formation of MN and NA in peripheral erythrocytes.This study also confirms that the comet, micronucleus and nuclear abnormality assays are useful tools in determining the potential genotoxicity of pesticides on non-target organisms. Furthermore, studies on QPinduced genotoxic eff ects in fishes are rare, and the present findings contribute to the limited literature on the exposure of fishes to sublethal levels of the pesticide.
6 DATA AVAILABILITY STATEMENT
The data used in the present investigation are available from the corresponding author upon request.
7 ACKNOWLEDGMENT
The author (D. HEMALATHA) wish to thank UGC, New Delhi for providing grant of Basic Science Research Fellowship (BSR) (No. F.7-24/2007).
 Journal of Oceanology and Limnology2021年2期
Journal of Oceanology and Limnology2021年2期
- Journal of Oceanology and Limnology的其它文章
- Predicting sediment flux from continental shelfislands,southeastern China*
- Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom*
- Development of high-resolution chloroplast markers for intraspecific phylogeographic studies of Phaeocystis globosa*
- Effects ofiron and humic acid on competition between Microcystis aeruginosa and Scenedesmus obliquus revealed by HPLC analysis of pigments*
- Effect of river plume on phytoplankton community structure in Zhujiang River estuary*
- An illustrated checklist of macrofaunal molluscs from softsediments of the Abrolhos Parcel (northeastern, Brazil) with 15 new records*
