Larva fish assemblage structure in three-dimensional floating wetlands and non-floating wetlands in the Changjiang River estuary*
Xiaofeng HUANG , , Feng ZHAO , Chao SONG , Yi CHAI, Qian WANG,Ping ZHUANG ,
1 College of Animal Science, Yangtze University, Jingzhou 434025, China
2 Engineering Research Center of Ecology and Agricultural Use of Wetland, Ministry of Education, Yangtze University, Jingzhou 434020, China
3 Chinese Academy of Fishery Sciences, East China Sea Fisheries Research Institute, Shanghai 200090, China
Abstract Fish populations have declined in many estuarine and freshwater ecosystems in part due to the loss of habitat in recent decades. Reconstructing lost habitat for larvae fish is a potential method for recovering larvae fish populations. Three-dimensional artificial floating wetlands (AFWs) on which Phragmites australis was planted were experimentally deployed to recover the lost habitat in the Changjiang(Yangtze) River estuary from May to July 2018. The AFW area was characterized by slow velocity, high transparency, low dissolved oxygen, and relatively constant water temperature. The total individuals oflarvae fish in the AFW area (12 122 in total) was higher than that in the non-AFW area (1 250 in total), and the densities of most larvae fish species were higher in the AFW habitat than in the non-AFW area. The distributions oflarvae fish species were positively influenced by habitat type because they were strongly related to the negative part of the first axis of the redundancy analysis, and Cyprinus carpio and Cyprinus auratus were inclined to habitat in the slow velocity and high transparency AFW habitat area. These results indicate that larvae fish species are inclined to inhabit the AFW habitat. The use of three-dimensional P.australis AFWs would be a potential method for enhancing the habitat oflarvae fish in the degraded habitats along the estuary.
Keyword: habitat rehabilitation; larvae fish; biodiversity conservation; artificial floating wetland;Changjiang (Yangtze) River estuary
1 INTRODUCTION
The loss and degradation of natural habitat is considered one of the greatest threats to biodiversity in many freshwater ecosystems. To improve the value of watercourses for human beings, great eff orts have been expended for agriculture, water supply, flood control, and transportation. These activities have changed the pattern of hydrology dynamics, and the natural environment and biodiversity distributions are threatened (Ficetola et al., 2012; Penha et al., 2014).Fish species have become confined to small and isolated habitat patches that can sustain only a limited number ofindividuals; as a result, approximately 1%oflarvae fish can survive in such freshwater ecosystems (Clavero and Hermoso, 2011; Figarski and Kajtoch, 2015). Therefore, recovering degraded habitat area is an important goal to protect the fish diversity.
Many scientific methods have been used to reestablish the deteriorated habitats of fish species.Vegetation is a method that has been used to create habitat for fish and crustaceans. The main reason for this is that these plants represent ideal food resources and suitable habitat for these species (Thom et al.,2004; Weinstein et al., 2009; Putnam et al., 2010).Larvae fish ontogeny is also influenced by the diff erent types of aquatic vegetation because the number of fish species in the created plant habitat was higher than that in uncreated habitat areas (Cheminée et al., 2017).and artificial device for larvae fish ontogeny may be influenced by the diff erent types of aquatic vegetation(Nicolle et al., 2010). The placement of artificial structures (such as submerged trunks, brushwood bundles, industrial structures, and artificial reefs) is another method used to protect fish and crustacean resources in areas where habitat has been lost because these artificial structures create suitable habitat for these species (Bolding et al., 2004; Smokorowski and Pratt, 2007; Gois et al., 2012). Constructing artificial devices is a useful method for creating habitats for larvae fish in freshwater ecosystems; as such structures have been shown to attract fishery resources (Ahmed et al., 2015; Godwin et al., 2015; Johnson et al., 2015;Pont et al., 2015). Shallow heterogeneous rocky bottoms were used to support larvae fish populations along 250 km of the Catalan coastline. The floating wetland was a special fish aggregating device (FAD),as it can be placed in the water to aggregate fish and crustaceans in many areas (Dagorn et al., 2010;Gutzler et al., 2015). It was proven that a Phragmites australis artificial floating wetland (AFW) belongs to a special FADs and it could serve as a habitat for nekton in an estuary (Huang et al., 2017). Because the main characteristic oflarvae fish is a low swimming ability, whether three-dimensional AFWs could serve as habitat for larvae fish in the Changjiang (Yangtze)River estuary should be further studied.
The floating wetlands have been applied to recover deteriorated habitat for various animals. Both the physical complexity of the simulated structures and their location in the water column could facilitate the formation of a suitable environment. Moreover, all kinds of cultivated plants were used in a previous study to attain nutrients directly from the water column because they were not rooted in any substrate,which may improve the rate of nutrient uptake into biomass. These plants facilitated the attachment of microorganisms, which favored the decomposition of organic matter and the entrapment of suspended solids. Larvae fish show high mortality in the early stages of their life cycles, and rebuilding the suitable habitat the practical conversation management of fish species in an ecosystem.
