Natural predators of polyps of three scyphozoans: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum*
Changsheng TANG , Song SUN ,5,**, Fang ZHANG 1, 2, 3, 4
1 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory of Marine Ecology and Environmental Sciences, Qingdao National Laboratory for Marine Science and Technology,Qingdao 266071, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
5 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Jellyfish blooms have become a hot research topic in recent decades because they pose a serious threat to fisheries, coastal industries, tourism, and the marine ecosystem. The life cycle of scyphozoan jellyfish consists of a pelagic medusa stage and a benthic polyp stage, where asexual reproduction and strobilation of the polyps directly aff ect the abundance of ephyra and subsequently medusa abundance. The dynamics of polyps are aff ected by both environmental and biological factors, and predation by natural predators is one of the most important biological factors. Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum are three scyphozoan species that are commonly found in Chinese coastal waters,and previous studies reported that the survivorship of polyps diff ers among the three species when they are exposed to the same benthic community. To identify potential natural predators of polyps of these three species in Chinese coastal waters and to determine whether the predation rates on polyps of the three species diff er, we collected 39 species of macrozoobenthos from the Bohai Sea, Yellow Sea, and East China Sea from May 2014 to June 2016 and conducted predation tests and predation rate measurements. We found that the nudibranchs Pleurobranchaea novaezealandiae, Okenia plana, and Chromodoris tinctoria and the sea anemones Paracalliactis sinica, Calliactis japonica, Anthopleura incerta, and Anthopleura midori could prey on the polyps of all three scyphozoan species. The predation rates increased with the body length of the predators. The predations rates were also related to the polyp species, although the diff erent predators showed no consistent preference for a particular species of polyp. Our results indicate that introducing predators to locations inhabited by polyps might be a way to control the benthic polyp populations and prevent subsequent jellyfish blooms.
Keyword: jellyfish bloom; polyps; predation rate; predators
1 INTRODUCTION
Reports of jellyfish blooms have increased worldwide in recent decades and have aroused public attention (Mills, 2001; Purcell et al., 2007; Holst,2012; Lee et al., 2017; Feng et al., 2018). Discussions of whether jellyfish population sizes are really increasing has been debated (Purcell, 2012; Condon et al., 2013), and Chinese coastal seas (i.e., Bohai Sea,Yellow Sea, and East China Sea) are now recognized as hot spots for regional jellyfish blooms (Sun et al.,2015a,b). Blooms of scyphozoans such as Nemopilema nomurai (Zhang et al., 2012; Uye, 2014), Aurelia coerulea (Feng et al., 2018; Dong et al., 2019), and the releasing species Rhopilema esculentum (Dong et al., 2009, 2014) is particularly common in these areas.Blooms of these previous two species can cause serious damage to fisheries by clogging and destroying fishing nets and preying on larval fish (de Lafontaine and Leggett, 1988; Kawahara et al., 2006; Uye, 2014;Miyajima-Taga et al., 2016), to coastal industries by clogging cooling water systems (Dong et al., 2010),to tourism by stinging tourists at beaches (Fenner et al., 2010), and to marine ecosystems by shifting from fish-dominated to jellyfish-dominated systems (Uye,2011).
The life cycle of scyphozoans consists of a pelagic sexual medusa stage and a benthic asexual polyp stage (Lucas et al., 2012; Thein et al., 2012). To better understand the sudden outburst of adult medusae, it is essential to study the polyp stage, as asexual reproduction and strobilation of the polyps directly determine the abundance of medusae (Arai, 2009;Hoover et al., 2012; Lucas et al., 2012). The population dynamics of polyps are determined by various factors,including temperature and food supply (Han and Uye,2010; Feng et al., 2015; Purcell et al., 2019), substrate preference and availbility of man-made surfaces(Holst and Jarms, 2007; Hoover and Purcell, 2009),space competition (Watanabe and Ishii, 2001),hypoxia (Condon et al., 2001; Shoji et al., 2010),climate conditions (Lucas, 2001; Purcell et al., 2007),and predation (Hernroth and Gröndahl, 1985; Hoover et al., 2012; Takao et al., 2014). Among these factors,little is known about the eff ects of predation.
Hernroth and Gröndahl (1985) reported that the nudibranch Coryphella verrucosa could prey on polyps of Aurelia aurita which led to the drastic decline of polyp abundance. Thiel (1962) found that the aeolid nudibranch Facelina bostoniensis was an important predator of polyps. Other polyp predators included caprellid amphipods, pycnogonids, and decapods (Uchida and Hanaoka, 1933; Oakes and Haven, 1971; Hutton et al., 1986). Takao et al. (2014)identified eight species as the key polyp predators,with predation rates ranging from 27 to 672 inds./(predator·d). However, the composition, quantity distribution, and biodiversity of the macrobenthic fauna in Chinese coastal waters (Peng et al., 2014)diff er from those in Japanese coastal waters (Okanishi et al., 2016), thus the potential predators of polyps likely diff er between the two regions.
Polyp distribution also can be aff ected by sessile zoobenthos, such as cnidarians and bryozoans, which can be food or space competitors of sessile polyps(Watanabe and Ishii, 2001; Suzuki et al., 2016).Sessile zoobenthos species and polyps can encounter each other in at least four ways: developing near each other, reproducing asexually and extending their spatial distribution, using stolons to ‘walk’ around diff erent surfaces, and detaching and floating to new locations (Hoover and Purcell, 2009; Tang et al.,2020). When competition occurs, intraguild predation might occur (Arai, 1997), which involves killing and eating among potential competitors for a resource(Polis and Holt, 1992; Arim and Marquet, 2004).Consequently, sessile zoobenthos species are potential intraguild predators of polyps.
