Effect of Thunbergia laurifolia water extracts on hepatic insulin resistance in high-fat diet-induced obese mice
Jarinyaporn Naowaboot, Urarat Nanna, Linda Chularojmontri, Pholawat Tingpej, Patchareewan Pannangpetch
1Division of Pharmacology, Department of Preclinical Science, Faculty of Medicine, Thammasat University, Pathum Thani 12120, Thailand
2Division of Microbiology and Immunology, Department of Preclinical Science, Faculty of Medicine, Thammasat University, Pathum Thani 12120, Thailand
3Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
ABSTRACT
KEYWORDS: Thunbergia laurifolia; Obesity; Insulin resistance; Inflammation
1. Introduction
A correlation between obesity-related insulin resistance and development of type 2 diabetes has been well established[1]. The insulin-resistant state responds to high-fat diet (HFD) feeding and obesity, leading to hyperglycemia and hyperlipidemia which result in the fatty liver condition[2]. Hepatic insulin resistance is associated with increased free fatty acid release in the liver. This elevation of hepatic free fatty acid influx can stimulate de novo lipogenesis, leading to an increase in the amount of triglyceride synthesis that causes hepatic steatosis[3]. As the liver is a major organ in the systemic regulation of glucose and lipid metabolism, the primary factor for insulin resistance is associated with hepatic insulin inactivity[4]. Peroxisome proliferator-activated receptor alpha (PPARα), a PPAR subtype that is mainly found in the liver, functions to control β-oxidation and fatty acid transport, responsible for the stability of lipid metabolism and energy[5]. PPARα also regulates glucose metabolism by inhibiting gluconeogenesis[5]. It was found that insulin resistance increases in mice fed with HFD, but this condition can be reduced by activation of PPARα[6,7].
Adenosine monophosphate-activated protein kinase (AMPK) is associated with the regulation of energy homeostasis[8]. In the liver, AMPK functions to control glucose and lipid metabolism by stimulating fatty acid oxidation, suppressing fatty acid and cholesterol synthesis, and reducing gluconeogenesis. AMPK inhibits gluconeogenesis mainly by suppressing phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (G6Pase) genes[9]. The lack of AMPK function in the liver relates to an increase in insulin resistance as well as metabolic disorders, including hepatic steatosis[10]. It has been reported that pterostilbene, a natural PPARα agonist, could activate AMPK function and suppress the expression of gluconeogenic genes, leading to a reduction of glucose production in H4IIE hepatoma cells[11]. It was also showed that, in type 2 diabetic and metabolic syndrome patients, a combination of metformin (AMPK agonist) and fenofibrate (PPARα agonist) was more effective in regulating serum lipid and glucose levels than using these agents alone[12]. AMPK is not the only pathway that is associated with an improvement in glucose metabolism and insulin sensitivity. The phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) pathway is also involved in improving glucose metabolism in the state of insulin resistance[13]. The PI3K/Akt pathway is downstream of insulin receptor and insulin receptor substrate (IRS) proteins and plays a major role in regulating metabolic insulin action[14]. Characteristics of obesity are correlated with an increase in body fat mass, oxidative stress, impaired glucose, and lipid homeostasis as well as chronic inflammation[15]. The proinflammatory cytokines such as tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein-1 (MCP-1) have been reported to be prominently produced in obesity-induced inflammation[16].
Thunbergia laurifolia (T. laurifolia) is a Thai medicinal plant. T. laurifolia has been reported to possess antimutagenic, anti-inflammatory, hepatoprotective, antioxidant, anticancer, neuroprotective, antidote, and antidiabetic properties[17-23]. However, there has been no data indicating whether the different parts of T. laurifolia have similar effects in regulating glucose and lipid homeostasis in obesity-induced insulin resistance conditions. Thus, the present study aimed to investigate the effect of T. laurifolia extracts (leaf, stem, and flower) on hepatic insulin resistance using an obese-mouse model in which the mice were fed with 16 weeks of HFD.