The aim of this study was to evaluate whether the P. australis AFW could create a suitable habitat for larvae fish in the degraded habitat area. Further objectives were to (1) compare the environmental variables between the AFW habitat and the non-AFW habitat area and (2) analyze larvae fish assemblage structures in the AFW habitat and the non-AFW habitat area.
2 MATERIAL AND METHOD
2.1 Experimental site
This experiment was conducted in the Changjiang River estuary, China. The P. australis AFWs were constructed with substrate-rooted plants and floating structures that consisted of aquatic or terrestrial plants growing in a hydroponic manner with buoyant frames floating on the surface of water bodies. Each AFW framework can be divided into two parts: artificial plant bed on the water surface and palm sheets hung over the frameworks (Fig.1c & d). The area of each frame unit was approximately 16 m2, and there were 10 kg/m2roots of P. australis in each AFW framework.
Due to agricultural development and metropolis expansion along this estuary, the course of this estuary has been highly regulated in recent decades. Few plants can grow along the estuary, so the habitat of some fish species has become degraded. P. australis AFWs were constructed to reestablish the lost habitat of fish species in the Changjiang River estuary and were anchored at the experimental sites (Fig.1a & b).Because two high tides and two low tides every day in the Changjiang River estuary, the water environment is very complicated. Nine AFW habitat sites (CA1-CA9) in the P. australis AFW area were selected as experimental sites, and nine non-AFW sites (NA1-NA9) surrounding the AFW area were established as control sites (Fig.1b). The distance between the sampling site and each AFW unit was 10 m. The distance between the sampling sites was 10.0 m, and each sampling area was 20 m2.
2.2 Sampling design
Most fish species spawned in March and April, and most juvenile fish grow up from May to July. Samples were collected on May 10-14, June 13-17, and July 12-16 of 2018, and each sampling time was set during low tide in the Changjiang River estuary. A 25-pound plankton net was designed to quantitatively assess the larvae fish in diff erent habitat types. The opening diameter of this net was 30 cm, the length was 70 cm,and the mesh size was 0.1 cm2. Each sample oflarvae fish was collected by horizontally hauling the net for 2 min at a speed of 5 m/min at -0.5 m below the water surface. Fifty-four samples were collected from each site in all months and then preserved in 10% formalin solution. The numbers oflarvae fish were recorded to establish the larvae fish relative abundance (N%) and composition of the larvae fish assemblages in the diff erent habitat types. Three replicate samples were collected each month.
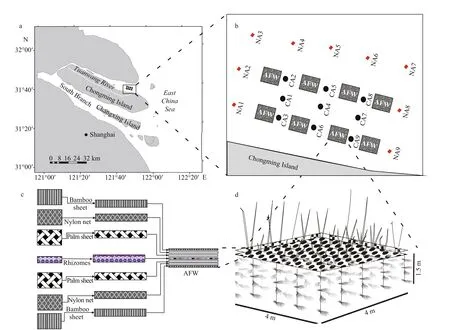
Fig.1 Illustration of experiment site in the Changjiang River estuary, showing its geographic location (a & b) and the framework of the artificial floating wetlands (c & d)
2.3 Environmental variables
Ten environmental variables were measured during each sampling period in the current study. All variables were measured within 0 to -1 m of the water surface at the sampling sites. Water transparency was measured with a Secchi disc and water velocity (m/s)was recorded (SQ-FP111, Global Water, USA). The mean depth (cm) at each site was measured three times in each sampling month. Water quality parameters, including temperature ( T, °C), dissolved oxygen (mg/L), pH, salinity, and total dissolved solids(TDS, mg/L), were measured with a YSI 85 handheld meter (YSI Incorporated, Yellow Springs, OH, USA).
2.4 Data analysis
All experimental data were tested for normality by examining model residuals and homogeneity of variance, the 2-sample t test was used to analysis the environmental variables in three months. All the environmental data are reported as the mean ± SE, and the significance level used was 0.001 unless otherwise indicated. The diversity of the larvae fish community was evaluated according to diversity indexes. The abundance oflarvae fish species was determined according to the number ofindividuals per 1 square meter (inds./m2), and the species dominance was also evaluated with dominance indexes.
