p-Coumaric acid alleviates adriamycin-induced hepatotoxicity in rats
Zeinab Rafiee, Maasoumeh Zare Moaiedi, Armita Valizadeh Gorji, Esrafil Mansouri
1Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2Department of Clinical Biochemistry, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
3Bone Marrow Transplantation Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
4Cellular and Molecular Research Center, Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
ABSTRACT
KEYWORDS: Adriamycin; Hepatotoxicity; p-Coumaric acid; Antioxidant; IL-1β
1. Introduction
Adriamycin is an anthracycline antibiotic that is effective in the treatment of a variety of cancers such as cancers of the ovary, uterus, in addition to hematological malignancies[1]. This chemotherapy drug is widely applied in both adult and pediatric patients as part of a combination regimen[2]. However, its application is limited due to its high toxicity[3]. Researchers have reported that free radicals are involved in toxicity caused by adriamycin[4]. Adriamycin’s chemical structure induces free radical formation and oxidative stress generation, which leads to cellular damage[5]. Adriamycin creates an imbalance between antioxidants and formation of reactive oxygen species (ROS). The disorder in oxidant-antioxidant systems leads to tissue damage, which is shown with protein oxidation and lipid peroxidation in tissue[6]. Endogenous antioxidant enzymes, including catalase (CAT) and superoxide dismutase (SOD), may inhibit the ROS effects; however, they quickly become quenched by a large amount of ROS. Many researches have revealed that oxidative stress, free radicals, and lipid peroxidation, along with inflammatory process, are often correlated with liver damage caused by toxic agents such as adriamycin. Regulation of these mediators has been regarded as a therapeutic requirement to avoid toxicity caused by adriamycin in different organs[7-9]. Using natural compounds with radical scavenging property can mitigate the damage caused by ROS produced by drugs. Natural antioxidant composites, including probucol, naringenin, epigallocatechin gallate, and quercetin have been examined with favorable outcomes with regards to their impact on toxicity induced by adriamycin in in vitro investigations and animal models[10-13]. p-Coumaric acid, a hydroxy derivative of cinnamic acid, can be available in an extensive range of edible plants, including barley grains, peanuts, tomatoes, navy beans, garlic, carrots as well as honey. It can also be found in vinegar and wine in substantial quantities[2,14,15]. p-Coumaric acid is one of the several phenolic composites that a majority of the world’s population receives every day by their dietary intake routine. p-Coumaric acid is an antioxidant with anti-inflammatory and antitumor effects. It is also effective in preventing various disorders such as cancers and cardiovascular diseases[16]. Although p-coumaric acid has an antioxidant effect, hepatoprotective property of this natural antioxidant has not been investigated.
This research aimed to assess the effect of p-coumaric acid on adriamycin-induced hepatotoxicity in rats.
2. Materials and methods
2.1. Reagents and chemicals
Adriamycin 50 mg vial (EBEWE Pharma Ges, Austria), p-coumaric acid powder (Sigma-Aldrich, St. Louis, Mo, USA), polyclonal primary antibody against interleukin 1-beta (IL-1β) and secondary antibody (Zellbio GmbH, Germany), glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) kits (Randox Lab, Crumlin, UK) were purchased. The rest of the chemicals were of analytical grade.
2.2. Ethical statement
All animal experimental protocols were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.ABHC.REC.1397.091) in agreement with guidelines of National Institutes of Health (NIH)[17].
2.3. Experimental design
Thirty-two Wistar rats weighing 190-210 g were procured from the animal house of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals were housed in standard conditions with the temperature (24 ± 2) ℃ and a 12-h dark/light cycle and free access to water and food. The rats were divided into 4 experimental groups (8 rats per group) as follows: Group 1: rats received solvent (10% propylene glycol) orally and served as normal control; Group 2: rats received 100 mg/kg[18] of p-coumaric acid (dissolved in 10% propylene glycol) orally for 5 d; Group 3: rats received a single dose of adriamycin (15 mg/kg i.p.)[19]; Group 4: rats received p-coumaric acid for five consecutive days and then treated with adriamycin. Twenty-four hours after the last administration, the rats were kept under anesthesia using ketamine (75 mg/kg) and xylazine (10 mg/kg) and sacrificed.
