Influence of NaOH Concentration on Sodium Storage Performance of Na0.44MnO2
Hui Li , Shuangyu Liu , Tianci Yuan , Bo Wang , Peng Sheng , Li Xu , Guangyao Zhao ,Huitao Bai , Xin Chen , Zhongxue Chen , Yuliang Cao ,*
1 State Key Laboratory of Advanced Power Transmission Technology, Global Energy Interconnection Research Institute Co.Ltd.,Beijing 102211, China.
2 Hubei Key Laboratory of Electrochemical Power Sources, College of Chemistry and Molecular Sciences, Wuhan University,Wuhan 430072, China.
3 Key Laboratory of Hydraulic Machinery Transients, Ministry of Education, School of Power and Mechanical Engineering,Wuhan University, Wuhan 430072, China.
Abstract: Aqueous sodium ion batteries (ASIBs) have attracted considerable attention for large-scale energy storage because of their prominent advantages of low cost, high safety, and environment-friendliness.Among the reported cathode materials for ASIBs, Na0.44MnO2 exhibits outstanding structural and hydrochemical stability, and hence is of much interest to research scholars.However, the reversible capacity of Na0.44MnO2 in most of the reported ASIBs was only 40 mAh·g-1 due to the restriction of stable working windows, although the in spite of theoretical capacity is121 mAh·g-1.Recently, we reported a Zn/Na0.44MnO2 dual-ion battery (AZMDIB) based on a Na0.44MnO2 positive electrode, Zn negative electrode, and 6 molL-1 NaOH electrolyte.The alkaline solution lowered the proton insertion potential and expanded the stable working window of the Na0.44MnO2 electrode, thus enhancing the reversible capacity to 80 mAh·g-1.Previous studies have demonstrated that the composition, concentration,and pH of the electrolytes have significant effects on the stable electrochemical window, rate performance, cycling performance, and other electrochemical properties of aqueous batteries.In addition, it has been reported that the cointercalation of hydrogen ions can be inhibited by increasing the pH of the electrolyte in order to improve the cyclic stability of the electrode.Therefore, exploring the effect of electrolyte concentration and pH on the electrochemical performance of Na0.44MnO2 can provide insight into the design and optimization of high-performance Zn/Na0.44MnO2 aqueous batteries.Hence, in this work, rod-like Na0.44MnO2 was synthesized by ball milling and subsequent high-temperature calcination, and the influence of NaOH concentration on the electrochemical performance of the Na0.44MnO2 electrode was investigated by adopting five different concentrated electrolytes, 1, 3, 6, 8, and 10 mol·L-1 NaOH.The results showed that an increase in NaOH concentration is beneficial for preventing the insertion of protons and improving the cycling performance and the rate performance of the electrode; however, it also leads to premature triggering of the oxygen evolution reaction.Moreover,the rate performance would decrease at high NaOH concentration.The Na0.44MnO2 electrode showed optimal electrochemical performance in 8 mol·L-1 NaOH.At a current density of 0.5C (1C = 121 mA·g-1), a reversible specific capacity of 79.2 mAh·g-1 was obtained, and a capacity of 35.3 mAh·g-1 was maintained even at a high current density of 50C.In the potential window of 0.2–1.2 V (vs.NHE), the capacity retention after 500 weeks was 64.3%, which increased to 78.2% when the potential window was reduced to 0.25–1.15 V, because of the fewer side reactions.In addition,Na0.44MnO2 showed an exceptional ability to sustain overcharging up to 30% in a concentrated alkaline electrolyte (based on the reversible capacity of 79.2 mAh·g-1), and the discharge capacity within 80 cycles was almost steady.The above mentioned results form the basis for possible technical directions toward the development of low-cost cathode materials to be used in ASIBs.