In a recent in-situ simulation experiment, Feng et al. (2017) exposed polyps of N. nomurai, A. coerulea,and R. esculentum to the same enviromental conditions and macrobenthic community and found that the survivorship of polyps diff ered. They reported that survivorship of A. coerulea polyps increased, whereas those of N. nomurai and R. esculentum decreased to diff erent degrees. The authors hypothesized that diff erent asexual reproduction modes of the three species and diff erent predation rates of a given potential predator on polyps of diff erent species might be important factors aff ecting polyp survivorship.
To the best of our knowledge, no previous study has investigated the predation rates of potential predators on polyps of diff erent scyphozoan species.The aim of this study was to identify potential predators of polyps of N. nomurai, A. coerulea, and R. esculentum in Chinese coastal waters and to determine whether the predation rates of these predators diff er among the three polyp types.
2 MATERIAL AND METHOD
2.1 Stock culture
Six mature females and four mature males of medusae of N. nomurai were captured using hand nets in Jiaozhou Bay in September 2013. They were transferred to a 30-m3pond for sexual fertilization in the laboratory of the Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China. The rearing temperature and salinity were maintained at 20±0.5 °C and 30±0.5, respectively. Parent medusae were removed after planulae were detected. Concatenated polyethylene corrugated plates were placed in the pond to allow for attachment of the planulae. Millions of N. nomurai polyps with two to four tentacles settled onto the plates within a week and grew into fully developed polyps with 16 tentacles within one month.
Polyps of R. esculentum were obtained from the Yancheng Jinyang Aquatic Products Breeding Company in Jiangsu, China, in June 2014. Polyps of A. coerulea were cultured at the Institute of Oceanology, Chinese Academy of Sciences, Qingdao.Mature medusa of A. coerulea were collected from Jiaozhou Bay in May 2014 and transferred to a circulating water tank with constant temperature(20±0.5 °C) and constant salinity (30±0.5) for fertilization. Polyethylene corrugated plates were used to allow for attachment of planulae. Fully developed polyps of A. coerulea were formed after a month.
2.2 Macrozoobenthos collection and maintenance
A total of 39 species of macrozoobenthos were collected (Table 1). Most of the macrozoobenthos samples were collected from 43 stations during eight cruises in the Bohai Sea, Yellow Sea, and East China Sea (Fig.1) by Agassiz trawls and bottom fishery resource trawls from May 2014 to June 2016. The Agassiz trawls were conducted onboard the R/Vs Science 1, Science 3, and Beidou. The fishery resource trawls were conducted only on the R/V Beidou. The Agassiz net width was 1.5 m, and the trawl duration was 15 min at every station at a speed of ~2 knots.The fishery resource net circumference was 167.2 m,and the trawl duration was 30-60 min at every station at a speed of ~3 knots. After retrieving each trawl, live macrozoobenthos were classified to species and transferred to an incubator onboard the vessel.
In the lab, the cultivation container (25 cm×20 cm×20 cm) for each species was half filled with filtered (160 μm) ambient seawater and oxygenated continuously. The cultivation temperature was maintained at 15±0.5 °C. The macrozoobenthos species were mainly fed with dried shrimp and newly hatched Artemia nauplii every 2 d. The seawater was replaced with freshly filtered seawater of the same temperature every 2 d. Ice bags were added to the cultivation containers to keep predators in good condition, and they were transported to laboratory of the Institute of Oceanology, Chinese Academy of Sciences, Qingdao.
In addition to the trawl sampling, three species of sea anemone ( Anthopleura incerta, Anthopleura midori, and Anthopleura qingdaoensis) were collected using a hand-held shovel from the intertidal zone of Huiquan Bay, Qingdao (S44 in Fig.1). Two species of crustacean ( Corophium major and Balanus amphitrite), one species of mollusc ( Mytilus edulis),and one species of bryozoan ( Bugula neritina) were collected from a floating dock in Jiaozhou Bay (S45 in Fig.1).
