Constitutive models and salt migration mechanisms of saline frozen soil and the-state-of-the-practice countermeasures in cold regions
YuanMing Lai,ZheMin You,Jing Zhang
1. State Key Laboratory of Frozen Soil Engineering, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
ABSTRACT A series of saline soil-related problems, including salt expansion and collapse, frost heave and thaw settlement, threaten the safety of the road traffic and the built infrastructure in cold regions. This article presents a comprehensive review of the physical and mechanical properties, salt migration mechanisms of saline soil in cold environment, and the countermeasures in practice. It is organized as follows:(1)The basic physical characteristics;(2)The strength criteria and constitutive models; (3) Water and salt migration characteristics and mechanisms; and (4) Countermeasures of frost heave and salt expansion.The review provides a holistic perspective for recent progress in the strength characteristics,mechanisms of frost heave and salt expansion,engineering countermeasures of saline soil in cold regions.Future research is proposed on issues such as the effects of salt erosion on concrete and salt corrosion of metal under the joint action of evaporation and freeze-thaw cycles.
Keywords: frozen saline soil; strength criteria and constitutive models; salt migration and crystallization; multiple field coupling model;countermeasures
1 Introduction
Permafrost, seasonally frozen soil, and saline soil regions account for approximately 22.4%, 55.0%, and 2.0% of China's total land area, respectively. Saline soils are widely distributed in the north, northeast, especially western cold regions of China (Wanget al.,1993) (Figure 1). With the variation of temperature and moisture, water and salt in the saline soil migrate,accumulate, and undergo phase change and crystallization processes, internal pressure induced by ice and salt crystals will lead to frost heave and salt expansion. The salt concentration of soil pore solution will rise due to the cooling effect, accelerating the precipitation of salt crystals and salt expansion. Also, the frost weathering can cause the recession of technogenic landform surfaces (Melnikovet al., 2019). Meanwhile, the construction of highways, railways, and power transmission lines in clod regions will lead to the ground temperature rising, frozen soil thawing,and water and salt flowing downward, eventually resulting in salt collapse and thaw settlement (Chaiet al., 2019; Xieet al., 2019). Owing to the salt expansion and collapse,frost heave and thaw settlement,tremendous engineering problems in saline permafrost and seasonally frozen regions have threatened the safety of the road traffic and caused severe damage to infrastructure construction, such as uneven uplifting,subgrade swelling,and concrete spalling(Figure 1).

Figure 1 Damage to the infrastructure in cold saline soil regions.(a)Subgrade uplifting,and(b)concrete spalling
Frozen saline soil is a porous medium composed of soil particles, liquid water, dissolved salt ions, ice crystals, salt crystals, and gas (Figure 2). The essential difference between frozen saline soil and ordinary unfrozen soil is ice and salt crystals (Xuet al., 1995,2010). There is a lot of research on the basic physical and mechanical characteristics of saline and frozen soil in past decades. Compared with unfrozen non-saline soil, frozen saline soil has significantly different basic physical characteristics, including variation of liquid and plastic limits, optimal water content, structural characteristics,thermal and hydraulic conductivities, freezing point and unfrozen water content. Such environmental and physical properties as temperature,confining pressure, water content, and salt content affect frozen saline soil's strength characteristics and its constitutive model. Water and salt migration characteristics and crystallization mechanisms have been verified experimentally.The freezing of saline soil involves heat transfer, mass transfer, deformation and chemical reaction, and thermo-hydro-salt and thermohydro-salt-mechanical models have been proposed.Comprehensive countermeasures of frost heave and salt expansion have been considered.
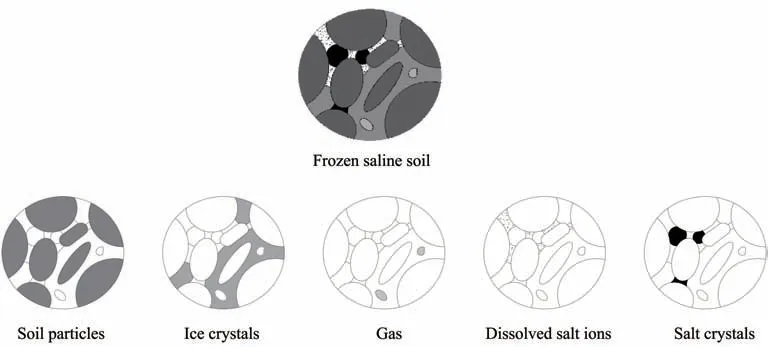
Figure 2 The multiphase compositions of frozen saline soil
The purpose of this article is to provide a comprehensive literature review on the physical and mechanical properties, salt migration mechanisms of saline soil in cold environment, consequences, and countermeasures. Four aspects are addressed as follows: (1)the basic physical characteristics, (2) the strength criteria and constitutive models,(3)water and salt migration characteristics and mechanisms, and (4) countermeasures of frost heave and salt expansion. Since intensive evaporation may occur in cold regions, the combined action of evaporation and cooling can drive water and salt to migrate and accumulate on the ground surface or beneath and around the engineering structures. The resulting consequences, including salt expansion and collapse, salt erosion on concrete, and corrosion of metal, can endanger related engineering assets, such as water conveyance project, subgrade,and pavement.In the end,this article recommends further investigation of issues related to saline soil in cold regions, including the effects of salt erosion on concrete and corrosion of metal under the combined action of evaporation and freeze-thaw cycles.
2 Physical characteristics of frozen saline soil
2.1 Basic physical property
The content of soluble salt affects the soil's liquid and plastic limits and optimal water content,which are proportional to the content of sodium sulfate and reversely proportional to the content of chloride sulfate (Li, 2006). The structural characteristics of saline soil change with temperature. As temperature drops, sodium sulfate soil expands, accompanied with a decreased density, whereas chloride sulfate soil initially shrinks and then swells with a decreasing density(Denget al.,2008).The volume of high-chloride saline soil only changes slightly without an overburden. However, it contracts significantly under static and dynamic-load conditions during cooling.Besides, volumetric expansion occurs in chloride saline soil with a high proportion of sulfate salt (Zhanget al.,2017).
2.2 Thermal and hydraulic conductivities
The thermal and hydraulic conductivities of saline soil are affected by salt types (Wang, 1993; Xu,2010). At positive temperatures, the thermal conductivities of silt and silty clay are proportional to chloride sulfate content but are inversely related to sodium sulfate content. However, at negative temperatures, soil thermal conductivity decreases with an increasing salt content (Denget al., 2004). The hydraulic conductivity of saline soil reduces as salt content increases, at a faster gradient for fine-grained soil than that of coarse-grained soil and with sodium sulfate salt resulting in more reduction (Denget al.,2006). The hydraulic conductivity increases apparently after freeze-thaw cycles (Wang, 2006). Furthermore, addition of sewage sludge or fly ash was found to enhance the stability of saline-sodic soil and increase the hydraulic conductivity (Sahinet al., 2008).Salt also affects soil's heat capacity. Particularly, during the phase transition of water or salt, the heat capacities of multiple soil components change, resulting in the variation of the overall heat capacity of saline soil.