2. Materials and methods
2.1. Chemicals and reagents
Primary antibodies against rabbit phospho-AMPKα (p-AMPK, Cat. No. 09-290), total AMPKα (tAMPK, Cat. No. 07-350) and tAkt (Cat. No. 07-416), and mouse p-Akt (Cat. No. 05-669) were purchased from Millipore (Billerica, MA, USA). Primary antibodies against goat G6Pase (Cat. No. sc-27198), PPARα (Cat. No. sc-1985) and β-actin (Cat. No. sc-1616), and horseradish peroxidaseconjugated anti-goat (Cat. No. sc-2020), anti-rabbit (Cat. No. sc-2004) and anti-mouse (Cat. No. sc-516102) secondary antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Low-fat diet (LFD: D12450H) and HFD (D12451) were purchased from Research Diets (New Brunswick, NJ, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant extraction
T. laurifolia leaf, stem, and flower were collected between October and December 2016 (Phayao, Thailand). The plant was identified by the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand (voucher specimen code: SKP 192 20 12 01). The dried plants were extracted three times with distilled water at 100 ℃ for 30 min. The aqueous extracts were filtered and then freeze-dried. The yields of the dry powder were 7.38%, 5.05%, and 8.36% for T. laurifolia leaf, stem, and flower, respectively.
2.3. Animals and obesity induction
Forty male ICR mice weighing (23 ± 2) g were purchased from the National Laboratory Animal Center (Nakhon Pathom, Thailand). They were housed in a condition of 12-hour light/dark cycle with a room temperature of (25 ± 2) ℃. Animals were provided with LFD and water ad libitum for a week. After that, they were fed with LFD or HFD for 16 weeks.
2.4. Experimental design
After 8 weeks of diet feeding, the mice were randomly divided into five groups (n = 8 in each group). T. laurifolia extracts were dissolved in distilled water, and the extract was orally administered using an intragastric tube daily for 8 weeks. The most effective dose of T. laurifolia extracts was chosen as described in a previous report by Phyu and Tangpong[24]. The five experimental groups were categorized as follows: GroupⅠ: normal control mice (LFD) treated with distilled water; GroupⅡ: obese control mice (HFD) treated with distilled water; Group Ⅲ-Ⅴ: obese mice treated with T. laurifolia leaf, stem, and flower extract, respectively at the dose of 200 mg/kg/day.
The body weight and food intake of the mice were recorded every week. Fasting blood glucose (FBG) was determined before and after the 8-week treatment.
2.5. Sample collection
After 8-week treatment, the 6-hour fasted mice were anesthetized using inhalant isoflurane. For biochemical studies, blood was collected from the heart by cardiac puncture. The liver was removed, weighed, and was further used for histological and Western blotting analysis.
2.6. Intraperitoneal glucose tolerance test (IPGTT)
The IPGTT was determined in 6-hour fasted mice by the method described previously[25]. Mice were intraperitoneally injected with 2.0 g/kg of glucose. The blood glucose was measured at 0, 20, 60, and 120 min.
2.7. Analysis of serum biochemical parameters
Concentrations of insulin, leptin, and TNFα were measured using
Millipore ELISA kit according to the manufacturer’s instructions
(Billerica, MA, USA). MCP-1 was measured using Thermo Scientific ELISA kit (Rockford, IL, USA). Total cholesterol (TC), triglyceride (TG), and non-esterified fatty acid (NEFA) were evaluated using Wako enzymatic colorimetric kit (Osaka, Japan).
2.8. Measurement of liver TG
The liver TG was extracted as previously described[26]. Briefly, 50 mg of the liver was homogenized and extracted with 1 mL of isopropanol. The supernatant from the extraction was measured using Wako enzymatic colorimetric kit (Osaka, Japan).
2.9. Western blotting assay
The protein lysates (20 μg) of the liver were separated using 12%
Mini-PROTEANTGX precast gel (Bio-rad, Brea, CA, USA). Western blotting analysis was performed as previously described[25] with p-AMPK (1:1 000), tAMPK (1:1 000), p-Akt (1:1 000), tAkt (1:1 000), G6Pase (1:1 000), PPARα (1:1 000) and β-actin (1:1 000) primary antibodies. Briefly, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:5 000). The bands were detected using ClarityWestern ECL substrate (Bio-Rad, CA, USA). The images were obtained with an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA), and band intensities were quantified by densitometry using a Gel-ProAnalyzer version 3.1 software. All quantitative analyses were normalized to β-actin.
2.10. Liver histological study
The liver was placed in 10% formalin and processed for paraffin embedding. Sections with 3-μm thickness were stained with hematoxylin and eosin for measuring lipid accumulation in liver tissue. Histological changes were observed using a light microscope (magnification 400×) (CX31; Olympus, Tokyo, Japan).
2.11. Statistical analysis
Data are expressed as mean ± SEM. Data analysis was done by one-way analysis of variance with Tukey’s post-hoc test (SigmaStat 4.0, Systat Software, San Jose, CA, USA). P<0.05 was considered significant.