All larvae fish species were analyzed based on their morphology in the laboratory; they were determined by the book Fish Resources of Early Life History Stages in Yangtze River (Cao et al., 2007). The density data for these larvae fish are also presented. When the assumption of homogeneity of variance was not met,abundance values were log transformed [log10( x+1)]and fish species richness values were square root transformed prior to analysis to meet the assumptions of parametric statistical procedures. Non-metric multidimensional scaling (NMDS) was used to analyze the relationship between the AFW area and the non-AFW habitat area based on the larvae fish structure. To interpret the relationship between the fish species and the environmental variables, the redundancy analysis (RDA) was used in this study.Analysis of similarities (ANOSIM) was used to analysis the environment variables in the diff erent habitat sites. The vegan package in R software (version 3.2.3) was used to interpret the relationship between the environmental variables and larvae fish species.According to the used procedure, if the maximum axis length is greater than 4, unimodal models are selected;when the axis length is between 3 and 4, both the unimodal model and linear model can be chosen; if the maximum axis length is lower than 3, linear models are recommended for selection(Clarke, 1993).
3 RESULT
3.1 Environment in the experiment sites
The values of the environmental variables in the experiment sites were changed by the AFW. The average dissolved oxygen in the AFW habitat was lower than that in the non-AFW habitat area at the diff erent sampling times. The mean value of dissolved oxygen varied from 6.49 mg/L in May to 5.05 mg/L in July in the AFW habitat area and from 7.36 mg/L in May to 5.23 mg/L in July in the non-AFW area(Fig.2), the main reason is the sunlight cannot be utilized by phytoplankton. During each sampling time, significantly lower water velocity was observed within the AFW area in comparison to that in the non-AFW area. However, water transparency was consistently higher in the AFW area than in the non-AFW area in each sampling month (Fig.2, 2-sample t test, P <0.05). The average water temperature in the AFW habitat was lower than that in the non-AFW area (Fig.2, 2-sample t-test, P <0.05) and ranged from 24.8 to 25.1 °C in the AFW habitat area and from 25.4 °C in May to 28.2 °C in July in the non-AFW area (Fig.2). The average values of some environmental variables (such as dissolved oxygen, water transparency, water temperature) considerably diff ered between the AFW habitat and non-AFW habitat area in each sampling month.
3.2 Larvae fish in AFW habitat area were redundant to non-AFW area
Thirteen fish species were captured in the experimental sites during the experimental period,and all belonged to three families: Engraulidae,Cyprinidae, and Taenioididae. There are 12 122 fish in the AFW habitat, 1 520 fish were caught in the non-AFW area in total (Table 1). According to the calculated abundance of fish species, the density of Cyprinus auratus ranked first in two habitat types(1.89 inds./m2in the P. australis AFW area and 0.18 inds./m2in the non-AFW area); Cyprinus carpio ranked second: 0.59 inds./m2in the AFW area and 0.06 inds./m2in the non-AFW area. Cyprinus auratus accounted for over 40% of the abundances (61.19%in the AFW area and 46.58% in the non-AFW area);C. carpio accounted for over 10% of the abundances(18.94% in the AFW area and 14.80% in the non-AFW area) (Table 1). Other larvae fish species were also captured in the two habitat types at the same time(Table 1). These assemblages significantly diff ered across all months despite the similarity in the density of some species and taxonomic groups.
The two diff erent habitat types can be distinguished significantly based on the fish species. The densities of all larvae fish were aff ected by the habitat type and sampling month according to two-way ANOVA(Table 2, P <0.001). Only four larvae fish species( Hypophthalmichthys nobilis, Pseudobrama simoni,Culter mongolicus, and Parabramis pekinensis) were not aff ected by habitat type; nine larvae fish species(e.g., Coilia nasus, Pseudobrama simoni, and Erythroculter dabryi) were not aff ected by sampling time. The abundance of all larvae fish species also showed a significant interaction between habitat type and sampling month (Table 3, P <0.001). Samples from the AFW habitat were significantly distinguished from the non-AFW area (ANOSIM: global test R=0.297, P=0.001), and ordination plots showed a clear separation of the samples from the two diff erent habitat types (Fig.3). The results show that the AFW habitat area is diff erent from the non-AFW habitat areas.