2.4. Serum collection and tissue preparation
Blood samples were collected from heart ventricle, centrifuged at 3 000 rpm (10 min), and the obtained serum was used for biochemical analysis. The livers of rats were removed immediately.
A portion of the liver was dissected and stored at -70 ℃ for assessment of oxidative stress markers. Another part of the liver was fixed in formalin (10%) for immunohistochemical and histopathological analyses.
2.5. Biochemical analyses
Serum biomarkers of the liver function including alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), cholesterol, triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), albumin, and total bilirubin (TB) were measured according to the methods described for the particular kit (Pars Azmoon, Iran). All biochemical assays were performed spectrophotometrically using an auto-analyzer (Vita lab Selectra).
2.6. Liver homogenate preparation and determination of protein concentration
For preparing liver tissue homogenate, the liver was homogenized (Heidolph Silentcrosher M, Germany), and a 5% w/v homogenate was prepared in potassium phosphate buffer (0.1 M, pH 7.4). The homogenate was centrifuged at 16 000 ×g for 20 min, and the supernatant was collected. Protein concentration was determined in the supernatant by Bradford’s method[20].
2.7. Determination of oxidative stress markers in tissue
The measurement of lipid peroxidation of the liver was done by a reaction with thiobarbituric acid as described by Nikravesh et al[21]. Randox antioxidant kits were used to measure the activity of GSHPx and SOD. Tissue CAT activity was determined according to the method described in our previous study[22].
2.8. Histopathological analysis
Fixed liver samples embedded in paraffin and sectioned at 5 µm were stained with hematoxylin-eosin. All sections were examined under a light microscope (Olympus, CX31). A minimum of five microscopic fields was evaluated to score the samples. Histopathological evaluation and scoring were done by an observer unaware of the experimental groups. The factors for evaluation of sections were based on intensity and diffusion of degenerative hepatocytes, inflammatory cells, eosinophilic cytoplasm, dilatation of sinusoids, and parenchymal necrosis. In the end, counted values were summed for every section and therefore the degree of degeneration was specified in conformity with the scoring. Scoring was applied as follows: 0 to 4 (no, low, moderate, high, and extremely high, respectively)[8].
2.9. Immunohistochemical examination
Immunohistochemical staining was performed for IL-1β using polyclonal rabbit/anti-IL-1β antibody. Briefly, liver sections were deparaffinized, followed by antigen retrieval and incubated with the primary antibody against IL-1β (dilution 1:400) overnight at 4 ℃. Then, sections were washed and incubated for 60 min with a peroxidaseconjugated secondary antibody (Goat anti-rabbit IgG/HRP; dilution 1:1 000). Diaminobenzidine was used to visualize the peroxidase. Finally, sections were counterstained with haematoxylin. For every slide, 15 microscopic fields were examined, and positive IL-1β was scored from 0 to 4. The degrees were computed based on intensity of staining as follows: 0 = no staining; 1 = low staining; 2 = moderate staining; 3 = severe staining; 4 = very severe staining[23].
2.10. Statistical analysis
Data were presented as mean ± standard deviation (SD). Oneway analysis of variance was used to test the statistical significance (SPSS version 16.0) between the different groups followed by Tukey’s test. Statistical significance was set at P-value < 0.05.
3. Results
3.1. Effect of p-coumaric acid on biochemical parameters
Effects of p-coumaric acid on biochemical parameters are shown in Table 1. The levels of serum ALT, AST, ALP, TG, cholesterol, LDL-C, and TB were significantly elevated while HDL-C and serum albumin levels were decreased (P < 0.01) in the adriamycin group compared with the normal control. p-Coumaric acid administration reversed adriamycin-induced changes in all biochemical parameters (P < 0.05). p-Coumaric acid administration alone did not affect biochemical parameters when compared with control animals.
3.2. Effect of p-coumaric acid on oxidative stress markers
The impact of p-coumaric acid on oxidative stress markers in rats is illustrated in Table 2. The MDA level was increased (P < 0.01), but antioxidant enzyme activities (GSH-Px, SOD, and CAT) were decreased significantly (P < 0.01) in the adriamycin group when compared with the normal control group. However, these alterations in lipid peroxidation level and antioxidant enzyme activities were recovered (P < 0.01) by pretreatment of p-coumaric acid. Administration of p-coumaric acid alone without adriamycin injection did not show any influence on oxidative stress markers in comparison with control rats.