Key Words: Sodium ion battery; Na0.44MnO2; Electrochemical performance; Concentration; Overcharging

1 Introduction
Sodium-ion battery (SIB) emerges as an appealing battery technology in recent years due to its similar operation mechanism to rocking chair lithium-ion battery (LIB).Contrastive to lithium resources, sodium reserves are more abundant, ubiquitous and easily accessible, SIB therefore shows great promising application prospect in large-scale energy storage systems (ESS).Despite the merit of potentially low cost,the use of non-aqueous electrolyte in SIBs will inevitably trigger safety concerns as raised in LIBs.Compared to nonaqueous batteries, aqueous SIBs show prominent advantages of high safety, toxicity free, environment-friendliness and low cost, thus have attracted considerable attentions as a promising battery technology for ESS1–3.In recent years, aqueous sodium-ion batteries (ASIBs) have developed rapidly and a variety of positive electrode materials have been found stable in aqueous system, such as Mn-based oxides4–6, polyanionic compounds7–9and Prussian blue analogs10–14, providing ample options for ASIBs.
Among these cathode materials, Na0.44MnO2with outstanding structural and hydrochemical stability attracts the keen interest of many researchers.Na0.44MnO2owns three-dimensional tunnel structure, which is not only favorable for the fast intercalation/de-intercalation of Na+ions, but also could relief the structural strain generated during charge-discharge process,thus improving the cyclic stability.It has been revealed that 0.44 Na+could be reversibly de/intercalated in the tunnel structure of Na0.44MnO2, corresponding to a theoretical capacity of 121 mAh·g-115–18.Whitacre et al.first revealed the electrochemical behavior of Na0.44MnO2in Na2SO4solution19.However, the reversible capacity of Na0.44MnO2in almost all the ASIBs was only 40 mAh·g-1, far lower than its theoretical capacity20–22.Recently, we reported a Zn/Na0.44MnO2dual-ion battery(denoted AZMDIB) based on Na0.44MnO2positive electrode, Zn negative electrode and 6 mol·L-1NaOH electrolyte.The alkaline solution lowered the proton insertion potential and expanded the stable working window of Na0.44MnO2electrode, thus enhancing the reversible capacity to 80 mAh·g-123.It has been demonstrated in some previous studies that the composition,concentration, and pH of electrolytes have significant effects on the electrochemical properties of aqueous batteries.Electrolytes with different concentrations have different ionic conductivities,leading to different rate performances of battery24.And highly concentrated electrolyte can reduce the reactivity of water and inhibit the dissolution of electrode materials, thus widening the stable electrochemical window and improving the cycling performance of electrode materials25,26.In addition, the pH of electrolyte would also affect the hydrogen/oxygen evolution potentials.And, it has been reported that the co-intercalation of hydrogen ions could be inhibited by tuning pH of electrolyte27–29in order to improve the cyclic stability of the electrode30,31.Therefore, exploring the effect of electrolyte concentration and pH on the electrochemical performance of Na0.44MnO2can provide insight into the design and optimization of highperformance Zn/Na0.44MnO2aqueous battery.
In this work, a three-electrode system was set up by using Na0.44MnO2as working electrode and zinc sheet as the counter and reference electrode.The electrochemical performances of Zn/Na0.44MnO2battery with five different concentrated electrolytes (1, 3, 6, 8 and 10 mol·L-1NaOH) were investigated and the influences of electrolyte concentration and pH on the stable working window, rate performance and cycle performance of Na0.44MnO2electrode were studied.
2 Experimental
2.1 Material preparation
Na0.44MnO2was prepared by a solid-state method.Firstly,Na2CO3(AR, ≥99.8%, Sinopharm) and MnCO3(AR,Sinopharm) were put into the agate grinding tank with a stoichiometric ratio of 0.242 : 1, the excess sodium source was to compensate the sodium loss upon high-temperature calcination, and acetone (AR, ≥99.5%, Sinopharm) was added as the dispersant at once.Then, the tanks were fixed on a planetary mill (XGB2, BYT) for 3 h at a speed of 300 r·min-1to obtain a homogeneous slurry.After milling, the slurry was transferred to oven, and dried for 10 h at 80 ℃.Finally, the solid was fully ground into a powder and heated in air atmosphere at 850 ℃ for 10 h with a heating rate of 3 ℃·min-1to obtain final product.
2.2 Material characterization
The morphology of Na0.44MnO2was characterized by scanning electron microscope (SEM, ZEISS Merlin 132 Compact) and high-resolution transmission electron microscope(HRTEM, JEM-2100FEF).And the structure characterization was conducted on X-ray powder diffractometer (XRD, Bruker D8 ADVANCE).