2.3 Freezing point and unfrozen water content
The freezing point of saline soil is affected by water content, grain size distribution, salt content,etc..The freezing point reduces with an increasing salt content and decreasing water content. The influence of common anions and cations on the freezing point follows the order of Cl->CO2-3>SO2-4and K+>Na+>Ca2+, respectively. Soil freezing point also decreases with an increasing fraction of finer particles (Bing and Ma, 2011). There is a difference between the freezing points of sodium sulfate solution and sodium sulfate soil. The freezing point of sodium sulfate soil is lower than that of sodium sulfate solution at the same concentration, and salt crystals are more likely to precipitate in the saline soil (Wan and Lai,2013). Wanet al.(2015) measured the freezing points of saline soil with a water content of 18% and salt contents varying from 0.1% to 3.2%, and they found that as the solution concentration rose, the freezing point of sodium sulfate soil initially declined, then rose, and finally decreased (Figure 3a),whereas that of chloride sulfate soil decreased almost linearly, and observed a lowest freezing point of-14.2°C(Figure 3b).
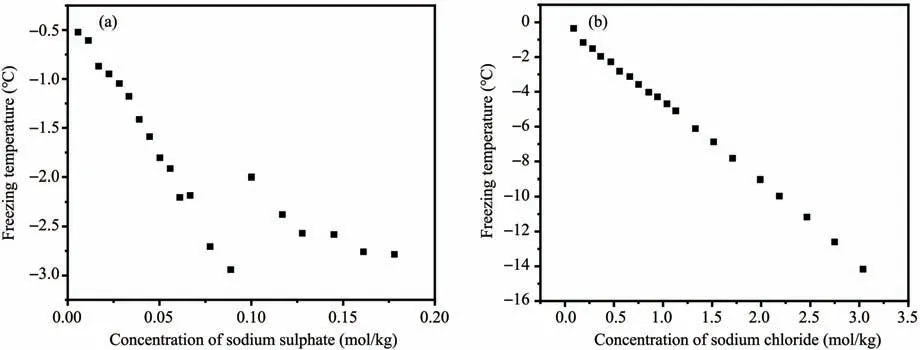
Figure 3 Variations of soil freezing point with salt concentration.(a)Sodium sulfate,(b)chloride sulfate(Wan et al.,2015)
The freezing point of a solution is affected by salt concentration and can be expressed as:
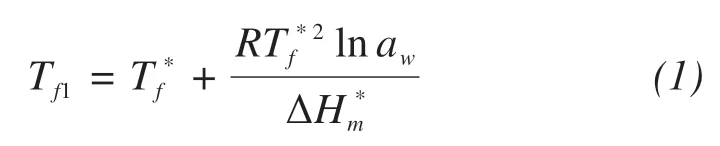
whereTf1andT *fare the freezing points of the solution and bulk water(K),respectively;Ris the gas constant, 8.314 J/(mol·K);awis water activity;is the fusion enthalpy of ice; and(Sl-Si)T. When temperature variation is small,can be regarded as a constant of 6,010 J/mol.
Saline soil's freezing point is affected by two factors. The first one is the water activity, which has a negative correlation with salt concentration.The other is pore size. The relationship between the freezing temperature and these two factors can be expressed as(Xiaoet al.,2017):
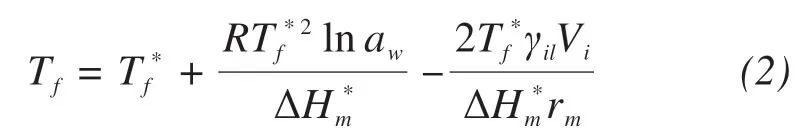
whereTfis saline soil's freezing point;γilis the surface energy of the ice/liquid solution interface (J/m2);Viis the molar volume of ice crystal (m3/mol); andrmis the mean curvature radius(m).
Equations(1)and(2)were used to obtain the relationships between the freezing points of the solution and saline soil and sodium sulfate concentration, and the results were compared with the experimental data when salt concentration was less than 0.5 mol/kg(Wanet al., 2015). There was a good agreement between the experimental and calculated results(Figure 4)(Maet al.,2017).
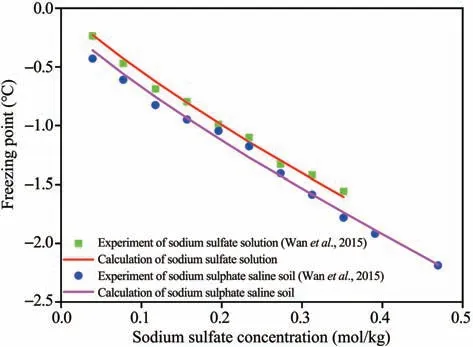
Figure 4 Variations of the freezing points of sodium sulfate soil and solution with salt concentration(Ma et al.,2017)
Based on the Frezchem model, the freezing temperature of the ternary pore-solution of saline soil,H2O-NaCl-Na2SO4, was investigated by the freezing temperature equation proposed by Xiaoet al.(2017), considering the common ion effect (Marion and Farren, 1999). The calculated and experimental results are shown in Figure 5, and a good agreement can be observed for NaCl concentration less than 0.5 mol/kg.
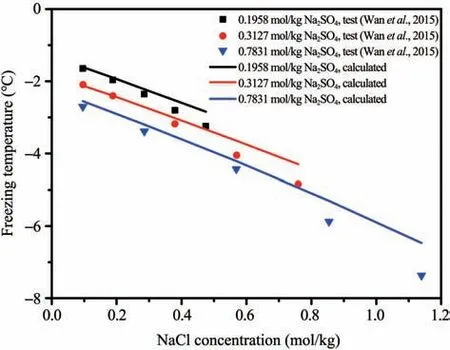
Figure 5 Freezing temperature of the ternary pore-solution of saline soil versus NaCl concentration
Due to the uncertainty of transformation products of sodium sulfate, heptahydrate (Na2SO4·7H2O) or decahydrate (Na2SO4·10H2O), it is difficult to determine the freezing point of sulfate saline soil (Xiaoet al., 2017). The relation between the remaining water content and the freezing point shift is applicable for silty clay with sodium chloride(Banin and Anderson,1974). An empirical equation relating the unfrozen water content to temperature and specific surface area was established, and the calculated and experimental results were in good agreement,particularly at temperatures below-5°C(Anderson and Tice,1972).
The unfrozen water content is closely related to sodium sulfate's solubility, which depends on the temperature. A relationship between the solubility and temperature has been determined based on the test results by(Koniorczyk and Konca,2013):
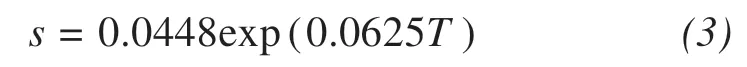
wheresis the solubility(mol/kg);Tis the temperature(°C), ranging from 5.0 to 32.5 °C. Notably, this equation only applies to salt crystallization over the range of positive temperatures.