Table 1. Effect of p-coumaric acid (100 mg/kg/day) on biochemical parameters in rats with adriamycin-induced hepatotoxicity.

Table 2. Effect of p-coumaric acid (100 mg/kg/day) on MDA level and antioxidant enzyme activity in rats with adriamycin-induced hepatotoxicity.

Table 3. Effect of p-coumaric acid (100 mg/kg/day) on histopathological changes in rats with adriamycin-induced hepatotoxicity.

Figure 1. Effect of p-coumaric acid on histopathological changes of liver tissue in different experimental groups (H&E ×300). (A) The normal control group, and (B) the group receiving p-coumaric acid treatment alone (100 mg/kg/day) show that the liver has a normal architecture. (C) The adriamycin group shows parenchymal necrosis (black arrow), dilatation of sinusoids (white arrow), hepatocyte degeneration (green arrow), eosinophilic cytoplasm (yellow arrow) and inflammatory cells (blue arrow). (D) The group treated with adriamycin and p-coumaric acid alleviates the damages induced by adriamycin.
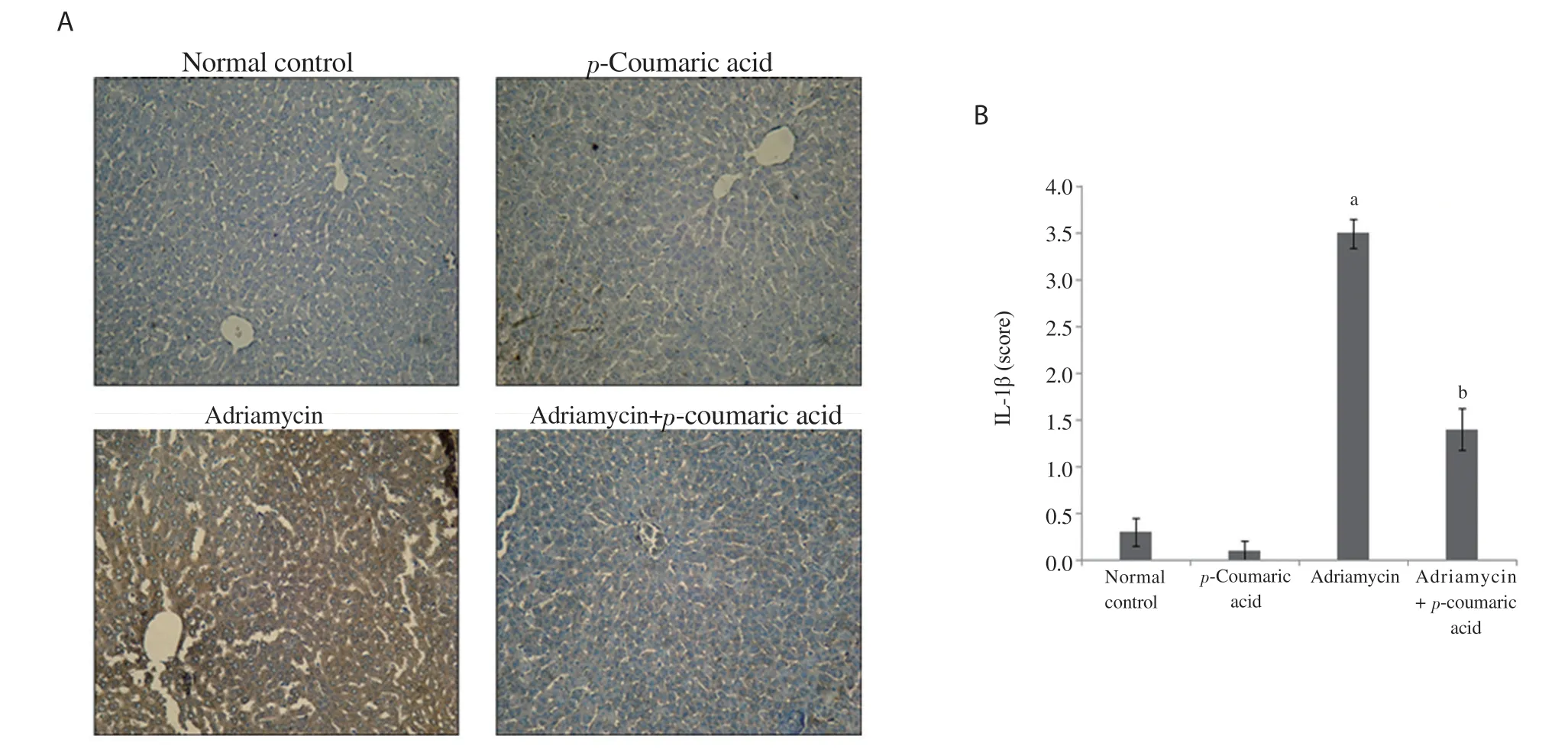
Figure 2. Effect of p-coumaric acid on IL-1β expression in liver tissue. (A) Immunohistochemistry analysis of IL-1β expression. (B) Bar graph. Data are expressed as mean ± SD (n=8). acompared to the normal control group, bcompared to the adriamycin group, P < 0.05.