2.3 Electrochemical measurements
The working electrode was prepared with rod-like Na0.44MnO2as the active material, Super P as conductive carbon and 60% (mass fraction) polytetrafluoroethylene (PTFE)emulsion as the binder in a weight ratio of 8 : 1 : 1.Firstly, the Na0.44MnO2and Super P were well mixed by grounding in an agate mortar.Then, PTFE emulsion and isopropanol (AR,≥99.7%, Sinopharm) were added into the above powder and stirred to form a gum-like mixture under infrared lamp.Finally,the electrode was obtained after rolled with twin rollers and was subsequently dried for 10 h at 100 ℃ in a vacuum drying oven.The current collector is stainless steel net and the mass loading of working electrode is about 5 mg·cm-2.Both counter and reference electrodes were Zn sheets with a thickness of about 180 μm.NaOH (AR, ≥96%, Sinopharm) solution with different concentrations was used as the electrolyte.Galvanostatic charge/discharge tests and cyclic voltammetry were carried out on LANDCT2001A and electrochemical workstation (Autolab PGSTAT128N,Eco Chemie,Switzerland), respectively, and the specific capacity of the battery was calculated based on the mass of active material of working electrode.
3 Results and discussion
Fig.1a is the XRD pattern of as-synthesized Na0.44MnO2.It can be seen that all diffraction peaks could be indexed to orthorhombic Na0.44MnO2(Pbamspace group, JCPDS No.27-0750) indicating that highly pure Na0.44MnO2was synthesized by solid-state method.Fig.1b displays the crystal structure of Na0.44MnO2.The structural framework is composed of double and triple rutile-type chains of edge-sharing MnO6 octahedra and corner-sharing MnO5square pyramids, forming two types of tunnels: large S-shaped tunnel and small pentagon tunnel.Na2 sites and Na3 sites are located in the large S-shaped tunnel, while Na1 is situated in the small pentagon tunnel.The large S-shaped tunnel is approximately half filled where sodium ions can be reversibly de/intercalated.The small pentagon tunnel is almost entirely occupied by sodium ions, but these sodium ions cannot be utilized32–34.

Fig.1 (a) The XRD pattern of Na0.44MnO2, (b) the schematic crystal structure of orthorhombic Na0.44MnO2.
We used SEM and HRTEM to characterize the morphology of Na0.44MnO2.As presented in Fig.2a, Na0.44MnO2consists of many short rods with a diameter of about 0.5 μm and length ranging from 2 to 5 μm.A lattice fringe with a spacing distance of 0.26 nm can be clearly observed from the HRTEM images in Fig.2b, corresponding to the (350) lattice plane of Na0.44MnO2.
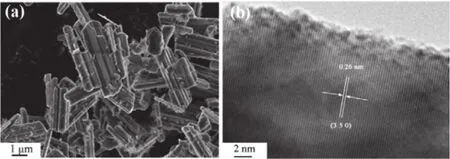
Fig.2 (a) The SEM image, (b) the high-resolution TEM image of Na0.44MnO2.
In order to determine the stable electrochemical window of NaOH solutions with different concentrations, the AZMDIB was charged to 2.0 V (vs.NHE) and discharged to 0 V, respectively.As shown in Fig.3a, all the batteries displayed multiple charge voltage plateaus below 2.0 V, corresponding to the Na+deintercalation from Na0.44MnO2.However, a quite flat voltage plateau appeared at ~1.2 V (vs.NHE) as the batteries were further charged, which should be attributed to the oxygen evolution reaction.The oxygen evolution potential varied as the concentration of electrolyte changed, the relative order is generally summarized asφ1>φ3>φ6>φ8>φ10.According to the Nernst equation:
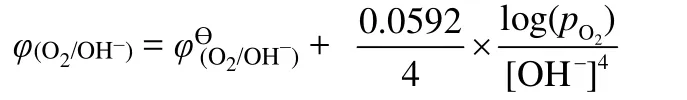
The oxygen evolution potential decreases as the [OH-]increases.Whereas the concentration of OH-varies within a small range (from 1 to 10 mol·L-1), the difference of oxygen evolution potential between each battery is not obvious.In this regard, the upper charge potential was limited to 1.2 V.The discharge profiles of the above five batteries are shown in Fig.3b.As is seen, multiple discharge voltage plateaus above 0.2 V,which are related to the Na+intercalation into Na0.44MnO2are clearly observed.Note that a flat discharge plateau emerged at~0.2 V (vs.NHE) as the batteries were further discharged, which is ascribed to the insertion of hydrogen ions into Na0.44MnO2.The proton insertion potential also varied with the alterations of electrolyte concentrations, and the relative order isφ1>φ3>φ6>φ8>φ10.This variation can also be interpreted by Nernst equation: boosting the concentration of OH–in the electrolyte means decreasing the concentration of H+, resulting in the delay of proton insertion process.