The threshold value of supersaturation (Ua) is selected as the criterion to judge the saturation state of the solution at a specific temperature. Additionally, it is assumed that when Uaequals 1, the solution is considered to be saturated. According to the Frezchem model, Sodium sulfate's solubility can be obtained by extending the solubility function from positive to negative temperatures. The calculated results are in good agreement with the experimental data (Spenceret al., 1990; Marion and Farren, 1999; Xuet al.,2001), illustrating that the above method can be applied to evaluate sodium sulfate's solubility at negative temperatures (Figure 6). Additionally, Zhanget al.(2020) proposed a fitting equation to describe their relationship by:
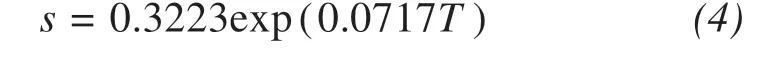
Xiaoet al.(2017) measured the liquid water fractions in saline soils with various concentrations of sodium chloride or sodium sulfate at different temperatures employing nuclear magnetic resonance (NMR). They compared the discrepancy of phase change between the pore solution and a free solution and found it was mainly affected by soil pore size distributions (PSDs).Furthermore, Xiaoet al.(2020) established a thermodynamic model for evaluating the unfrozen water content by considering its dependence on the pore radius,nonfreezable layer, and pore shape, and confirmed this model providing a reasonable approximation.
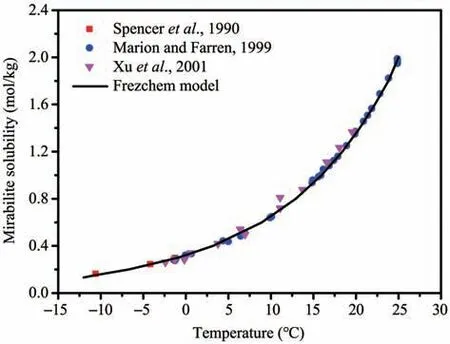
Figure 6 Mirabilite solubility versus temperature
3 Strength criteria and constitutive models of frozen saline soil
3.1 Strength criteria
Material strength theory expresses the relationship between the stress state and the critical failure strength.The Mohr-Coulomb strength criterion with a linear strength envelope is one of the most widely used criteria,which is not applicable for most geomaterials,such as frozen soil and sandy soil. It is more reasonable to adopt the nonlinear strength criterion for geomaterials.Based on the Spatial Mobilized Plane (SMP) conception, the M-N strength criterion (Matsuoka and Nakai,1974) was put forward in 1974. The Lade-Duncan strength criterion for sandy soil was proposed in 1975(Lade and Duncan, 1975). The revised Hoek-Brown strength criterion has become a widely used criterion in engineering (Hoek and Brown,1992). Based on the M-N strength criterion and the related stress transition method, the Unified Hardening (UH) model was brought forth(Yaoet al.,2009).
Frozen soil strength depends on many factors, including temperature, confining pressure, water content,etc.. Besides, salt ion distribution and concentration should be taken into account for frozen saline soil. The uniaxial compressive strength of frozen soil is inversely related to the temperature by a power function (Sayles and Haines, 1974; Bragg and Andersland, 1981; Zhu and Carbee, 1984). The shear strength of frozen sandy soil follows the power law with temperature ranging from -2 °C to -15 °C (Wuet al., 1994). However, other studies (Haynes and Karalius, 1977; Ma, 1996) reported that the uniaxial compressive strength of frozen silt has a linear relationship with temperature.
The strength of frozen soil is sensitive to confining pressure (Chamberlainet al., 1972; Parameswaran and Jones,1981).The shear strength increases approximately linearly with confining pressure of relatively lower values, which can be described by the linear Mohr-Coulomb strength criterion (Alkire and Andersland, 1973; Jessberger, 1981). However, a relatively large confining pressure leads to ice melting and particle slippage and breakage. The strength criterion changes substantially compared with at lower confining pressure and can be expressed as a hyperbola (Maet al.,1996;1999).
Other studies(Fish,1991;Zhanget al.,2008)proposed parabolic strength criterion for frozen soil,which degenerates to the classical linear and constant strength criteria under certain conditions (Shen and Wu, 1999).A revised Mohr-Coulomb strength criterion was proposed to describe the nonlinear variation of strength with confining pressure (Qi and Ma, 2007).By multiplying the critical state function in the p-q plane with a shape function in the π plane, Laiet al.(2007, 2010) established a strength criterion in 3-D stress space for frozen sandy soil. By the introduction of a new parameter and instantaneous friction angle for the nonlinear failure envelope, a united strength criterion was established, of which the deviatoric section is constrained by the Mohr-Coulomb and Drucker-Prager criteria as the low and upper bounds,respectively. This strength criterion takes the intermediate principal stress into account and can well reflect the nonlinear strength characteristic of frozen soil under the influence of pressure melting and crushing phenomena (Yanget al., 2013). Chenet al.(2019) conducted torsional shear tests in a hollow cylinder apparatus at-10 °C with different shear rates and intermediate principal stress coefficients and established a failure criterion with its deviatoric stress defined by a selected strain-hardening curve.
The uniaxial compressive strength of frozen soil grows with increasing water content until reaching 100% saturation and then decreases with increasing ice content until arriving at the strength of ice (Pekarskaya, 1963; Wuet al., 1983). The uniaxial compressive strength of frozen clay and clayey silt also exhibits an initially ascending but later declining trend with increasing water content within a temperature range between-10°C and-55°C(Shusherina and Bobkov,1969). Sayles and Carbee (1981) found that the "ice matrix strength" is approximately proportional to the volumetric ice content of saturated Fairbanks silt with total water content ranging from 0.28 to 0.58.
It is obvious that salt affects the strength of frozen saline soil. The shear strength of frozen sandy and clay soil descends with increasing salt content(Chamberlain, 1985; Miller and Johnson, 1990). When the salt content varies from 0% to 3%, the uniaxial compressive strength declines exponentially for frozen chloride sulfate soil, while it decreases initially and then increases for frozen sodium sulfate soil (Chenet al., 2013). The strength of frozen saline soil is influenced by stress conditions and the basic physical properties of soil, its chemical composition,etc.(Roman,1995). Its dependence on salt and temperature can be explained by the unfrozen water content(Hivonet al.,1995). The compressive strength of saline soil is remarkably sensitive to freeze-thaw cycles, with a significant reduction after three cycles and a trendency to stablize after ten cycles (Fanget al., 2016). Liaoet al.(2016) established a strength criterion based on the generalized nonlinear strength theory,which can reflect the major nonlinear strength characteristics of frozen saline soil resulted from salt content, pressure melting and ice crushing. The salient features of the above strength criterion mainly include: a modified generalized effective stress function for describing the pressure melting under high hydrostatic pressures, the degenerative effective stress multiplied by the initial critical pressure ratio for describing the critical strength curve.
The yield function of the frozen saline soil can be expressed as follows.

whereθis the stress Lode angle, which can be calculated as follows.