3.3. Effect of p-coumaric acid on histopathology of the liver
Histopathological examination revealed that normal control and p-coumaric acid alone groups had normal structure in hepatic lobules and sinusoids (Figure 1; Table 3). On the other hand, the adriamycintreated group showed dilated sinusoids and marked degeneration of hepatic cords. Furthermore, eosinophilic cytoplasm, inflammatory cells, and necrotic parenchyma were also shown in this group. Pretreatment with p-coumaric acid prevented the histopathological damages induced by adriamycin (Figure 1).
3.4. Effect of p-coumaric acid on IL-1β expression
The expression of IL-1β was measured by immunohistochemical staining (Figure 2A). Semiquantitative analysis was further accomplished to calculate the degree of significance (Figure 2B). Immunohistochemical staining of hepatic tissues indicated that adriamycin injection induced a significant increase (P < 0.001) in IL-1β expression compared to the normal control and p-coumaric acid alone groups. Administration of p-coumaric acid before adriamycin significantly decreased IL-1β expression in hepatic tissue (P < 0.001). No significant difference was shown in IL-1β expression between the normal control and p-coumaric acid alone groups (Figure 2B).
4. Discussion
Adriamycin induced an injury in the liver tissue shown by microscopic and biochemical tests in this study. Cancer treatment with chemotherapy drugs such as adriamycin and other quinone anthracyclines is drastically restricted by intense toxicity[24]. Metabolites of semiquinone by delocalization of Fe (Ⅱ) from ferritin and generation of hydrogen peroxide cause hydroxyl radical formation and oxidative damage of the cellular systems[8]. The present findings reveal that administration of adriamycin caused a marked increase in levels of ALT, ALP, AST, and TB. Furthermore, adriamycin administration leads to a considerable decline in serum albumin levels. These findings are in agreement with many studies that have shown that rats treated with adriamycin exhibited significant increases in ALT, AST, as well as bilirubin levels as compared to the untreated control group[25,26]. The higher AST and ALT activities reported in the current research can be due to intense conditions caused by the toxic activity of doxorubicin accumulations in the liver; and in turn, this may stimulate cellular destruction or increase the hepatic cell permeability. There was a relative increase in the concentration of serum TB, which can be ascribed to protection of defense mechanism against free radical-induced oxidative damage, such as a decrease in free radicals by raising electron donors like bilirubin[27]. The occurrence of hepatic damage was confirmed by the rise in the activities of ALT, AST, ALP, as well as TB level. The present study showed that p-coumaric acid treatment reversed the changes induced by adriamycin effectively, which indicates the hepatoprotective effects of p-coumaric acid.
Moreover, adriamycin strikingly elevated the level of TG, cholesterol, LDL-C, and decreased the level of HDL-C. Lipids have a pivotal role in CVD[28]. Adriamycin interferes with lipid biosynthesis and metabolism; thus, the levels of TG, cholesterol, as well as LDL-C, were raised, and the level of HDL-C was reduced in serum. Treatment with p-coumaric acid that restored these parameters towards normal suggested that hypolipidemic effect of p-coumaric acid could be likely mediated by a local impact on the hepatic cholesterol esterification and its elimination by bile[29]. Ragab et al. observed the same impact in his study[30].