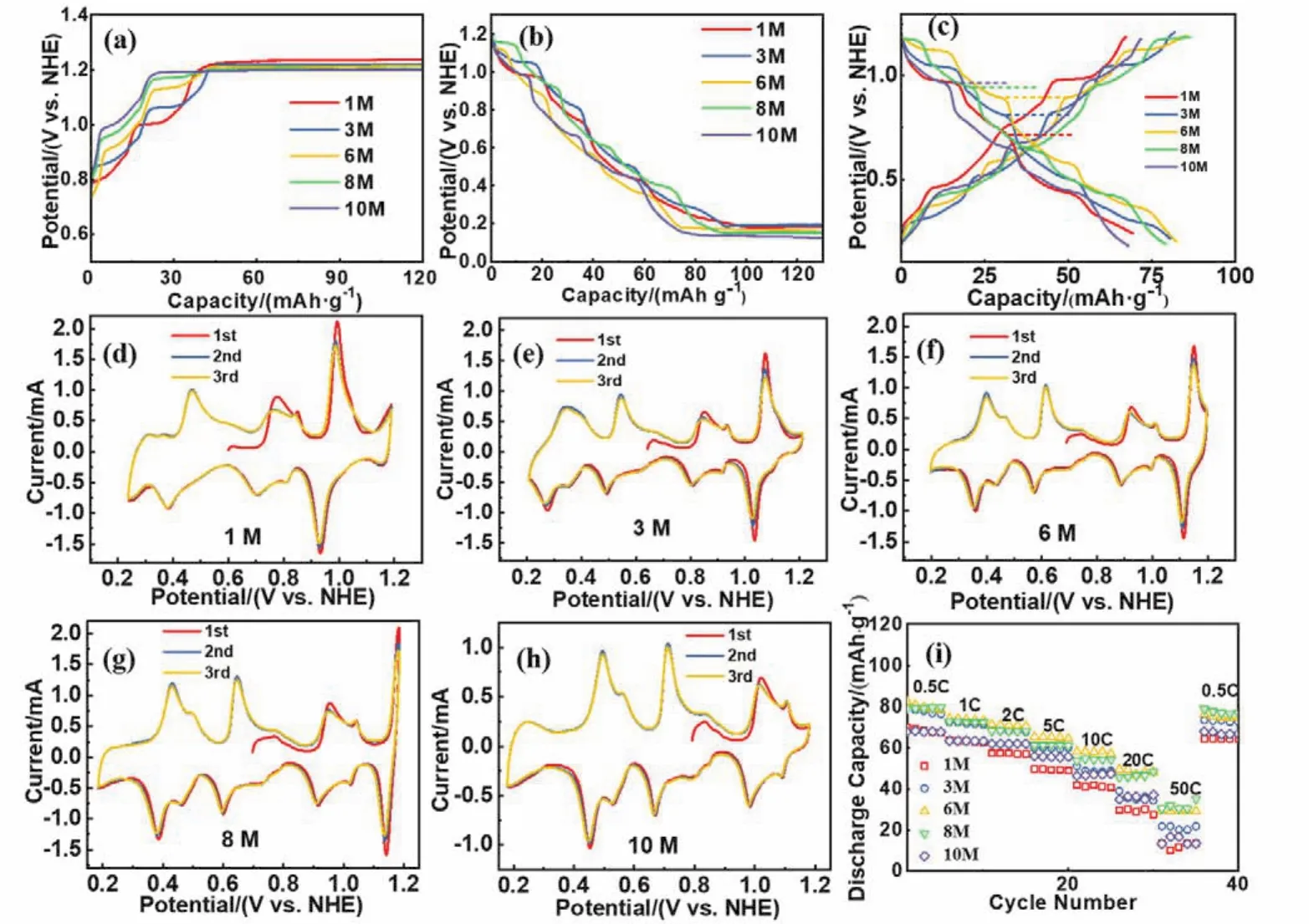
Fig.3 The electrochemical performances of AZMDIB in different electrolytes with various NaOH concentrations (1, 3, 6, 8 and 10 mol·L-1 NaOH).