2.12. Ethical statement
This study was approved by the Animal Ethics Committee of Thammasat University, Pathum Thani, Thailand (AE 004/2015). Animal experiments were conducted according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC accreditation).
3. Results
3.1. Metabolic parameters
As shown in Figure 1A, the mean food intake was significantly (P < 0.05) decreased in all of the obese groups when compared to the normal control group, but it was not significantly different among the obese groups. After 8-week HFD feeding, the baseline body weight was significantly (P < 0.05) higher in all obese groups than that in the normal control group (Figure 1B). At the end of 16-week HFD feeding, the body weight of the obese control group was significantly increased when compared with the normal control group and its baseline body weight. After 8 weeks of T. laurifolia treatments, the body weight was reduced when compared with the obese control group. In addition, the body weight remained unchanged in T. laurifolia-treated groups when compared with the baseline body weight of each group.
After 8 weeks of diet feeding, the baseline of FBG was significantly higher in all the obese groups when compared with the normal control group (Figure 1C). T. laurifolia extracts markedly decreased the FBG (P < 0.05) when compared with the baseline levels. Moreover, the treatment groups had significantly lower levels of FBG in comparison with the obese control group. Glucose tolerance test demonstrated that the normal control mice and the HFD-induced obese mice treated with T. laurifolia stem and flower extracts significantly improved glucose tolerance (Figure 1D).
The significant elevations of serum insulin and leptin were observed in the obese control group (P < 0.05) (Figure 1E and 1F). The HFD-induced hyperinsulinemia and hyperleptinemia were significantly suppressed by T. laurifolia treatments. Moreover, the T. laurifolia extracts also reduced the serum TNFα and MCP-1 (P < 0.05) induced by HFD (Figure 1G and 1H).

Figure 1. Food intake (A), body weight (B), fasting blood glucose (C), blood glucose in IPGTT (D), serum insulin (E), serum leptin (F), serum TNFα (G) and serum MCP-1 (H) after 8-week treatment with Thunbergia laurifolia extracts. Data are expressed as mean ± SEM (n = 8). *P < 0.05 vs. the normal control group. #P < 0.05 vs. the obese control group. ‡P < 0.05 vs. baseline in each group. **P < 0.05 vs. baseline of normal control group. †P < 0.05 vs. after treatment of the obese control group. NC: normal control mice, OB: obese control mice, TLL: Thunbergia laurifolia leaf extract, TLS: Thunbergia laurifolia stem extract, TLF: Thunbergia laurifolia flower extract, IPGTT: intraperitoneal glucose tolerance test, TNFα: tumor necrosis factor alpha, MCP-1: monocyte chemoattractant protein-1.

Figure 2. Effect of Thunbergia laurifolia extracts on the levels of serum TC (A), TG (B) and NEFA (C). Data are expressed as mean ± SEM (n = 8). *P < 0.05 vs. the normal control group. #P < 0.05 vs. the obese control group. NC: normal control mice, OB: obese control mice, TLL: Thunbergia laurifolia leaf extract, TLS: Thunbergia laurifolia stem extract, TLF: Thunbergia laurifolia flower extract, TC: total cholesterol, TG: triglyceride, NEFA: non-esterified fatty acid.

Figure 3. Effect of Thunbergia laurifolia extracts on liver weight (A), liver TG (B) and liver histological structure (H&E staining, 400×) (C). In the liver histological study, Thunbergia laurifolia extracts reduce lipid deposition in obese mice vs. the control group. Data are expressed as mean ± SEM (n = 8). *P < 0.05 vs. the normal control group. #P < 0.05 vs. the obese control group. NC: normal control mice, OB: obese control mice, TLL: Thunbergia laurifolia leaf extract, TLS: Thunbergia laurifolia stem extract, TLF: Thunbergia laurifolia flower extract.
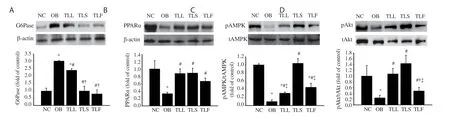
Figure 4. Liver protein expressions of G6Pase (A), PPARα (B), phosphorylated AMPK (C) and phosphorylated Akt (D) by Western blotting analysis. Data are expressed as mean ± SEM (n = 8). *P < 0.05 vs. the normal control group. #P < 0.05 vs. the obese control group. †P < 0.05 vs. the TLL group. ‡P < 0.05 vs. the TLS group. NC: normal control mice, OB: obese control mice, TLL: Thunbergia laurifolia leaf extract, TLS: Thunbergia laurifolia stem extract, TLF: Thunbergia laurifolia flower extract, PPARα: peroxisome proliferator-activated receptor alpha, G6Pase: glucose-6-phosphatase, tAMPK: total adenosine monophosphate-activated protein kinase, tAkt: total Akt.