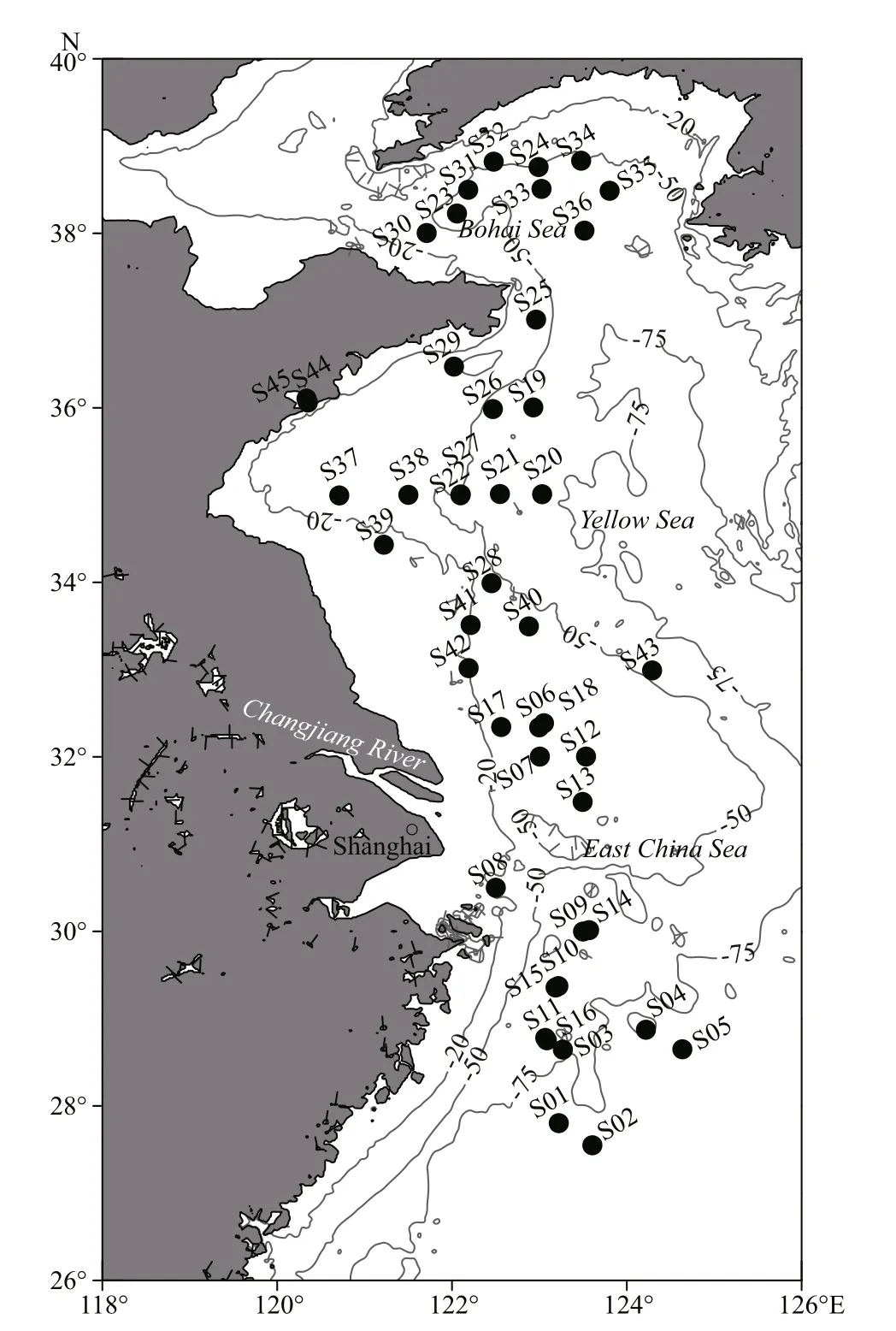
Fig.1 Sample stations of macrozoobenthos in the Bohai Sea,Yellow Sea, and East China Sea from 2014 to 2016
2.3 Experimental design
The corrugated plates with polyps of N. nomurai,A. coerulea, and R. esculentum attached were cut into small pieces (ca. 8 cm×10 cm) and separately linked together. They then were cultivated in an incubator(15±0.5 °C) and fed with newly hatched Artemia nauplii every 2 d. The water in the tank was replaced with filtered (160 μm) ambient seawater every 2 d.
The macrozoobenthos were starved for 24 h before conducting the predation experiments to standardize the feeding conditions. To identify which of the 39 macrozoobenthos species are potential predators of polyps, we conducted the following experiment for each type of polyp. After counting the number of polyps present on a piece of corrugated plate, the plate was transferred to a tank containing the macrozoobenthos. The predation behaviors were monitored for 1-3 d. The macrozoobenthos species then were starved for 1 day before the predation test was conducted for the second polyp species, and the process was then repeated for the third polyp species.Seven potential predator species were identified(Table 1). Only one or two specimens of Chromodoris tinctoria, Calliactis japonica, and A. midori were collected, so it was impossible to have replicate measurements. Therefore, predation rates were measured only for the other four species:Pleurobranchaea novaezealandiae, Okenia plana,Paracalliactis sinica, and A. incerta.
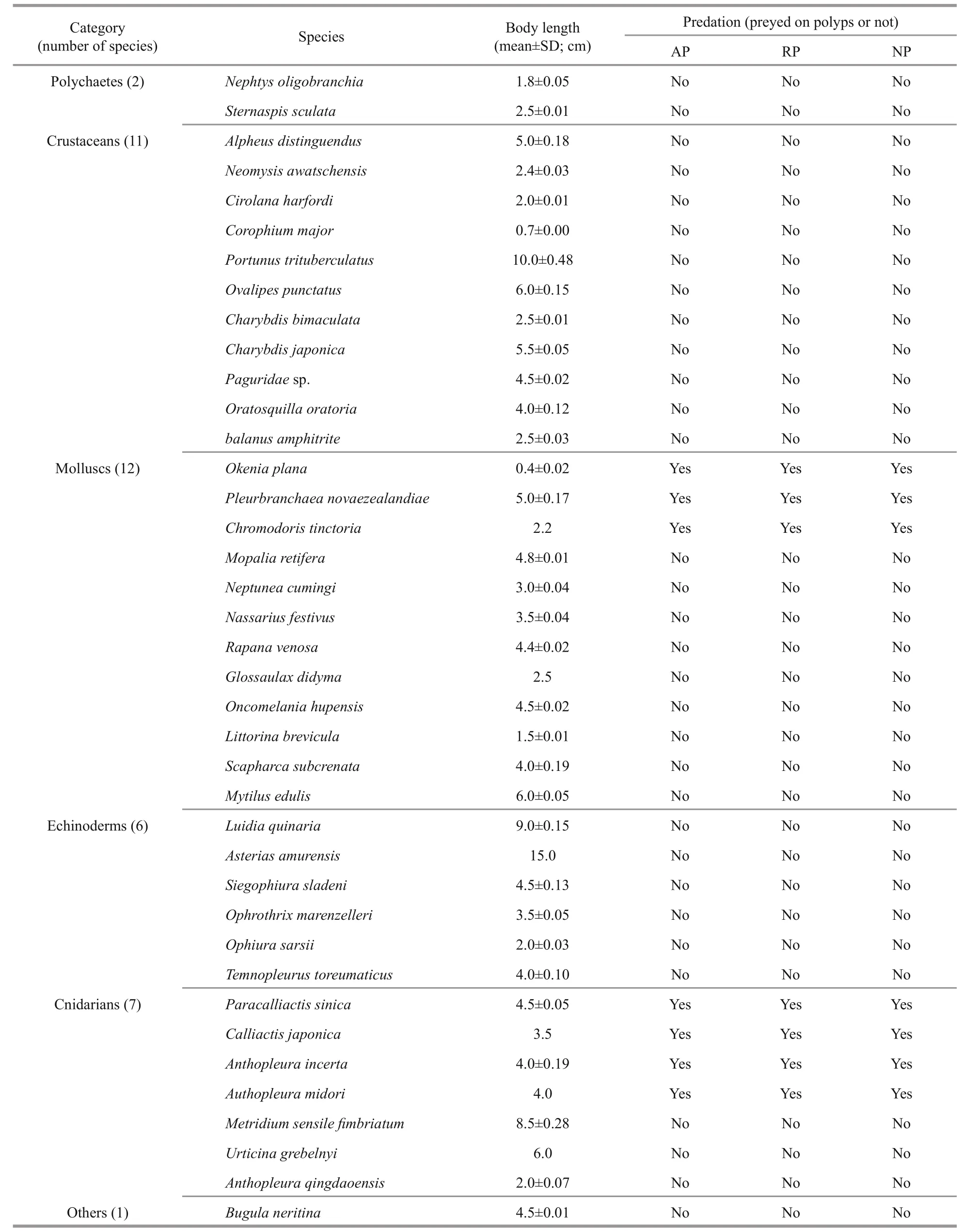
Table 1 List of macrozoobenthos collected and their predation activity on polyps of A. coerulea, R. esculentum, and N.nomurai
For the predation rate experiment, the body length of the predators was measured and they were starved for 24 h. Freely moving P. novaezealandiae and O. plana were introduced to a plate (ca. 15 cm×18 cm)with polyps of A. coerulea attached (average density 8.5±1.6 inds./cm2). The culture temperature was 15±0.5 °C. The number of missing polyps was counted after 24 h to calculate the predation rate of the predators. The sessile sea anemones P. sinica and A. incerta were fed with polyps of A. coerulea, which were detached carefully from the root of the pedal disc with a dissecting needle under a dissecting microscope. For every feeding, ten polyps were collected into a pipette and released within the tentacles of the sea anemones. The anemones were fed every hour for 18 h during a single day (6꞉00-24꞉00). The number of polyps ingested was counted to calculate the predation rate. The experimental temperature was maintained at 15±0.5 °C, and the seawater in the plastic container (25 cm×20 cm×20 cm) was replaced with fresh sand-filtered seawater of the same temperature daily. Two to four body sizes(i.e., lengths) for each of the four predator species were tested, with three replicates per body length level.