Based on the preliminary battery results, we can determine that the stable working voltage window of AZMDIB should be confined between the oxygen evolution potential and proton insertion potential due to the poor reversibility of proton insertion21,35.Therefore, the potential window of AZMDIB in 1 mol·L-1NaOH was set as 0.25–1.2 V, and the other systems (3,6, 8 and 10 mol·L-1NaOH) used 0.2–1.2 V.Fig.3c presents the typical charge-discharge curves of AZMDIB in the above five electrolytes, and the typical discharge potentials of five batteries at the same discharge depth were marked, respectively.It can be found that the relative order of potential isφ10>φ8>φ6>φ3>φ1.The operating potential is affected by the activity of sodium ions in the electrolyte36.According to the Nernst equation, the potential of sodium ions insertion into Na0.44MnO2electrode should climb when the concentration of sodium ions increases.The relative order of reversible capacity for five batteries is 6 mol·L-1(82.5 mAh·g-1) > 3 mol·L-1(80.6 mAh·g-1) > 8 mol·L-1(79.2 mAh·g-1) > 1 mol·L-1(69.3 mAh·g-1) > 10 mol·L-1(67.9 mAh·g-1).Among them, 3, 6 and 8 mol·L-1possess similar discharge capacity, which is about 80 and ~10 mAh·g-1higher than that of 1 and 10 mol·L-1.The specific discharge capacity is mainly affected by the thermodynamics of sodium ions de/intercalation.When the concentration of sodium ions is low,the activity of sodium ions falls, resulting in lower operating potential.Therefore, the amount of sodium ions that could be reversibly de/intercalated in higher voltage range diminished,leading to lower specific capacity.For instance, there is hardly obvious charge-discharge potential platform in the potential range of 1.0–1.2 V for AZMDIB in 1 mol·L-1NaOH.Likewise,when the concentration of sodium ion grows, both the activity of sodium ion and potential platform increase accordingly.As a result, the number of reversibly inserted sodium ions drops in the lower voltage range, bringing lower specific capacity.For example, no obvious potential platform was observed in the potential range of 0.05–0.2 V for AZMDIB in 10 mol·L-1NaOH.Thus, the discharge capacity of Na0.44MnO2electrode firstly increases and then decreases as the NaOH concentration varies from 1 to 10 mol·L-1.It should be noted that the ionic conductivity of electrolyte has little influence on the discharge capacity of Na0.44MnO2electrode since the charge-discharge current density is as low as 0.5C.Fig.3d–h display the cyclic voltammograms of Na0.44MnO2electrode in different concentrated NaOH solutions.It can be seen that redox peaks potential affected by the activity of sodium ions shift to the higher potential with the increase of NaOH concentration and more redox peaks appear in 3, 6 and 8 mol·L-1NaOH corresponding to the higher reversible capacity which are consistent the result observed form the typical charge-discharge profiles.