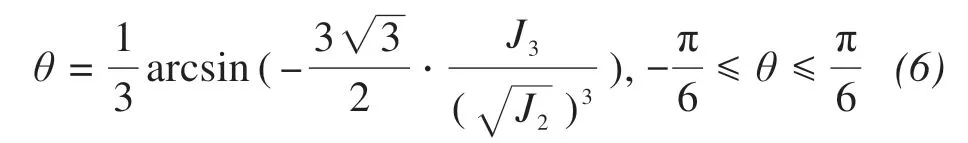
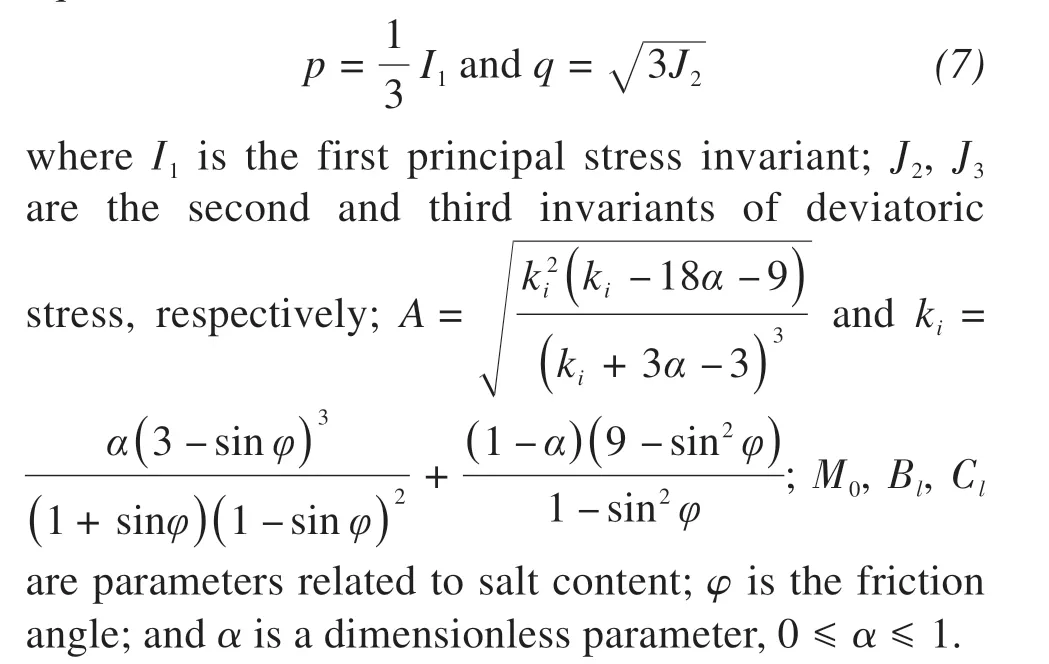
p,qare the hydrostatic pressure and deviatoric stress, respectively.They are defined by the following equations:
Zhaoet al. (2020a) put forward a dynamic strength criterion for frozen sulfate silty clay under cyclic loading, which provides a method for determining dynamic strength parameters. This criterion can account for the effects of dynamic loading stress ratio, salt content and high confining pressure on the dynamic strength.A salient feature of this dynamic strength criterion is that the dynamic critical state line evolves with the mass content of sodium sulfate.
The yield funciton can be expressed as:
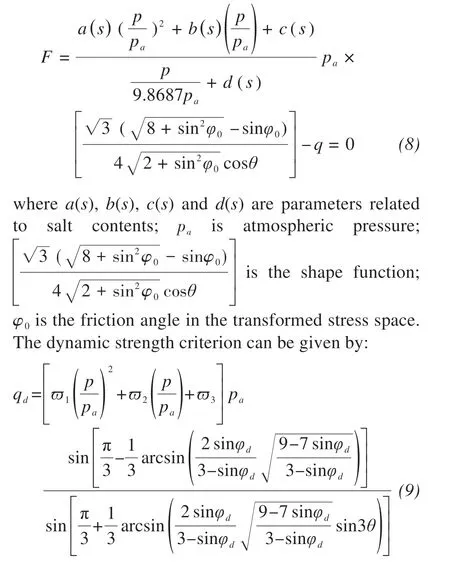
whereϖ1,ϖ2,ϖ3are parameters associated with salt content, axial strain, and loading dynamic stress ratio, respectively; andφdis the effective kinetic friction angle.
3.2 Constitutive models
Various types of constitutive models for soil have been developed over the past several decades. The Cam Clay model (CCM), based on critical state soil mechanics theory, was proposed for saturated clay by adopting a cap yield surface and an associated flow rule, with the hardening parameter composed of plastic volumetric strain (Roscoeet al., 1963). By adjusting the yield surface of the CCM, the Modified Cam Clay model(MCCM)was presented(Roscoe and Burland, 1968). Based on the experimental results of sandy soil,Lade and Duncan(1978)established the Lade-Duncan model, which employs a non-associated flow rule and adopts the plastic workWpas the hardening parameter. The model can depict dilatation properly.Xie and Shao (2006)proposed a constitutive theory to describe the elastoplastic deformation properties of soils,considering both plastic shearing and plastic volumetric compression mechanisms. Also, Peric and Ayari (2002) recommended a constitutive model with a cap compression yield surface and a conical shear yield surface that can nicely reflect the above two plastic mechanisms.
The power function can be used to depict the stress-strain relationship of frozen soil (Viyalov,1962). Nevertheless, both elastoplastic and viscoelastic-plastic behavior should be considered in the stressstrain relationship of frozen soil. Heet al. (2000) proposed an elasto-viscoplastic isotropic damage model for frozen soil and introduced a damage evolution law. Laiet al.(2009) adopted an anisotropic damage variable and presented an elastoplastic constitutive model for frozen soil. Based on the generalized plastic mechanics theory,Laiet al.(2010)offered an elastoplastic constitutive model for frozen sandy soil, in which the compression mechanism was described by an elliptic yield surface and a non-associated flow low, while the shear mechanism was considered by a parabola yield surface and a non-associated flow low.Further, Laiet al.(2014) presented an incremental elastoplastic constitutive model for frozen loess by applying the hyperplastic theory and provided a method for determining model parameters.
The effects of the salt contents and confining pressures on the strength and deformation properties of frozen sulfate soils are significant. Regarding the effect of salt content, a new constitutive model with double yield surfaces was suggested for frozen saline soil, which also accounted for the effects of the initial anisotropic rotational angle and load-induced anisotropy on the rotational hardening of the yield surfaces under plastic volumetric compression (Laiet al.,2016). Meanwhile, they adopted a paraboloid yield surface to describe plastic shear.The above model can properly describe the deformation properties of frozen saline soil under static loading.The flow rule for each loading mechanism can be expressed as:
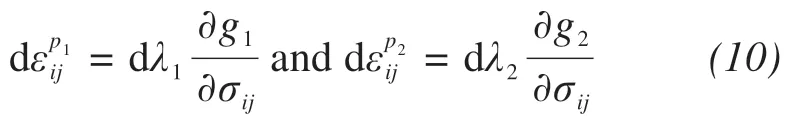
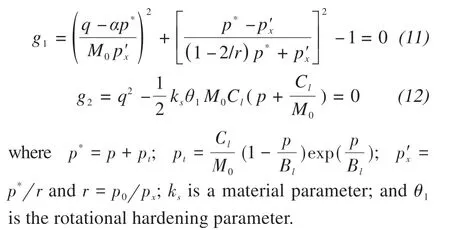
Zhaoet al.(2020b)presented an elastoplastic constitutive model for frozen saline silty clay under cyclic loading based on the bounding surface plasticity theory, which included the effects of salt content and confining pressure. This model introduced critical stress ratio and bounding surface shape parameters into the plastic potential surface and bounding surface to describe pressure melting and crushing characteristics of frozen saline soil under high confining pressures.The model can capture the stress-strain relationship of frozen saline soil with varying salt contents under dynamic cyclic loading.