The predators were starved for 24 h and their predation rates on polyps of R. esculentum and N. nomurai were measured using the same predators and the same method. The average densities of polyps of R. esculentum and N. nomurai were 6.6±1.2 inds./cm2and 1.3±0.4 inds./cm2, respectively.
2.4 Statistical analysis
Normality and homogeneity of variance of the data were tested (SPSS 23.0) before conducting statistical analysis. Because the data met normality and homogeneity of variance criteria, two-way analysis of variance (ANOVA) was used to test four null hypotheses: The predation rate of P. novaezealandiae,O. plana, P. sinica, or A. incerta on polyps was not related to predator body length or prey polyp species.If an interaction between body length and polyp species was detected, simple eff ect analysis was used for further pairwise comparison. If no interaction between the two factors was detected, the Sidak method of one-way ANOVA for each factor was used for further pairwise comparison. The figures were created using GraphPad Prism 8. Relationships between predator body length and predation rate were explored using linear fitting regression analysis in GraphPad Prism 8.
3 RESULT
3.1 Predators of polyps
In total, 39 species of macrozoobenthos were collected, and they survived for over a month under the culture conditions used. Of species, three of nudibranch ( O. plana, P. novaezealandiae, and C. tinctoria) and four of sea anemone ( P. sinica,C. japonica, A. incerta, and A. midori) were found to prey on polyps of all three scyphozoans (Table 1).The crustaceans Alpheus distinguendus, Charybdis bimaculata, and Oratosquilla oratoria, and the echinoderms Siegophiura sladeni and Ophiura sarsii occasionally damaged polyps but did not prey on them. The gastropods Nassarius festivus, Rapana venosa, and Littorina brevicula crawled on the plates with polyps attached, but they retracted their tentacles after contacting the polyps and moved away in other directions.
3.2 Predation rate
Figure 2 shows the predation rate of diff erent sizes of the four predators on diff erent polyp species as well as the significant linear fitting regression models.P. novaezealandiae and O. plana were able to completely scrape off the polyps at the root of pedal disc using their radula and then ingest the polyps.Their predation rates were 27-78 inds./(predator·d)and 30-118 inds./(predator·d), respectively. Both predators had strong adhesion ability and were able to crawl to the bottom surface of the plates and prey on the polyps.