3.3 The AFW served as an ideal habitat for larvae fish species

Fig.2 Average values of environmental variables in two habitat types from May to June
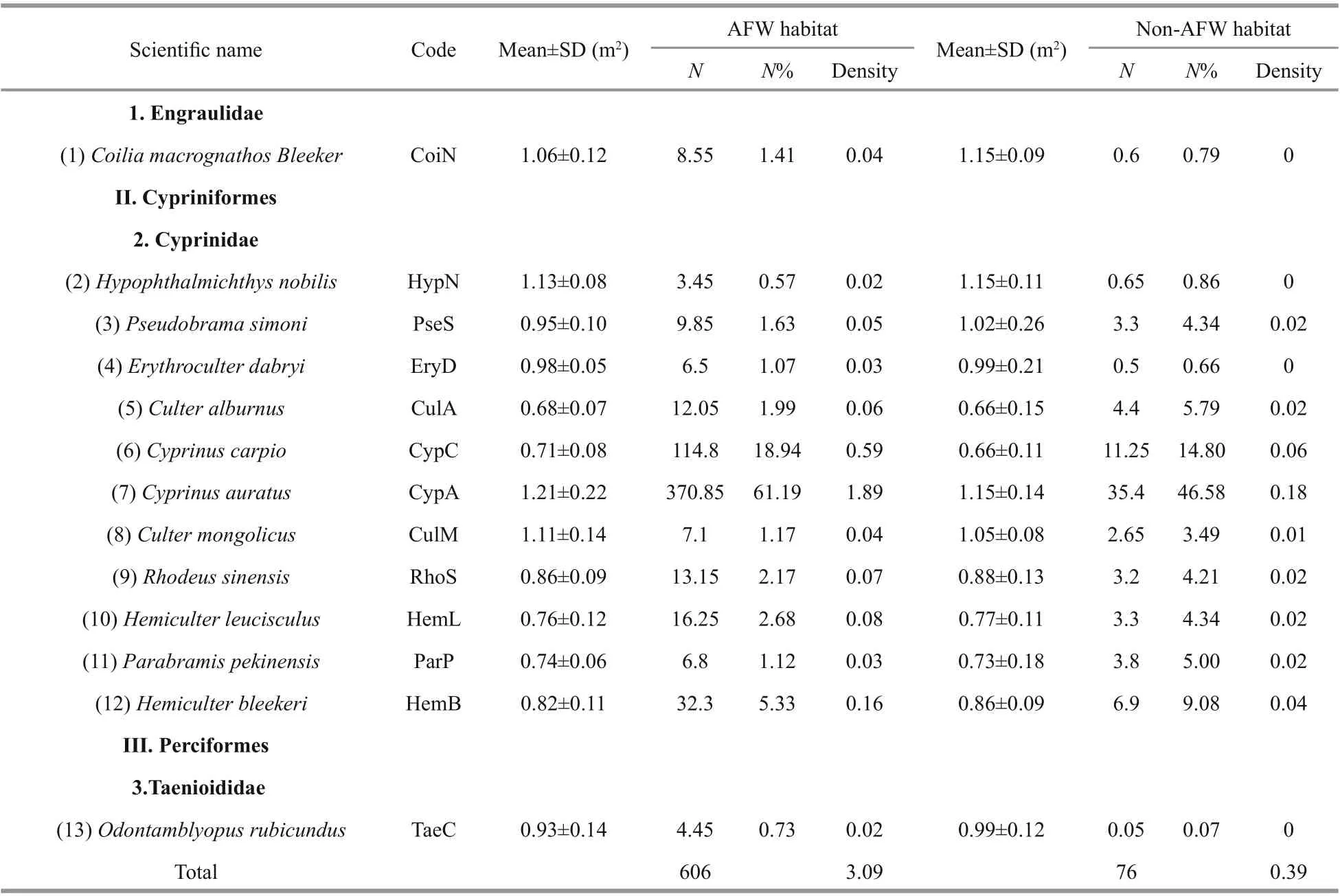
Table 1 List oflarvae fish species collected in the non-AFW habitat and the AFW habitat from May to June 2018

Fig.3 NMDS ordination plot oflarvae fish assemblages in the AFW area and non-AFW area ( n=27)
The relationships between the larvae fish species and environmental variables were interpreted by redundancy analysis through the vegan package. The maximum value of the axis length was 1.545 5(eigenvalue=0.109 1) in May, 0.870 5 (eigenvalue=0.116 4) in June and 1.823 8 (eigenvalue=0.060 2) in July (Fig.4). Referring to the results of the maximum axis length value, redundancy analysis (RDA) was used to analyze the larvae fish assemblage structure and the environmental variables in each sampling month. The first and second axes of the RDA cumulatively accounted for more than half of the variance in all sampling months (Fig.4). The RDA of the entire dataset indicated a strong relationship between the larvae fish assemblage structure and environmental variables, C. carpio and C. auratus were inclined to habitat in the slow velocity and high transparency AFW habitat area (Fig.4a & b); Other larvae fish species were also correlated with the P.australis AFW habitat area in July (Fig.4c). The eigenvalue of the first axis in May was 14 904 and was strongly correlated with AFW habitat (Fig.4). C.carpio and C. auratus were strongly related to the negative part of the first axis in May (RDA1=14 904,RDA2=159), June (RDA1=36 816, RDA2=6 026)and July (RDA1=10 260, RDA2=64) (Fig.4).According to the above analysis, larvae fish were inclined to inhabit the AFW area because it represented a suitable environment where their habitat was degraded.
4 DISCUSSION
4.1 The P. australis AFWs altered the environmental variables in degraded habitat area
Protecting the larvae fish is an important aspect of fisheries management, yet the prerequisite oflarvae fish conservation is to create a suitable habitat forlarval fish species. The artificial structures had the function of changing the hydrology and environmental variables in the habitat area (Cristofor et al., 2003;Matheson and Sukias, 2010; Jacobs and Harrison, 2014),so they can create suitable habitat for some fish.