The proposed bounding surface and plastic potential surface can be expressed as:
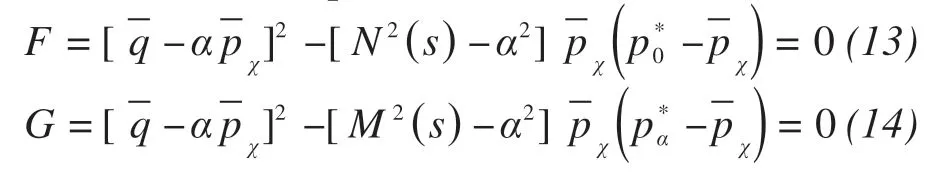
By applying the non-associated flow rule and mixed hardening law, the total stress-strain relationship is given by:
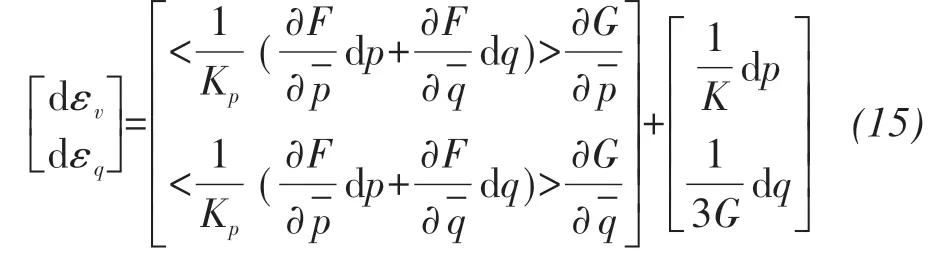
whereF,Gare bounding surface function and plastic potential surface function, respectively;is the point on the plastic potential surface or bounding surface of the presented model;is the revised hydrostatic pressure;αis a dimensionless quantity representing the rotation of bounding surface or plastic potential surface;M(s) is the critical stress ratio;N(s) is a parameter related to the shape of bounding surface;are the modified hydrostatic pressures of the interactions between the anisotropic line with the bounding surface and plastic potential surface,respectively.
Recongnizing the dependence of soil mechanical properties on the rotation of principal stress axes (RPSA), Zhaoet al.(2020c) formulated a constitutive model, which can reflect the influences of the principal stress amplitude increment and RPSA on the mechanical and deformation behaviors of frozen saline soil. This model considers the plastic deformations in two parts: one induced by the increment in the principal stresses and the other by the rotation of the principal stress axes.
For the first, the plastic deformation can be obtained based on the plastic volume compression and shear mechanisms. The bounding surface and plastic potential surfaces for the plastic volume compression can be expressed as:
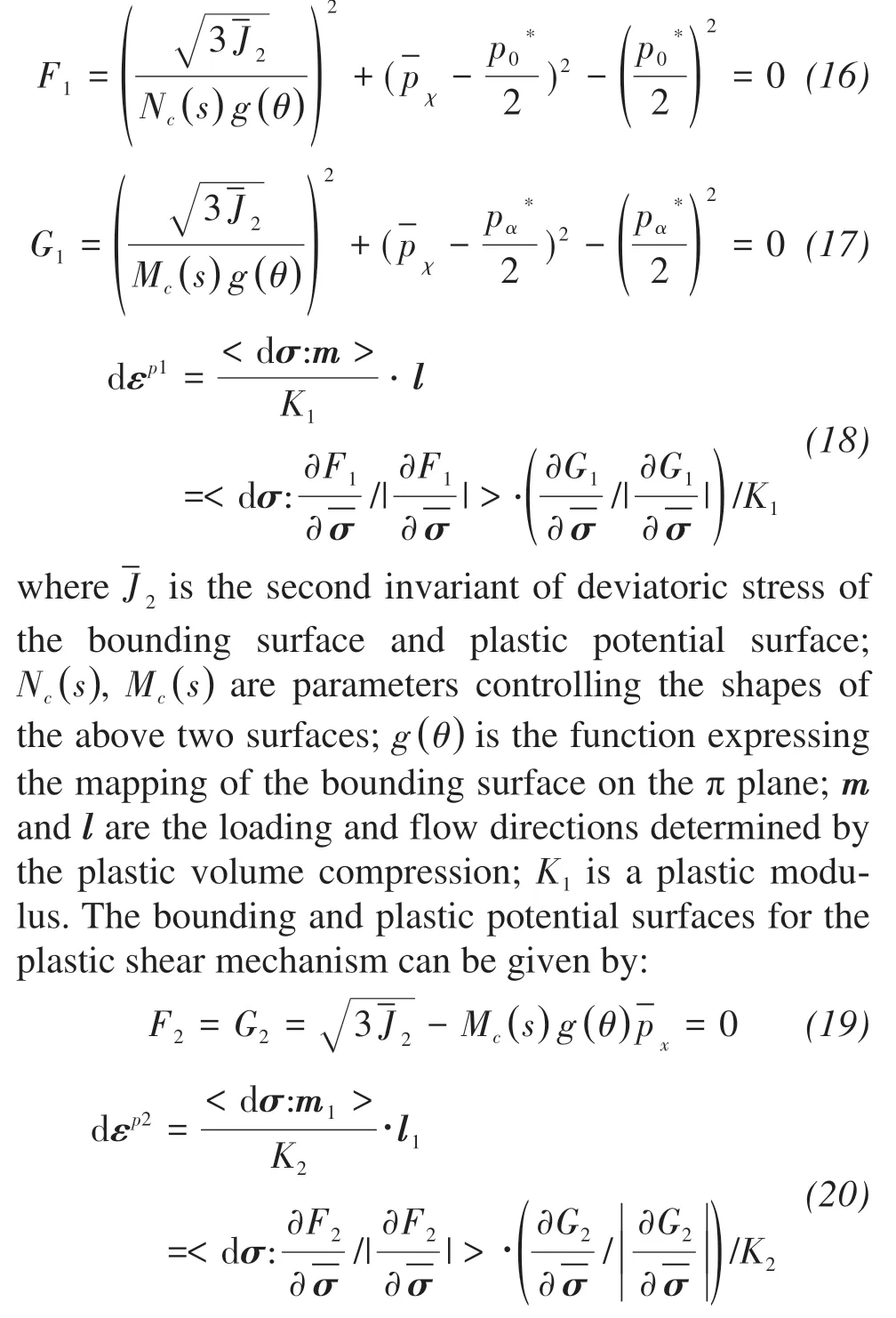
wherem1,l1are the loading and flow directions determined by the plastic shear; andK2is plastic modulus.The total plastic strain due to the increment of principle stress amplitude can be evaluated as follows.