Fig.3i shows the rate performances of AZMDIB in five different electrolytes.The potential range for 1 mol·L-1NaOH is 0.25–1.2 V, and the others is 0.2–1.2 V.As can be seen, the relative order of rate performance is 6 mol·L-1≈ 8 mol·L-1> 10 mol·L-1> 3 mol·L-1> 1 mol·L-1.At the current density of 50C,Na0.44MnO2can still release a specific capacity of about 30 mAh·g-1in 6 and 8 mol·L-1NaOH.However, when the NaOH concentration is 1 or 10 mol·L-1, the specific capacity at 50Cis only 13 mAh·g-1.Obviously, the high rate performance is related to the ionic conductivity of electrolyte.When the concentration of electrolyte is low, the ionic conductivity of electrolyte gradually improves with the increase of concentration from 1 to 6 mol·L-1.However, when the concentration of electrolyte exceeds 6 mol·L-1, the viscosity increases, leading to the decrease of ionic conductivity.Therefore, the rate performance of Na0.44MnO2electrode firstly increases and then decreases as the NaOH concentration varies from 1 to 10 mol·L-1.
Fig.4a is the cyclic performance of AZMDIB at the current density of 5Cin different electrolytes with various NaOH concentrations.It can be seen that the capacity retention of AZMDIB after 500 cycles in 10, 8, 6, 3 and 1 mol·L-1NaOH is 65.8%, 64.3%, 54.2%, 33.2% and 27.4%, respectively.The battery in higher NaOH concentration exhibited better cyclic stability, this is because increasing the concentration can reduce the redox activity of water, and inhibit the side reactions during charge-discharge process37–42.In general, as shown in Table 1,AZMDIB in 8 mol·L-1NaOH showed the most outstanding electrochemical performance in terms of high reversible capacity, outstanding rate performance and superior cyclic stability

Fig.4 (a) The cycling performance of AZMDIB in different concentrated NaOH solutions (1, 3, 6, 8 and 10 mol·L-1 NaOH) at a current density of 5C,the potential range for 1 mol·L-1 is 0.25–1.2 V, and the others is 0.2–1.2 V; (b) the cycling performance and Coulombic efficiencies of AZMDIB in 8 mol L-1 NaOH at a current density of 5C, the potential range is 0.25–1.15 V.
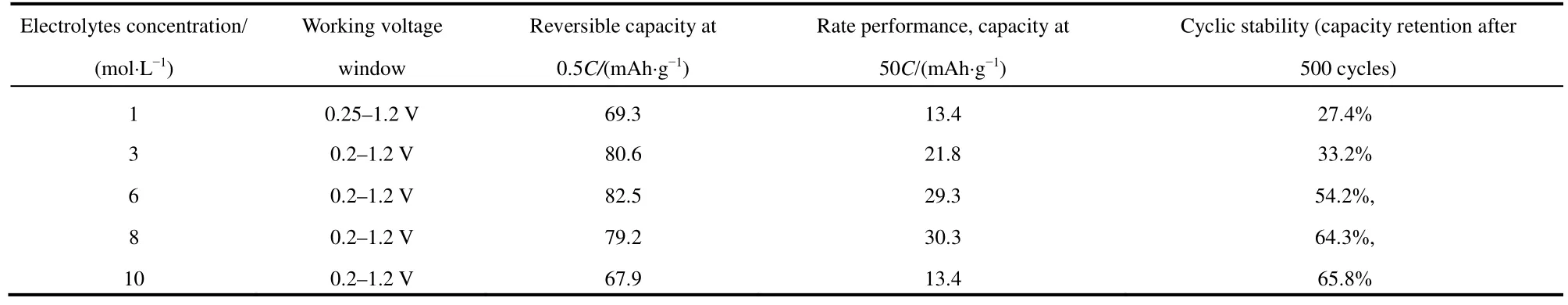
Table 1 Sodium-storage performance of Na0.44MnO2 in different concentrated electrolyte.
In order to verify whether the charge-discharge voltage window has an impact on the cyclic performance, we tested AZMDIB in 8 mol·L-1NaOH within a narrower window of 0.25–1.15 V.As shown in Fig.4b, the capacity retention after 500 cycles is 78.2%, 13.9% higher than that in 0.2–1.2 V (64.3% after 500 cycles).Widening the potential window will result in more side reactions, such as oxygen evolution and proton insertion, which might lead to the deterioration of electrode material and corrosion of current collector.