Damage is attenuated via antioxidant enzymes, such as GSHPx, SOD, CAT, at the cellular level[31]. One of the main enzymatic antioxidant mechanisms facing superoxide radicals is SOD that inhibits adriamycin-induced liver toxicity[8]. GSH-Px and CAT catalyze the superoxide anion dismutation (O) into hydrogen peroxide (HO), then transform HOinto the water, which can play a protective role against ROS[32]. The decrease in activity of such enzymes can be due to the increase in the production of free radicals during adriamycin metabolism[33]. Existing data revealed that adriamycin dramatically enhanced lipid peroxidation and declined levels of SOD, GSH-Px, as well as CAT in liver tissue. It was also revealed that adriamycin not only increases the free radical production but also reduces its capability to detoxify ROS[34]. p-Coumaric acid treatment dramatically reduced the level of lipid peroxidation and raised the activity of SOD, GSH-Px, and CAT compared to the adriamycin group, which may be attributed to its ability to scavenging free radicals[35]. The results of the present study are in line with those of previous research[36]. In addition, the findings of the current research show that adriamycin induces significant structural alterations in hepatic tissue of rats. The damage to this organ might be owing to the induced oxidative stress by the reactive intermediates of semiquinone made from adriamycin. It has been reported that the anthracyclines form semiquinone radical intermediates that react with molecular oxygen to form ROS interacting with cell macromolecules to cause cellular injury[37]. Treatment with p-coumaric acid remarkably attenuates adriamycininduced liver damages, perhaps by minimizing oxidative stress caused by adriamycin. Recent work confirms this hypothesis[38]. Tissue inflammation is a primary adverse response after adriamycin exposure. Studies have shown that adriamycin causes a series of inflammatory reactions in tissues via upregulating NF-κB and inducing the release of various pro-inflammatory cytokines[39]. It has been demonstrated quite lately that the gradual rise of proinflammatory cytokines in tissues may be the pathological cause for adriamycin-induced toxicity[40]. In accordance with these findings, the results of the present study showed a significant increase in IL-1β expression in the adriamycin group compared with the normal control, confirming a key role of inflammation in the pathogenesis of adriamycin-caused hepatotoxicity. Impaired antioxidant capacity of tissue, elevated levels of ROS, as well as lipid peroxidation, may be the major factors that cause such changes. Increases in inflammatory mediators were recently reported to be associated with elevated oxidative stress that is thought to trigger inflammatory reactions[41]. The present study demonstrated that treatment with p-coumaric acid caused a significant reduction in hepatic expression of IL-1β. p-Coumaric acid has been indicated to possess anti-inflammatory effects in addition to its antioxidant properties[42]. Earlier research also had recorded a mild inhibitory impact for p-coumaric acid on the NF-κB activation[43]. These features of p-coumaric acid indicate a cytoprotective function against the release of inflammatory mediators by adriamycin within liver tissue. This conclusion is in line with the results described by Pragasam et al[42].
Our research confirms the useful effect of a natural phenolic acid in protecting animals against adriamycin-induced hepatic oxidative injury. This property of p-coumaric acid may be attributed to its free radical scavenging and anti-inflammatory ability. p-Coumaric acid could be used in cancer chemotherapy with adriamycin.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Funding
This work was supported by a grant from the Deputy of Research and Technology Development of Ahvaz Jundishapur University of Medical Sciences (Grant No. 97s55).
Authors’ contributions
EM designed the work. ZR and MZM performed experiments and collected data. AVG and EM analysed the data and interpreted the results. EM wrote the manuscript in consultation with ZR. All authors have read and approved the final manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年3期
Asian Pacific Journal of Tropical Biomedicine2021年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Phytochemical analysis of Berberis lyceum methanolic extract and its antiviral activity through the restoration of MAPK signaling pathway modulated by HCV NS5A
- Celastrus paniculatus oil ameliorates synaptic plasticity in a rat model of attention deficit hyperactivity disorder
- Effect of Thunbergia laurifolia water extracts on hepatic insulin resistance in high-fat diet-induced obese mice
- Apoptotic and cytostatic actions of maslinic acid in colorectal cancer cells through possible IKK-β inhibition