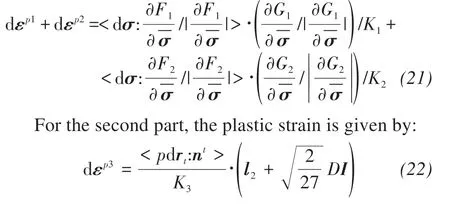
where drtis the increment of stress ratio perpendicular to the deviator stress axis;ntandl2are the loading and plastic flow directions, respectively;K3is plastic modulus;andDis the dilatancy equation.
The total stress-strain relation can be expressed as:dε=dεe+dεp=dεe+dεp1+dεp2+dεp3
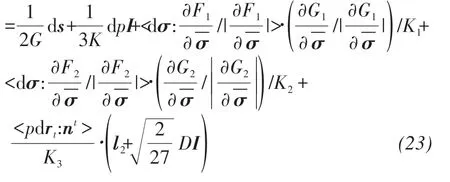
4 Water and salt migration characteristics and mechanism in freezing saline soil
4.1 Water and salt migration characteristics
Exploring water and salt migration characteristics is crucial to understand saline soil's frost heave and salt expansion behaviors. Numerous studies have shown that water and salt within saline soil migrate from the warm end to the cold end during freezing(Qiuet al.,1986).When a freezing temperature gradient forms in saline soil, the unfrozen water and the solute will migrate and accumulate at the frozen fringe, resulting in increased water and salt contents(Zhang and Wang, 2000), and hence frost heave and salt expansion.The solute and water flux are positively correlated with the initial water content, salt content, and temperature gradient and have a parabolic correlation with soil dry density (Xuet al., 1995).There are two ways for salt migration: convection with pore water and diffusion driven by a concentration gradient (Bing and He, 2011). Saline soil pore solute will crystallize during the processes of cooling and evaporation, resulting in cumulative, irreversible salt expansion within a specific temperature range(Chenet al.,1989).
Water and salt migration in unsaturated frozen soil include the mass flow of dissolved salts in a liquid film of water,vapor,and salt diffusion.Thermal diffusion and salt sieving appear to be unimportant (Cary and Mayland, 1972). Cary (1987) presented a numerical model coupling flow of heat, water, and solute in freezing unsaturated soil, and they verified that increasing solutes can decrease frost heave by reducing water flow into the ice lens. Salt redistribution consists of salt rejection and brine drainage from an extended partially frozen region just above the freezing front during downward freezing. The amount of salt rejected near the freezing front is proportional to soil salinity but inversely proportional to the freezing rate.Baker and Osterkamp (1989) proposed an effective distribution coefficient for describing salt redistribution during the freezing of saline sands.
Numerical methods are useful to study water and salt migration in saline soil during unidirectional freezing under low salinity conditions. Kadlec (1984) suggested a semi-analytical model to describe the isolation of solute by the freezing front. Padilla and Villeneuve(1992)presented a MELEF-3v model based on Miller's theory, which accounts for the presence and formation of ice lenses in saturated, partially saturated, colloidal or non-colloidal soils.Also,it models the main processes involved during soil freezing, including dispersion,convection,solute redistribution,overburden,etc..
4.2 Theoretical models of water and salt crystallization
Molecular dynamics theory has been applied to analyze soil pore solute migration in the formation process of ice lens during freezing (Nada and Furukawa, 1996).The thermal molecular pressure gradient is the driving force for water migration. The interaction between the driving force and the molecule shows that the spacing, thickness, and water content of ice lenses are closely related to pressure potential and temperature.While salinity alters soil freezing temperature, salt transport and crystallization affect the position and thickness of the ice lens(Agudoet al.,2007).
The crystallization pressure induced by water and salt is a common cause of material damage. It is particularly destructive to porous media containing sodium sulfate solution (Becker and Day,1916).Based on thermodynamic, Correns (1949) researched the relationship between the crystallization pressure and solution concentration and put forward a formula for evaluating the pressure from linear crystal growth which is a milestone in the study of salt crystallization pressure.The stability of salt crystal is affected by crystal size. Based on thermodynamics, a larger crystal is more stable than a smaller one under the condition of low chemical potential (Everett, 1961), and crystallization occurs in larger pores and then in smaller pores.
The stresses generated on the pore walls depend on several factors, including PSDs, the free energy at the pore wall/crystal interface, and the crystal yield stress or buckling strength (Scherer, 1999). The thermodynamic and kinetic factors influencing crystallization pressure include capillary rise, evaporation, and wetting-drying cycles. High crystallization pressure requires a substantial supersaturation of the pore liquid. For the crystal surrounded by a solution film,high stress is expected in small pores and even in larger pores if the film is discontinuous (Scherer, 2004).Additionally, the crystallization pressure is related to solution concentration and composition saturation(Navarro and Doehne, 1999; Scherer, 1999; Koniorczyk and Gawin, 2012). Based on PSDs, the crystallization pressure of different salt crystals at room temperature can be calculated by Pitzer's model (Steiger,2005a,b)as follows:
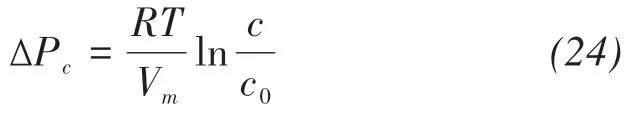
where ΔPc=Pc-Plis the crystallization pressure;PcandPlare the pressure on the surface of the growing crystal and transient pressure,respectively;Tis the absolute temperature;Vmis the molar volume of the solid phase; andcandc0are solution concentrations of super-saturated and saturated solutions,respectively.
With a combination of neutron radiography and X-ray micro-tomography, the transport process of water and salt solution in a layered oolitic limestone was revealed, and the locations of precipitated crystals were visualized. The deformation and crack mechanisms were analyzed based on a poromechanical model (Derluyn, 2012). A stone containing thenardite(Na2SO4), exposed to water below the temperature limit of stable mirabilite formation (Na2SO4·10H2O),suffers severe damage, due to a transition from thenardite to mirabilite, instead of thenardite reprecipitation. Thenardite dissolution can produce solutions highly supersaturated with mirabilite, and there exists a salt content threshold, beyond which damage increases substantially(Tsuiet al.,2003).
The Gibbs free energy was applied for exploring the effects of pressure, temperature, and PSDs, and the crystallization pressure can be modeled by transformation kinetics(Koniorczyk and Gawin,2012;Koniorczyk and Konca, 2013; Koniorczyk, 2015). The damage caused by mirabilite is most serious due to the larger molar volume and contact area with the pore wall compared with anhydrous sodium sulfate and other phase states(Steiger and Asmussen,2008).
Crystal formation and growth in the supersaturated solution are not in thermodynamic equilibrium(Chatterji, 2005). The temperature on the crystal surface is higher than that of the surrounding solution.The temperature difference is affected by the heat released during crystal growth, with a faster crystallization rate inducing a larger difference.