3.2. Lipid profiles and liver histological examination
T. laurifolia treatments significantly reduced the high levels of serum TC, TG, and NEFA (P < 0.05) (Figure 2A-C), liver to body weight ratio and liver TG accumulation (Figure 3A and 3B). Moreover, the liver histological study revealed that the obese mice treated with all the extracts decreased lipid deposition compared with the obese control group (Figure 3C).
3.3. Liver protein expression
As shown in Figure 4A, the G6Pase protein expression was significantly (P < 0.05) increased in the obese control group. Interestingly, all T. laurifolia extracts significantly (P < 0.05) decreased the expression of G6Pase and the stem and flower extracts showed better improvement.
The downregulated expression of PPARα, and the ratio of pAMPK to tAMPK and pAkt to tAkt was observed in the obese control group (Figure 4B-D). However, treatment with T. laurifolia extracts reversed the change induced by HFD. The T. laurifolia stem extract showed significant improvement in upregulating the ratio of pAMPK to tAMPK and pAkt to tAkt.
4. Discussion
The current study presents evidence that the administration of T. laurifolia extracts could improve the impairment of glucose and lipid homeostasis as demonstrated in the HFD-induced obese mouse model with hepatic insulin resistance. In this mouse model, HFD feeding for 16 weeks could lead to the development of insulin resistance, glucose intolerance, hyperglycemia, hyperleptinemia, and hyperlipidemia which is similar to the obesity-induced insulin resistance found in humans.
The treatment with three parts (leaf, stem, and flower) of T. laurifolia extracts could control food consumption as well as body weight. This study showed that body weight was significantly reduced after 8 week treatments when compared with the obese control group. Moreover, the liver weight was markedly reduced in all T. laurifolia groups, suggesting that treatment with T. laurifolia extracts may control the metabolic process in obese conditions.
There is a link between hepatic insulin resistance, which promotes elevated fasting blood glucose, and the progression to diabetes[27]. The liver acts as an important organ in decreasing glucose production and improving glucose homeostasis in diabetic patients[28]. The treatment with T. laurifolia extracts was responsible for a decrease in hyperglycemia, hyperinsulinemia, and hyperleptinemia conditions. After 8 weeks of T. laurifolia treatment, we found that the extracts (especially from stem and flower parts) significantly decreased insulin resistance as shown by IPGTT. This may be due to the positive impact of T. laurifolia extracts on glucose homeostasis and insulin sensitivity. We further investigated the status of AMPK phosphorylation that has a function in the regulation of glucose and lipid homeostasis[9]. Activation of AMPK can improve insulin action in conditions related to diabetes and obesity[29,30]. As an important regulator in suppressing lipogenesis, gluconeogenesis, and protein synthesis in the liver, AMPK can suppress expression of G6Pase, a key enzyme for hepatic gluconeogenesis[31]. This study showed that hepatic phosphorylated AMPK proteins were increased and the expression of G6Pase was decreased in T. laurifolia-treated groups, indicating that T. laurifolia extracts could improve hyperglycemia through the regulation of AMPK activity in gluconeogenesis. Akt, a key effector of the IRS/PI3K pathway, also acts as a suppressor of hepatic glucose production[13]. Our study found that T. laurifolia extracts increased the phosphorylation of Akt in the liver of obese mice. This evidence shows that the activation of Akt by T. laurifolia extracts may also be involved in the inhibition of hepatic glucose production. It is thus possible that T. laurifolia extracts improve glucose homeostasis and insulin sensitivity by activating AMPK and Akt.