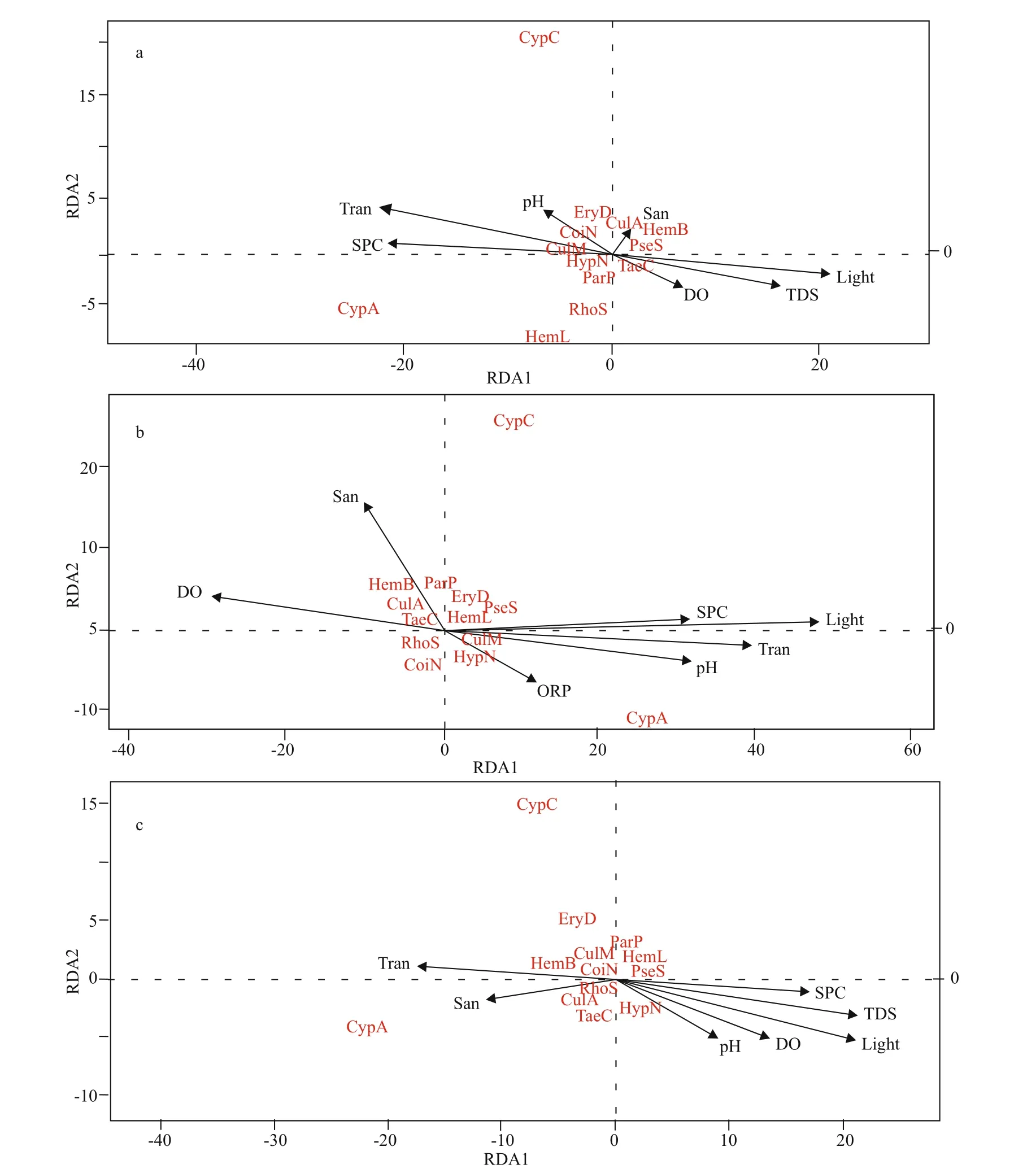
Fig.4 Associations oflarvae fish species and environmental variables between the AFW area and non-AFW area according to a RDA oflarvae fish species in May (a), June (b), and July (c)
Both creating new habitat and changing the water environmental variables were the main functions of constructed artificial structures in an ecosystem.Common eff ects of habitat degradation include impervious surface cover, the alteration of drainage density and flow dynamics, and decreasing groundwater renewal and sediment supply (Haase et al., 2013). An artificial wetland on which Typha latifolia was planted was fed with reconstituted hydrocarbon wastewater, the pH ranged between 6.9 and 8.0, and the total suspended solids were below 10 mg/L (Salmon et al., 1998). As the total suspended solids decreased in the artificial structure area, the water transparency increased, and the biomass of zooplankton was higher in the littoral zone than in thepelagic zone (Cazzanelli et al., 2008; Estlander et al.,2009). When artificial vegetation was installed in oligomesotrophic Lake Paro, the total phosphate concentration in this lake ranged from 1 060 mg/L in an uncreated area to 190 mg/L in the created habitat,and the total nitrogen concentration ranged from 9.1 mg/L in the unvegetated area to 4.9 mg/L in the created area (Seo et al., 2013). In addition, freefloating plants formed dense surface mats in the upper Mississippi River, and the water velocity changed from 0.542 to 0.095 m/s when the aquatic macrophytes covered 65% of the vegetated area (Giblin et al.,2014). Those studies indicated that the artificial structures could alter the fish habitat environment in an ecosystem.