As the crystallization pressure is developed within pores, the macroscopic tensile stress resulted from such pressure is needed for assessing material failure(Flattet al., 2014).According to the theory of porous mechanics and assuming a uniform distribution of crystals in the sample (Coussy, 2006), the stress over a representative volume element (REV) can be averaged to give the macroscopic tensile stress:

whereσ*is the macroscopic tensile stress;σris the radial compressive stress, which equals the crystallization pressure;bis the Biot coefficient; andScis the volumetric fraction of the pore space filled with crystals. Failure occurs when the macroscopic tensile stress exceeds the critical tensile stress according to the strain energy criterion.The critical stress is
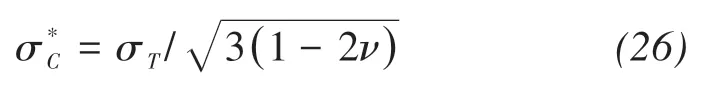
whereis the critical tensile stress;σTis the tensile strength of the materials;andνis Poisson's ratio.
4.3 Multi-field Coupled Thermo-hydro-salt-mechanical (THSM) models
The freezing of saline soil involves heat and mass transfer, deformation, and chemical reaction.As shown in Figure 7, water and salt migration are caused by the temperature gradient. The temperature variation leads to ice-water phase change and salt crystallization. The effective stress will change due to the crystallization of ice and salt, leading to deformation including frost heave and salt expansion. Furthermore, the negative pore-water pressure, due to phase change, is the main driving force for water and salt migration. Therefore,the freezing of saturated saline soil is a dynamic,thermo-hydro-salt-mechanical interacting process.

Figure 7 Thermo-hydro-salt-mechanical interaction mechanism in saline soil
Many researchers have used different laboratory,analytical, and numerical methods to investigate the coupling mechanism of heat and mass transfer and crystallization deformation in freezing saline soil. Salt migrates with pore water, which will affect water transport and heat transfer in porous media, and the stress will also change due to salt crystallization in pores. The salt is rejected from the solution and accumulates at the freezing front in the freezing process,and the salt flux decreases with the increase in cooling rate (Baker and Osterkamp, 1989; Konrad, 1989;Konrad and Mccammon, 1990). By employing the mass balance and energy conservation equation for water and solute in a saturated porous medium, Panday and Corapcioglu (1991) formulated a thermo-hydro-salt (THS) mathematical model for salt rejection and used it to elucidate salt redistribution during soil freezing. Pavliket al.(2011) incorporated advection and diffusion effects in a coupled water-salt transport model. However, this model neglected the phase change of salt.Nguyenet al.(2008)considered the influence of ions on liquid-vapor equilibrium and the salt crystallization on transport properties, and developed and validated a physically-based model for coupled ion-moisture transport. It is well known that a substantial amount of heat is released and consumed during crystallization and dissolution. Therefore, any model that neglects heat transfer might be erroneous.
Notably,Epsinosaet al.(2008)formulated a mathematical model for the kinetics of phase change of salts in pores to simulate the effect of coupled heat,moisture and salt transport in capillary porous materials and used the nonequilibrium Freundlich isotherm to describe the porosity growth. Another model was proposed to describe salt, moisture, and energy transport process in non-isothermal solution concerning the salt phase change kinetics, which accounted for the heat release and consumption during salt crystallization and dissolution (Koniorczyk, 2012). Nevertheless, both models ignored the impact of crystallization pressure on the stress field.
Suitable modelling of the crystallization/dissolution and hydration/dehydration processes allows considering salts with hydrous and anhydrous crystals.Castellazziet al.(2013, 2015) put forward a novel coupled multiphase model for hygrothermal analysis of masonry structures, and demonstrated that it promised to provide an effective prediction of stress induced by salt crystallization. However, this model is only applicable in unfrozen media. For frozen media,Zhanget al.(2018) introduced a solute conservation equation into the traditional three-field coupling model and formulated a THSM model by using the Clapeyron equation to illustrate the phase equilibrium in the multiphase system of soil,ice,and water.
Scholars have preliminarily explored the coupling mechanism of saline soil according to the analyses mentioned above. However, there are some obvious disadvantages. In many cases, the influence of salt on the heat and mass transfer is ignored, and the moisture and temperature fields in the THSM coupling models are still dominated by the three-fields (hydro,thermal and mechanical)addressed in traditional models.Moreover,the effects of salt crystallization and accumulation are rarely considered in the solute transport equation. Largely, insufficient studies exist on the interaction of water and salt migration, heat transfer, salt crystallization and accumulation, and soil deformation during freezing of sulfate saline soil.Therefore,it is extremely important to provide a more complete THSM coupled mathematical model for frozen saline soil.
Based on crystallization kinetics, Wuet al.(2017)derived a THSM coupled model for saturated porous media. This model considered the coupled process of heat and mass transfer, deformation, and crystal phase transition and provided a new approach to build coupled models of freezing saline soil. The simulated frost heave, temperature, and salt redistribution were in good agreement with the test results, which confirmed the model's effectiveness. Based on the above model, Zhanget al.(2020) proposed an ice-water phase change kinetics model for saturated freezing sulfate saline soil, with the consideration of the solute concentration and temperature influences on water activity and solution supersaturation. Based on the Frezchem model,this model also improved the application of supersaturation in the modeling of crystallization kinetics during solution-salt crystal phase change.
The salient features of the coupled model proposed by Zhanget al.(2020) include the consideration of the formation rate of crystal water,the growth rate of salt crystals, and the latent heat of water/salt crystal phase change. This model consists of a few key equations, such as mass balance equation, energy balance equation, and stress balance equation, which are given as:
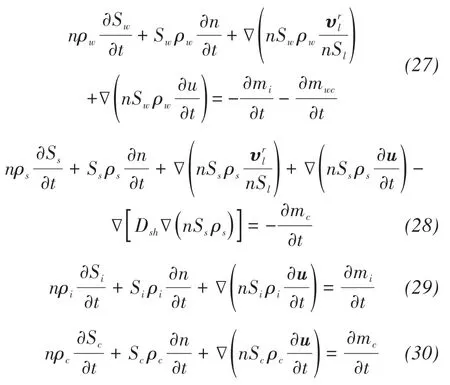
Sulfate and carbonate saline soil is prone to dilation and shrinkage due to variation of temperature and relative humidity in cold regions, which cause the superjacent structure to uplift and settle. Typical countermeasures aim to prevent water and salt beneath a structure from migration and protect the frozen soil in which the infrastructure is founded from thawing.