In the practical application of battery, there will inevitably be some transitory overcharge conditions.For neutral aqueous batteries, overcharging will cause oxygen evolution reaction on the surface of positive electrode.Even a slight overcharge may significantly change the pH of the electrolyte, accelerating the corrosion of the current collector and other metal components,finally resulting in the dissolution of electrode materials.In addition, when the generated oxygen diffuses to the negative electrode, it can easily oxidize anode in the discharge state,resulting in the deactivation of the anode material1,30.In order to investigate the ability of AZMDIB against overcharge, we overcharged the battery until 30% higher than the nominal capacity (75 mAh·g-1) of the battery.As shown in Fig.5a, the battery can maintain exceptional cycle stability in 80 cycles,indicative of a high overcharging tolerance.Fig.5b displays the corresponding charge-discharge curves of AZMDIB.As can be seen, the charge-discharge profiles kept almost unchanged in 80 cycles, demonstrating the AZMDIB has high tolerance for overcharging.Several factors may contribute to the high tolerance.Firstly, Na0.44MnO2itself has an outstanding structural stability in aqueous electrolyte.Secondly, there are sufficient OH-in highly concentrated NaOH electrolyte to maintain alkaline environment, consequently the transitory oxygen evolution reaction can hardly change the pH of the electrolyte.Lastly, the alkaline system has self-protection function; the oxygen generated at the cathode side can be consumed by anode,the same mechanism is demonstrated in Ni-Cd and Ni-MH batteries43,44.Therefore, the reactions of Na0.44MnO2cathode and Zn anode upon overcharge could be speculated as follows:
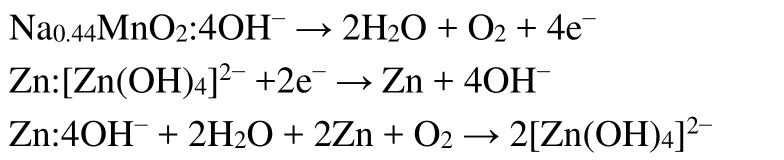
OH-was oxidized to O2at the cathode side and [Zn(OH)4]2-was reduced to Zn at the anode side.The overcharge product, O2diffused to anode, and was reduced to OH-again by Zn.The overall reaction almost could be considered as zero.
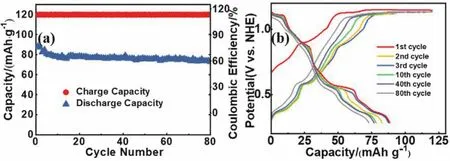
Fig.5 (a) The cycling performance of AZMDIB in 8 mol L-1 upon 30% overcharge; (b) the corresponding charge-discharge profiles.
4 Conclusions
Na0.44MnO2was synthesized by solid-state method and its electrochemical properties in NaOH solutions with different concentrations were investigated.The Zn/Na0.44MnO2dual-ion battery in 8 mol·L-1NaOH showed the most outstanding electrochemical performance in terms of high reversible capacity, outstanding rate performance and superior cyclic stability.A reversible capacity of 79.2 and 35.3 mAh·g-1can be obtained at the current density of 0.5Cand 50C, respectively.When the potential window is narrowed from 0.2–1.2 V to 0.25–1.15 V, the capacity retention after 500 cycles is improved from 64.3% to 78.2% due to the lessened side reactions.In addition,the AZMDIB shows high tolerance for overcharging in alkaline electrolyte.Our work not only demonstrates that the electrochemical performance of AZMDIB can be greatly improved by optimizing the electrolyte and working potential window, but also justifies its potential use as a low cost and high safe battery technology for large-scale energy storage system.
- 物理化学学报的其它文章
- SnO2表面卤化提高钙钛矿太阳能电池光伏性能
- ZrO2包覆高镍LiNi0.8Co0.1Mn0.1O2正极材料提高其循环稳定性的作用机理
- Regulating Electron Transport Band Gaps of Bovine Serum Albumin by Binding Hemin
- 基于血红素衍生的中空非贵金属催化剂氧还原反应电催化活性
- 一类受生物启发的双膦双硒镍配合物的合成及其电催化产氢性能
- Photocathodic Protection on Stainless Steel by Heterostructured NiO/TiO2 Nanotube Array Film with Charge Storage Capability