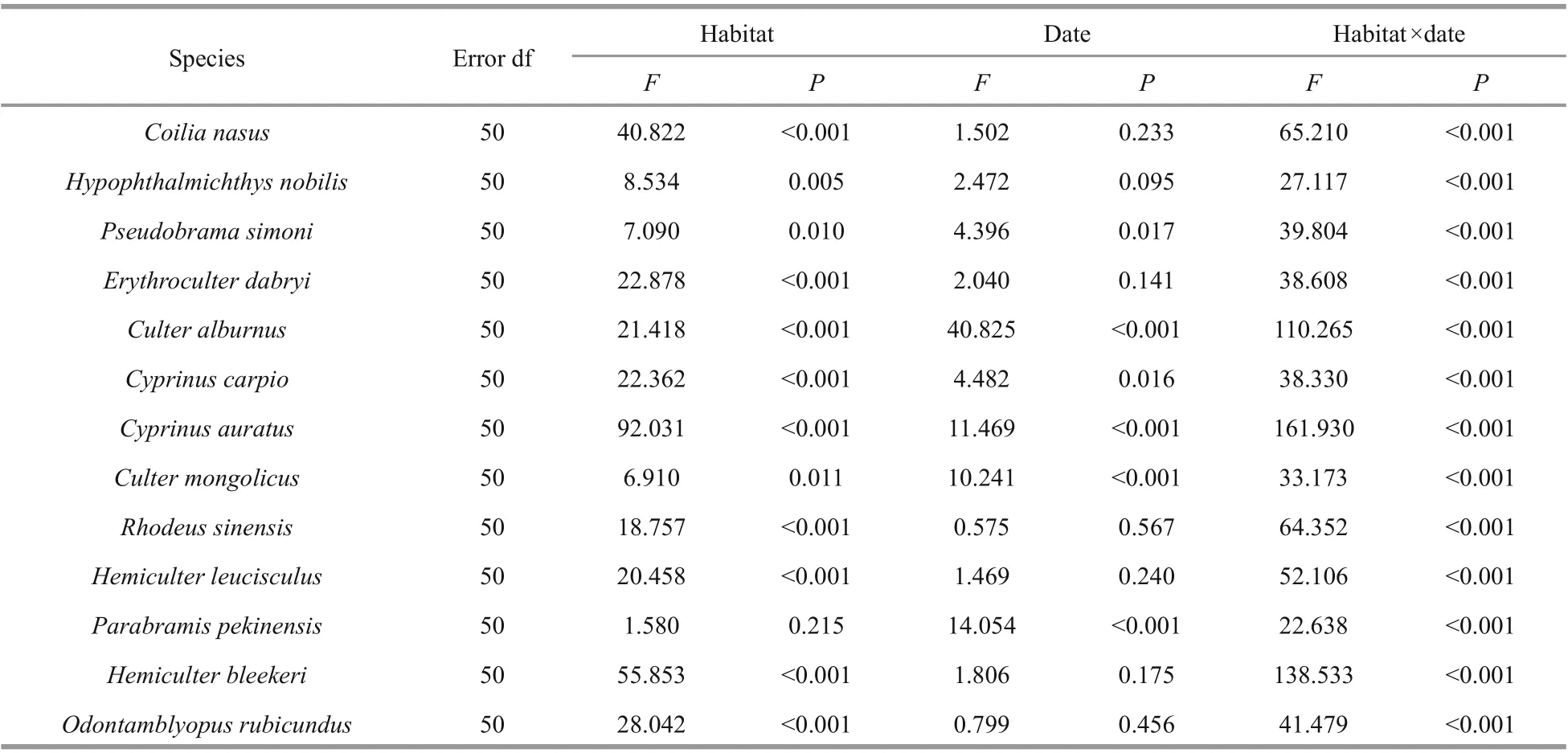
Table 2 Summary of two-way ANOVA results testing the eff ects of habitat and date on the density oflarvae fish
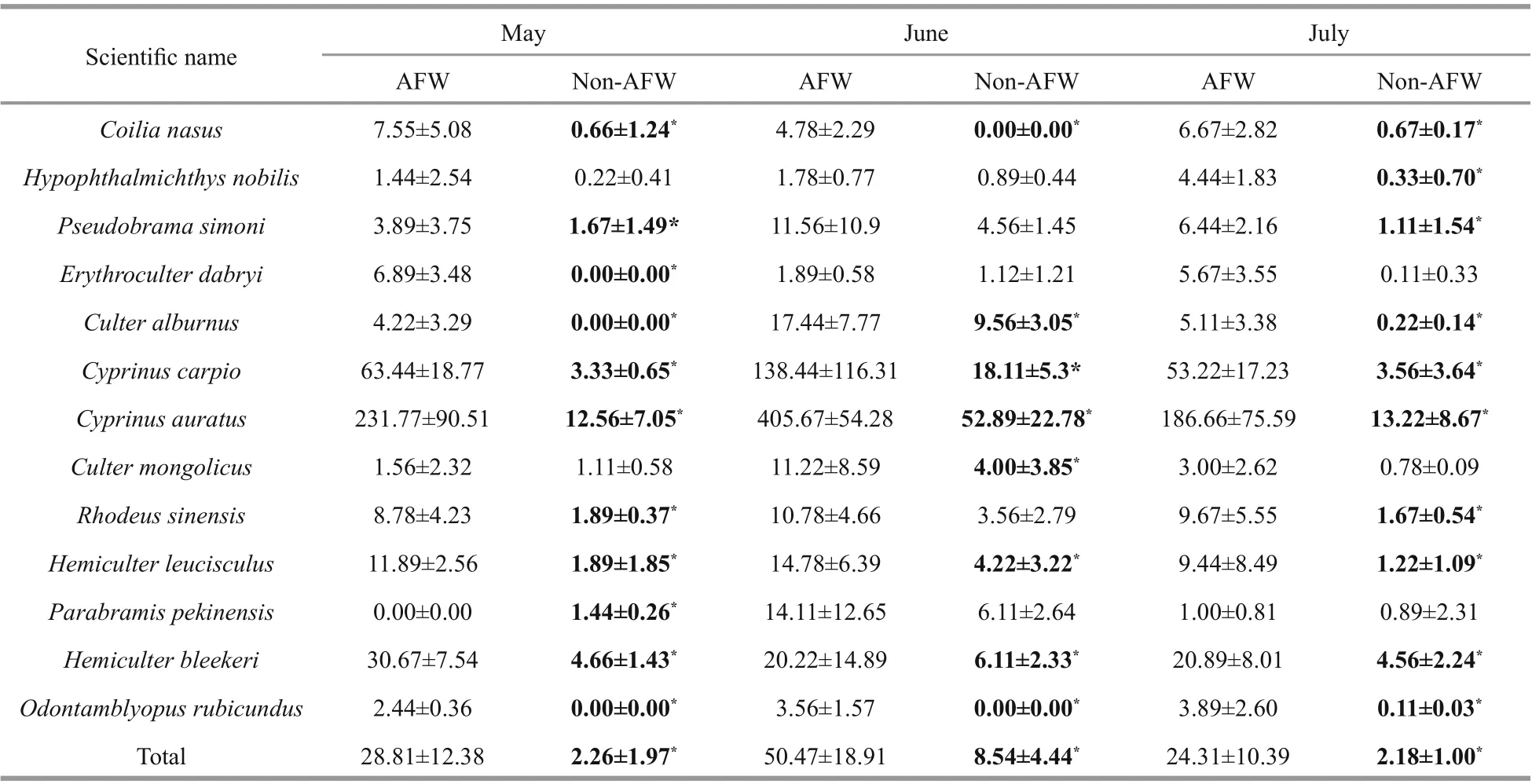
Table 3 Comparisons of the monthly average density oflarvae fish species between the AFW habitat and non-AFW habitat
Floating wetlands is a special artificial structure; it is a useful method to change the environments in an ecosystem. In the current study, the AFW habitat area was significantly diff erent with the non-AFW habitat area by the NMDS ordination (Fig.3). For example,water transparency in the AFW area was higher than that in the non-AFW area, and the water velocity was lower in the AFW sites than in the non-AFW sites(Table 3). Therefore, the P. australis AFWs changed the environmental variables of the degraded habitat area. The current study also suggested that the construction of AFWs would be an appropriate strategy for preserving and expanding the ideal habitat environment for larvae fish species.
4.2 Larvae fish species prefer habit in the P. australis AFW area to non-AFW area
Creating suitable habitat for larvae fish is a key target in fish management and conservation. Larvae fish species belong to the early stage of the life cycle,and whether they can survive in water ecosystems is a key point of fish resource management and protection(Ren et al., 2016). Because the early stages of many fish species lack swimming ability, they are inclined to inhabit low-velocity water environments with suffi cient light.