There are reports on the effect of PPARα agonist (fenofibrate) in improving glucose homeostasis in prediabetic patients[32]. PPARα is a metabolic sensor that has a positive effect on the reduction of plasma TG by stimulating fatty acid uptake, fatty acid oxidation, and lipoprotein lipase activity[33]. Activation of PPARα can inhibit postprandial hyperlipidemia by stimulating fatty acid oxidation in intestinal epithelial cells[34]. Moreover, activation of PPARα affects intestinal lipid and lipoprotein metabolism by reducing cholesterol esterification, suppressing chylomicron production, and increasing high-density lipoprotein synthesis by enterocytes[35]. The present study showed a stimulatory effect of T. laurifolia extracts on hepatic PPARα protein expression. As PPARα is known to regulate circulating lipid profile, treatment with T. laurifolia extracts can reduce the levels of serum TC, TG, and NEFA. In a state of insulin resistance, the antilipolytic function of insulin is impaired, resulting in the stimulation of hepatic TG synthesis[36]. This study showed that T. laurifolia extracts could decrease the hepatic TG content in HFD-induced obese mice. Furthermore, the lipid droplets in the liver were found to be reduced in T. laurifolia-treated groups. These findings suggest the action of T. laurifolia extracts in regulating lipid homeostasis in the condition of obesity-related hepatic insulin resistance. It is reported that the activation of AMPK is related to the activation of PPARα signaling in a fasting state[37]. Hence treatment with T. laurifolia extracts may regulate glucose and lipid homeostasis during insulin resistance by stimulating hepatic phosphorylated AMPK proteins.
The condition of HFD-induced inflammation has been shown to be related to the pathogenesis of insulin resistance[38]. Obesity is a cause of chronic low-grade inflammation, leading to alteration of inflammatory cytokines such as TNFα and MCP-1[39]. These cytokines are produced primarily from adipose tissue macrophages in obesity-induced insulin resistance[40]. This study demonstrated that T. laurifolia extracts have an anti-inflammatory effect as shown by the reduction of TNFα and MCP-1. The reduction of inflammatory cytokines by T. laurifolia extracts may have a role in improving insulin resistance conditions.
There are some limitations to the present study. The study only investigated the AMPK and Akt activity of T. laurifolia extracts in regulating glucose and lipid homeostasis in mice with obesityrelated hepatic insulin resistance. The beneficial effect of this extract on insulin sensitivity is involved in the stimulation of AMPK and Akt pathway. However, the metabolic effects of AMPK and Akt can be found in many tissues, including skeletal muscle and adipose tissue. Therefore, to determine its possible effect on these pathways, further study should be conducted in vitro (HepG2, L6 skeletal muscle, and 3T3-L1 adipocyte cells) using activators or inhibitors. Moreover, as previously mentioned, the inflammatory cytokines are involved in chronic low-grade inflammation and insulin resistance. Even though this study has shown the effect of T. laurifolia extracts in the reduction of the circulating inflammatory cytokines, it does not necessarily represent the inflammatory process occurring in the adipose tissue. Further investigation on the anti-inflammatory effect of the T. laurifolia extracts in adipose tissue is thus needed.
In conclusion, the present study indicates that T. laurifolia extracts (leaf, stem, and flower) act as a useful agent for regulating obesityrelated hepatic insulin resistance. The data suggest that T. laurifolia extracts may improve glucose and lipid homeostasis by reducing hyperglycemia, hyperinsulinemia, hyperleptinemia, hyperlipidemia, hepatic lipid accumulation, and inflammatory cytokines. These beneficial effects may be due to the stimulation of phosphorylated AMPK and Akt protein expressions. Among the three parts of T. laurifolia, the stem tends to be the most effective in improving glucose and lipid homeostasis. Therefore, T. laurifolia extracts can be considered as one of the therapeutic alternatives for the control of impaired glucose and lipid homeostasis in obesity-related hepatic insulin resistance and type 2 diabetes.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
We thank the kindness of Dr. Benjaporn Buranrat for providing the source for collecting this medicinal plant. We kindly thank Mr. Sebastien Maury for helping with the manuscript preparation and proofreading.
Funding
This work was supported by the research grant from the National Research Council of Thailand (Contract number: 37/2560).
Authors’ contributions
JN designed the experiment, collected and analyzed the data, and wrote the manuscript. UN and LC collected and analyzed the data. PT and PP analyzed the data and wrote the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年3期
Asian Pacific Journal of Tropical Biomedicine2021年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Phytochemical analysis of Berberis lyceum methanolic extract and its antiviral activity through the restoration of MAPK signaling pathway modulated by HCV NS5A
- Celastrus paniculatus oil ameliorates synaptic plasticity in a rat model of attention deficit hyperactivity disorder
- p-Coumaric acid alleviates adriamycin-induced hepatotoxicity in rats
- Apoptotic and cytostatic actions of maslinic acid in colorectal cancer cells through possible IKK-β inhibition