When A. incerta was fed with polyps, its whole body inflated and the oral disc twisted and folded,which allowed it to move the polyps towards and then into the central mouth. P. sincia used its outer tentacles to capture polyps and then transfer them to the inner tentacles, which moved them into the mouth. Some of the anemones stopped ingesting polyps when they were satiated, but after 2-3 h of digestion, they began to ingest polyps again. The predation rates of these two species were 20-76 and 40-240 inds./(predator·d), respectively.
3.3 Statistical results
Two-way ANOVA results (Table 2) show that the predation rates of the four predators diff ered significantly with predator body length and polyp species, thus all four null hypotheses were rejected.Interactions between predator body length and prey polyp species were detected for P. novaezealandiae and O. plana, so simple eff ect analysis was used for further pairwise comparison. No interactions between the two factors were detected for A. incerta and P. sinica, so the Sidak method was used for further pairwise comparison.
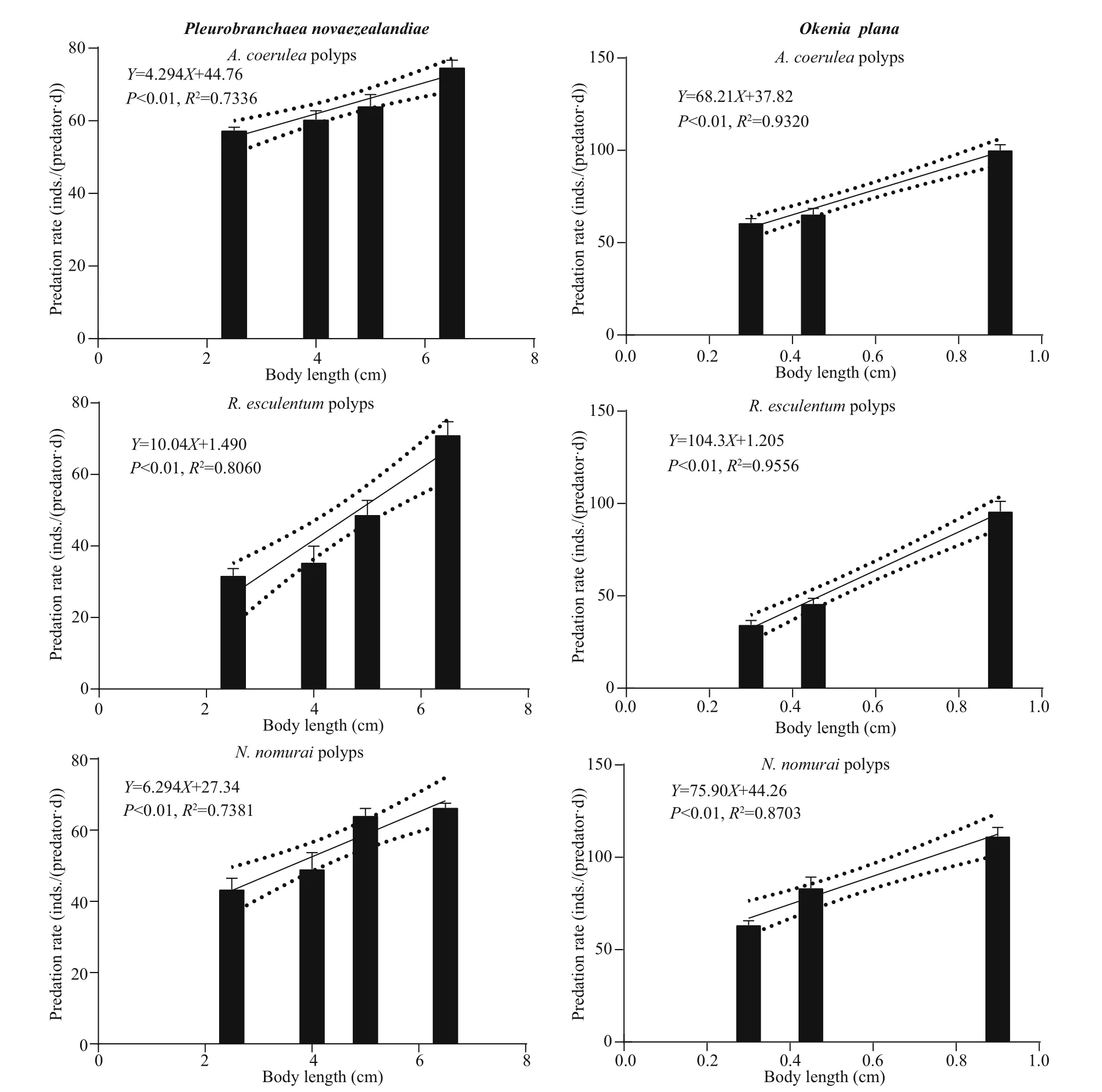
Fig.2 Predation rates of diff erent sizes of the four predators on polyps of A. coerulea, R. esculentum, and N. nomurai
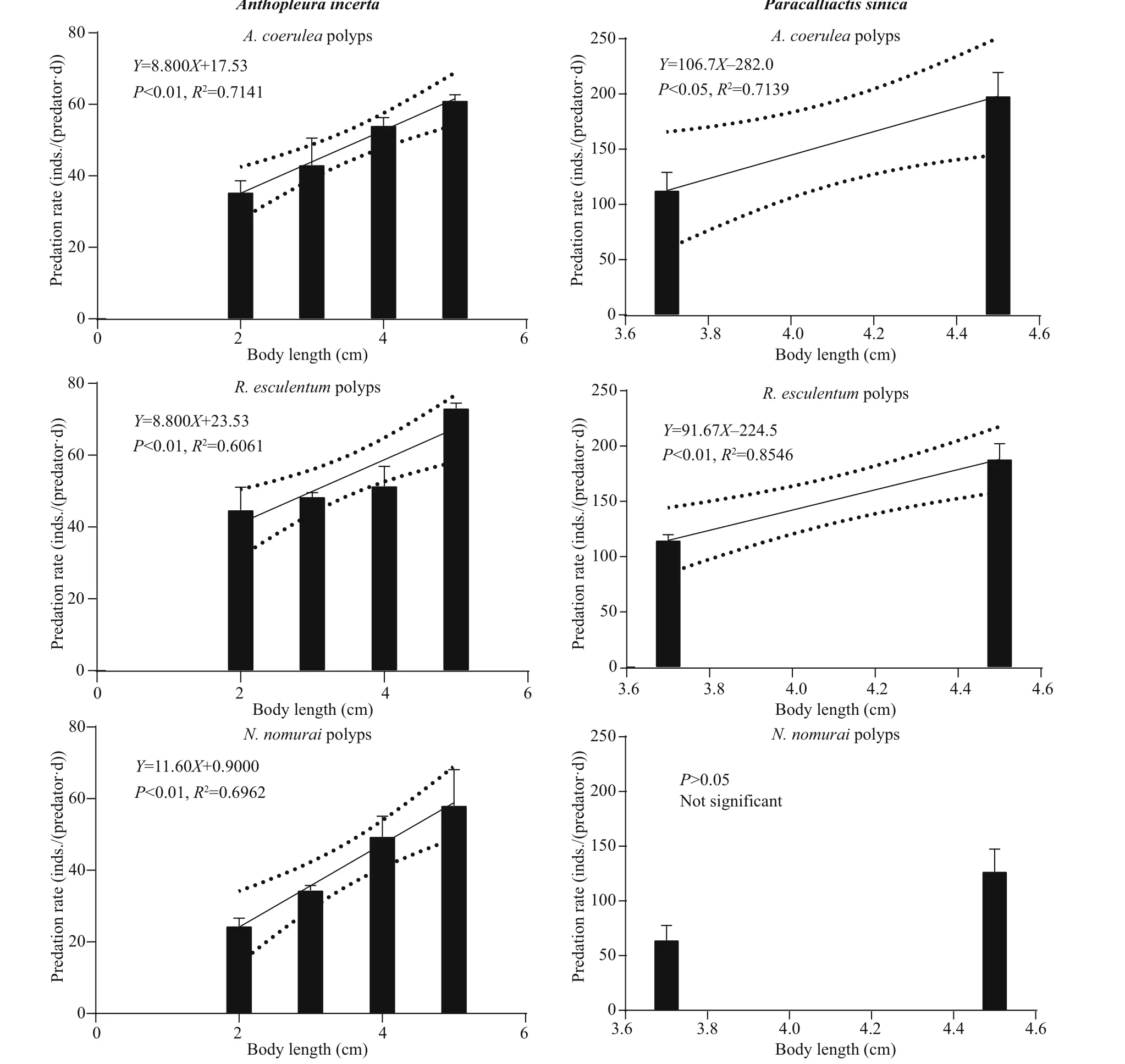
Fig.2 Continued
The linear fitting regression results showed that the predation rates were all positively correlated ( P <0.05)with predator body length (Fig.2), except for the predation rate of P. sinica on N. nomurai polyps( P >0.05). However, the predators showed no consistent predation preference for polyps of the three species (Fig.3). Further pairwise comparison results(not shown) revealed that for the same species of predator, the predation preference for polyp species diff ered for diff erent body lengths. Additionally,diff erent predator species preferred diff erent polyp species.
4 DISCUSSION
Three species of nudibranchs and four species of sea anemones were identified as potential predators of polyps of A. coerulea, R. esculentum, and N. nomurai.Nudibranchs are well-known predators of polyps of cnidarian species (Hernroth and Gröndahl, 1985;Martin, 2003; Hoover et al., 2012; Takao et al., 2014).Nudibranchs have a thick cuticle in their mouth to avoid being stung by the nematocysts of cnidarians(Cattaneo-Vietti et al., 1993; Martin, 2003;Greenwood, 2009). They can inhibit discharge of nematocysts by secreting mucus (von Salvini-Plawen,1972; Conklin and Mariscal, 1977; Bertsch, 1984)and can store and excrete indigestible nematocysts(Miller and Byrne, 2000). Three species of nudibranchs collected in Chinese coastal waters were able to prey on polyps of A. coerulea, R. esculentum,and N. nomurai, but their predation rates were lower than those reported by Hernroth and Gröndahl (1985)and Takao et al. (2014), indicating that their predationeffi ciency was relatively low. Predation effi ciency likely varies among species, as previous studies showed that the predation rate of diff erent predators on polyps diff ered (Hoover et al., 2012; Takao et al.,2014), and Pteraeolidia ianthina, Sakuraeolis gerberina, Aeolidina sp. (Takao et al., 2014), and Dendronotus arborescens (Feng et al., 2017) did not feed on A. aurita polyps at all.