In permafrost regions with lower ground and annual average temperatures, frozen soil can be protected from thawing by adjusting the thermal resistance of subgrade, such as increasing the height of roadbed and use of thermal insulation materials (Shenget al.,2002; Zhanget al., 2004; Wenet al., 2005; Maet al.,2015). However, in regions with higher air temperature or pavement surface temperature above 0 °C, a subgrade with active cooling must be adopted to protect the permafrost table (Laiet al., 2009).According to the air natural advection theory in the porous medium, Laiet al. (2003, 2006) proposed to use crushedrock layers as embankment and confirmed an optimal particle size for protecting permafrost (Zhanget al.,
5 Countermeasures of frost heave and salt expansion
2007). The size, shape and boundary conditions of an embankment have an inevitable effect on the natural convective effect in ballast embankments. A natural convective index was proposed to determine the height of crushed-rock layers and has been verified by experiments (Konget al., 2006). Besides, a ventilated embankment built in ducts was proposed for application in the field and the cooling effect was verified numerically.The area of thawing bulbs under the ventilated embankment declined, and the largest thawing depth shrunk, indicating that the ventilation pipes were able to reduce the base temperature and ensure the stability of embankments in permafrost regions(Laiet al., 2004). Zhanget al.(2006) presented a three-dimensional numerical model composed of air convective and coupled heat transfer to analyze the stability of the duct-ventilated embankment under wind action and validated the applicability of the model. Wenet al.(2005) proposed a combination of thermal-insulation and two-phase closed thermosyphon to protect the permafrost from thawing and proved its effectiveness. Chen (2016) optimized the design parameters of anti-freeze underground water pipelines in seasonally frozen areas by employing a Quasi-3D method and proposed five easy-to-assess parameters, including the maximum frozen penetration, burial depth, pipeline diameter, flow velocity and inflow water temperature. Additionally, Yang(2019) reported that electrical resistance-heating deicing technologies have been widely used in urban areas with concentrated traffic, which have apparent superiority, such as the same annual energy cost as the average annual maintenance and operation cost, minimal delay time,and improved safety for pedestrian and vehicular traffic.
Frost heave and thaw settlement, salt heave and dissolution settlement of saline soil occur under the periodically fluctuating temperature. While the deformation is mainly caused by salt heave with higher salinity, it mainly results from frost heave and thaw settlement with lower salinity (Liet al., 2009). Many countermeasures against salt migration and expansion have been attempted in saline soil regions. One of them is replacing saline soil with non-saline soil,which is extremely expensive.Another example is stabilization with chemical additions,e.g., NaCl, CaCl2,BaCl2, and Ca(OH)2(Ding and Chen, 1992; Huet al.,2014). Additionally, regulating the salt content and the compaction degree of filling materials, packing embankment, drainage, and slope reinforcement were adopted along the Lanzhou-Xinjiang Railway in China (Tanet al., 2011). Dynamic compaction with gravel column replacement was adopted in Qarhan-Golmud Highway of Qinghai Province in China and has been proved to be an effective reinforcement measure to prevent embankment settlement in saline soil(Zhanget al.,2011).
For road in saline soil with sufficient water and salt supply, a combination of water-proof geotextile and elevated embankment can effectively weaken the water and salt migration. Semirigid base board can also reduce salt expansion (Huanget al., 1997). Yuet al.(2009) reported that frost heave and salt expansion can be efficiently inhibited by mixing calcium chloride or calcium chloride and hydrated lime with saline soil and completely avoided with the addition of cement,hydrated lime and fly ash.
Maet al.(2017) conducted model tests to compare the cooling performance of two selected embankment configurations in saline soil regions, including an ordinary fill embankment and crushed-rock embankment with impermeable geotextile and aeolian sand,as shown in Figure 8.The variations of temperature, moisture, salt, and deformation all confirmed that the crushed-rock embankment configuration can efficiently avoid water and salt migration into the subgrade and prevent frost heave, salt expansion and longitudinal cracks from occurring in the embankment or pavement.
6 Conclusions and future research
This article provides a comprehensive review of existing research of saline soil behavior in cold regions, including the physical and mechanical characteristics, water and salt migration mechanisms, thermo-hydro-salt-mechanical coupled models, and countermeasures of frost heave and salt expansion. The conclusions can be made as follows.
(1)The liquid and plastic limits and optimal water content of soil are proportional to the sodium sulfate content but reversely proportional to the chloride sulfate content.As temperature decreases, the volume of sodium sulfate soil increases, while that of chloride sulfate soil decreases initially and then increases. The thermal and hydraulic conductivities of soil reduce with increasing salt content.
(2) The uniaxial compressive strength of frozen soil is inversely related to temperature by a power function. It increases with the confining pressure of relative lower values but decreases with the confining pressure at relatively larger values. The shear strength of frozen soil falls with increasing salt content. Many constitutive models have been presented for frozen soil,e.g., elasto-viscoplastic isotropic damage model, viscoelastic-plastic model, and elasticplastic model. Besides, some constitutive models have been proposed for frozen saline soil, such as the double yield surface model and a bounding surface elastoplastic model.
(3) Water and salt in saline soils migrate from the warm end to cold end during freezing. The solute and water flux are positively correlated with the initial water content, salt content and temperature gradient but increase parabolically with the dry density. Crystallization first occurs in larger pores and then in smaller pores.The damage induced by crystallization pressure is attributed to a transition between thenardite and mirabilite based on thermodynamics theory. Thermo-hydro-salt and thermo-hydro-salt-mechanical coupled models have been proposed for freezing saline soil and verified with experimental data.

Figure 8 The two physical embankment models(unit:cm).(a)The crushed-rock embankment model with impermeable geotextile,(b)The ordinary fill embankment model.Pink,green,blue marks indicate temperature,moisture and salt concentration,and deformation sensors,respectively(Ma et al.,2017)
(4) Numerous anti-freeze measures for roads have been put into practice in cold regions, including increased roadbed height, use of thermal insulation materials,incorporation of crushed-rock layers in the embankment, ventilated embankment, and a combination of thermal-insulation and two-phase closed thermosyphon.Salt expansion can be inhibited by mixing calcium chloride or calcium chloride and hydrated lime with saline soil. The crushed-rock embankment combining impermeable geotextile with aeolian sand can prevent frost heave, salt expansion and longitudinal cracks from occurring in the embankment or pavement.
Strong evaporation may exist simultaneously in cold regions,e.g., Qaidam Basin, China. Evaporation and cooling drive the water and salt to migrate and accumulate. Salt expansion and collapse, frost heave and thaw settlement, and salt erosion and corrosion of concrete and metal threaten engineered assets, such as water conveyance project, subgrade and pavement.Mechanisms of salt erosion of concrete and salt corrosion of metal in saline soil regions under the joint action of evaporation and freeze-thaw cycles should be focused on in the future.
Acknowledgments:
This research was supported by the National Key Research and Development Program of China (Grant No. 2018YFC0809605); the National Natural Science Foundation of China(Grant Nos.41230630,41601074);the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (Grant No.QYZDY-SSW-DQC015); Science and Technology Plan Project of Tibet(XZ201801-GB-07).
 Sciences in Cold and Arid Regions2021年1期
Sciences in Cold and Arid Regions2021年1期
- Sciences in Cold and Arid Regions的其它文章
- Simulated effect of soil freeze-thaw process on surface hydrologic and thermal fluxes in frozen ground region of the Northern Hemisphere
- Direct incorporation of paraffin wax as phase change material into mass concrete for temperature control:mechanical and thermal properties
- Response of revegetation to climate change with meso-and micro-scale remote sensing in an arid desert of China
- The evapotranspiration and environmental controls of typical underlying surfaces on the Qinghai-Tibetan Plateau
- Evaluating effects of Dielectric Models on the surface soil moisture retrieval in the Qinghai-Tibet Plateau