The biomass, densities, and categories of nekton were significantly higher in artificially created habitat area than that in the degraded habitat area.Barletta et al. (2010) identified habitat degradation as a principal mechanism through which urbanization impacts fish, especially when essential habitat structures for fish spawning, nursing and recruitment are lacking (Barletta et al., 2010). The densities oflarvae fish and post-larval Farfantepenaeus aztecus and Litopenaeus setiferus were significantly greater in vegetated areas than in non-vegetated areas, and 82% of all penaeids were found in vegetated areas(Howe et al., 1999). In the current study, the experiments were performed during the summer(i.e., after the reproductive season of spring), and the P. australis AFW habitat was primarily colonized by larvae fish species in along the estuary. Thirteen larvae fish species occurred in both the P. australis AFW area and the non-AFW habitat area. In the AFW habitat area, 606 inds./m2were caught, while in the non-AFW area, 76 inds./m2were found in total. Nine larvae fish species (e.g., Coilia nasus,Cyprinus carpio, and Erythroculter dabryi) were not aff ected by habitat type. Larvae fish preferentially selected AFWs over habitats naturally available in the Changjiang River estuary, as revealed by differences in richness and abundance. This evidence suggests that larvae fish species are inclined to assemble and habitat in the P. australis AFW area.
4.3 The P. australis AFWs facilitate larvae fish conservation in the habitat degraded area
Habitat fragmentation represents one of the most important global threats to biodiversity in recent decades. Fish habitat areas are strongly influenced by human settlements and agricultural and industrial land use, making them particularly prone to degradation.
Fish habitat fragmentation originates from the behavior of human beings. Estuaries, lakes, and their floodplains have always been preferential sites for human settlement because they provide access to drinking water, fishing, agriculture, and transport.The environments and biodiversity distributions along the Changjiang River estuary were seriously threatened in recent decades. Because ofland reclamation, floodplain drainage, hydropower production, and channelization for navigation,freshwater systems were heavily changed and destroyed during recent centuries (Zhou et al., 2015).Because the banks were rebuilt by concrete to utilize the water resources, hydrophytes cannot propagate and/or grow along the estuary. As a result, the larvae fish habitat in these freshwater ecosystems was destroyed.
A low survival rate oflarvae fish occurs under natural conditions because of the lack of suitable environmental conditions because of the destruction of their habitat areas. There is growing concern over whether artificial structures can rehabilitate larvae fish habitat, and the eff ectiveness of synthetic structures has been increasingly investigated in terms of providing suitable habitats for fish species(Knaepkens et al., 2004). Therefore, knowledge of their foraging ecology is fundamental to understanding the processes that function at the individual,population and community levels and in the management and conservation of their populations and habitats. Artificial structures improve the quality and variety of degraded habitats, and their implementation is a suitable measure for preserving the populations oflarvae fish species. This study demonstrated that the larvae fish habitats might be expanded by constructing P. australis AFWs. The deployment of AFWs is a good alternative measure for creating suitable habitats for larvae fish in the estuary where their habitat has been degraded. When the P. australis AFWs were applied in large-scale fishery management, they significantly contributed to improving larvae fish recruitment along the Changjiang River estuary.
5 CONCLUSION
The P. australis AFWs were constructed to recover the lost habitat for the larvae fish species in the Changjiang River estuary. Water transparency was consistently higher in the AFW area than in the non-AFW area in each sampling month, and the average water temperature and velocity in the AFW habitat was lower than that in the non-AFW area. Most larvae fish were inclined to assemble in the AFW habitat area. This AFW served as an ideal habitat for larvae fish species through changing the environment. The construction of AFWs would be an appropriate strategy for preserving and expanding the larvae fish populations in the habitat degraded estuary.
6 DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
7 ACKNOWLEDGMENT
We thank the Engineering Research Center of Ecology and Agricultural Use of Wetlands, Ministry of Education for help in the field work, the laboratory workers from the key laboratory of Fisheries Ecology of the Yangtze Estuary, CAFS, and the East China Sea Fisheries Research of Agriculture and Wildlife Service for assistance. Finally, we are also grateful for the comments from anonymous reviewers.
8 AUTHORS’ CONTRIBUTION
Conceived and designed the investigation: HXF,ZP. Performed field and/or laboratory work: SC, CY.Analyzed the data: HXF, ZF. Contributed materials,reagents, and/or analysis tools: WQ, ZF.
 Journal of Oceanology and Limnology2021年2期
Journal of Oceanology and Limnology2021年2期
- Journal of Oceanology and Limnology的其它文章
- Predicting sediment flux from continental shelfislands,southeastern China*
- Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom*
- Development of high-resolution chloroplast markers for intraspecific phylogeographic studies of Phaeocystis globosa*
- Effects ofiron and humic acid on competition between Microcystis aeruginosa and Scenedesmus obliquus revealed by HPLC analysis of pigments*
- Effect of river plume on phytoplankton community structure in Zhujiang River estuary*
- Exploring the sublethal genotoxic effects of class II organophosphorus insecticide quinalphos on freshwater fish Cyprinus carpio