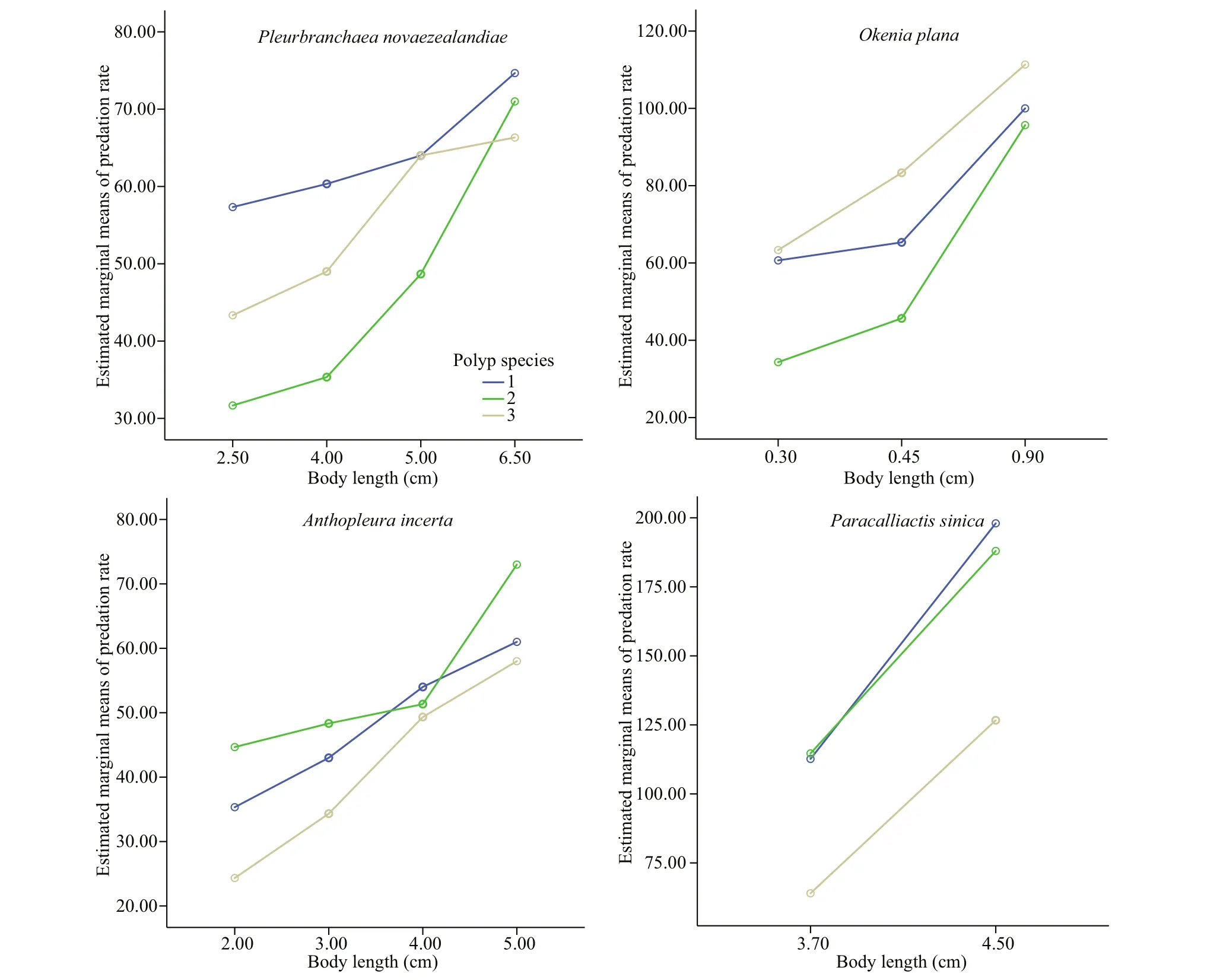
Fig.3 Predation rates and predator sizes on polyps of A. coerulea, R. esculentum, and N. nomurai based on the estimated marginal means
In our study, the four species of sea anemone ingested the polyps of A. coerulea, R. esculentum, and N. nomurai, suggesting that a comprehensive assessment of the eff ects of sea anemone communities on the distribution of polyps is needed. Sea anemones are soft animals, and polyps cannot attach to them because they require a hard substrate for attachment(Lucas, 2001; Holst and Jarms, 2007; Hoover and Purcell, 2009). Thus, competition for space between sea anemones (and other benthic organisms) andpolyps could aff ect the population dynamics of polyps(Watanabe and Ishii, 2001; Feng et al., 2017). Our predation experiments showed that A. incerta,P. sinica, C. japonica, and A. midori were potential intraguild predators of the polyps. Consequently,locations inhabited by sea anemones were unfavorable for the formation and development of the polyps.
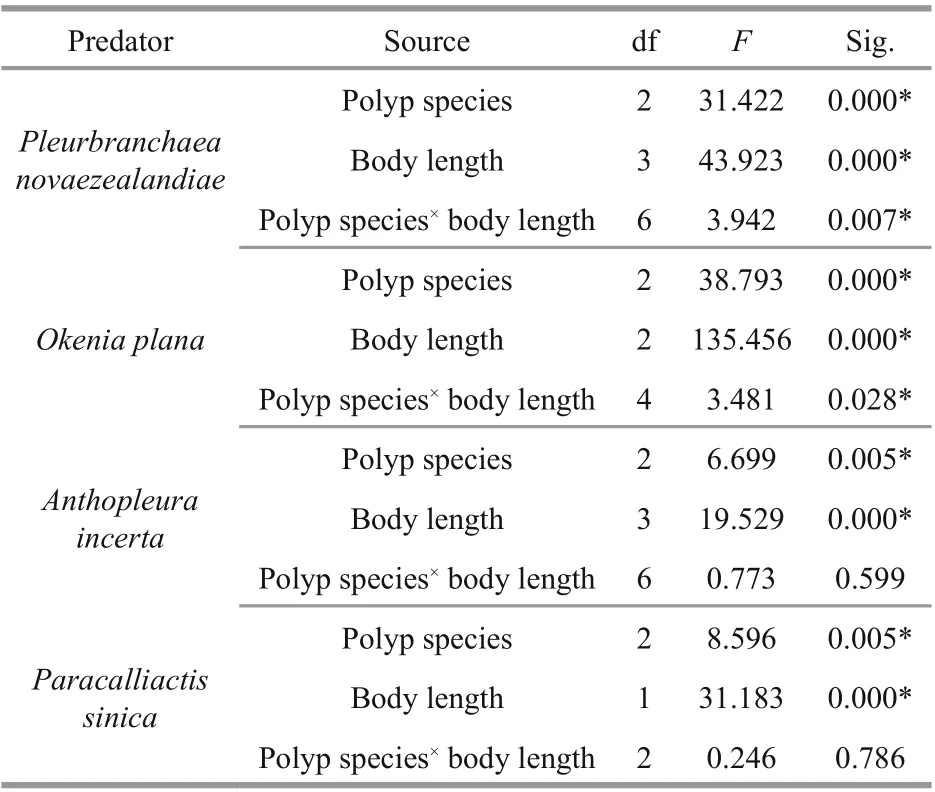
Table 2 Summary of two-way ANOVA results for the predation rate of the four predators on polyps
The predation rate increased with predator body length, which is in consistent with previous reports(Hoover et al., 2012; Takao et al., 2014; Feng et al.,2017). The statistical analysis results showed that the predation rate of the specific predators on polyps of N. nomurai, A. coerulea, and R. esculentum diff ered significantly. However, the predators showed no consistent preference for a particular species of polyp(Fig.3). For example, the predation rate of P. novaezealandiae was highest for A. coerulea polyps,but the predation rate of O. plana was highest for N.nomurai polyps. For the same predator, the predation preference also diff ered among diff erent body lengths.At a body length of 2.5 cm, the predation rate of P.novaezealandiae on N. nomurai polyps was higher than that on R. esculentum polyps, but at a length of 6.5 cm, the predation rate on N. nomurai polyps was lower than that on R. esculentum polyps. A similarity pattern was observed for the other predator species.
Nudibranchs can completely remove polyps at the root of pedal disc, leaving no basal part that could regenerate new polyps under favorable environmental conditions (Laurie-Lesh and Corriel, 1973). Although polyps usually are found hanging upside down (Holst and Jarms, 2007), the strong adhesion ability of nudibranchs allows them to prey on polyps in hidden sheltered locations. Thus, the predation capacity of nudibranchs might be used to regulate populations of benthic polyps (Takao et al., 2014). Introducing these predators to locations inhabited by polyps might be an important way to control the benthic polyp population and the subsequent abundance of pelagic medusae.However, enclosure experiments in the field are needed to simulate and evaluate the possible negative ecological impacts caused by the introduction of predators.
Benthic organisms can aff ect jellyfish population dynamics by providing settling substrate for the planulae (positive eff ects), competing with polyps for space and preying on polyps (negative eff ects), and preying on the planulae and the ephyrae (negative eff ects) (Suzuki et al., 2016). The results of our predation experiments showed that most species of macrozoobenthos do not directly prey on the polyps.Therefore, whether a change of benthic community structure would aff ect the survival of polyps and the degree of that influence would depend on the specific structure of a given benthic community. Sea areas dominated by nudibranchs and sea anemones likely would be unfavorable for formation and reproduction of polyps, whereas sea areas dominated by other organisms, such as crustaceans, echinoderm, and gastropods, would be more appropriate for polyp development. This study was a first step towards a deeper understanding of the relationship between long-term changes in benthic community structure and jellyfish blooms.
5 CONCLUSION
Of the 39 species of macrozoobenthos collected,seven were identified as predators of polyps of N. nomurai, A. coerulea, and R. esculentum. The predation rate was related to predator body length and polyp species. The predation rates increased with predator body length, but the predators showed no consistent preference for polyp type. Transplanting these predators to locations inhabited by polyps might be a way to control the benthic polyp population and subsequent blooms of pelagic medusae. Based on our results, zoobenthos communities dominated by nudibranchs and sea anemones would be unfavorable for the formation and reproduction of polyps, whereas those dominated by other taxa, such as crustaceans,echinoderm, and gastropods, would be more appropriate for the development of polyps.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available upon request by contacting with the corresponding author.
 Journal of Oceanology and Limnology2021年2期
Journal of Oceanology and Limnology2021年2期
- Journal of Oceanology and Limnology的其它文章
- Predicting sediment flux from continental shelfislands,southeastern China*
- Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom*
- Development of high-resolution chloroplast markers for intraspecific phylogeographic studies of Phaeocystis globosa*
- Effects ofiron and humic acid on competition between Microcystis aeruginosa and Scenedesmus obliquus revealed by HPLC analysis of pigments*
- Effect of river plume on phytoplankton community structure in Zhujiang River estuary*
- Exploring the sublethal genotoxic effects of class II organophosphorus insecticide quinalphos on freshwater fish Cyprinus carpio
