Celastrus paniculatus oil ameliorates synaptic plasticity in a rat model of attention deficit hyperactivity disorder
Khushboo G Faldu, Snehal S Patel, Jigna S Shah✉
1Zydus Research Centre, Cadila Healthcare Ltd., Ahmedabad, Gujarat, India
2Department of Pharmacology, Institute of Pharmacy, Nirma University, Ahmedabad, Gujarat, India
ABSTRACT
KEYWORDS: Attention deficit hyperactivity disorder; Celastrus paniculatus; Biogenic amines; Synaptophysin; Nerve growth factor
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is a paediatric disorder, which is now prominent in adolescents and adults due to lifestyle changes[1]. Studies showed that the prevalence of ADHD in adolescents ranges from 16.5% to 40.8% worldwide[2], while in India, the prevalence of ADHD ranges from 13.7% to 50.0%[3,4]. The prevalence exhibits rising trends in recent years by about 11%, which may cause an increasing burden on health care systems[5]. The etiological factors include genetic aberrations in dopamine transporter genes and in serotonin transporter genes, which causes neurotransmitter imbalance. Plasma membrane reuptake transporter and vesicular monoamine transporter are the crucial regulators of the neurotransmitter release and signalling pathways of dopamine[6]. The neurotransmitters related to ADHD include dopamine (DA), 5-hydroxytryptamine (5-HT), and noradrenaline (NE)[7]. Perinatal and prenatal exposure to environmental pollutants like lead, mercury, and polychlorinated biphenyls or head injury are implicated in ADHD pathophysiology[1]. The usual symptoms include hyperactivity, aggressiveness, and impulsivity. Current treatments include amphetamine, atomoxetine, and methylphenidate[8]. Further, pharmacological agents related to nicotine are also being extensively studied in various cognitive fields including attention and impulsive action of humans[9]. WHO estimates that 20% of adolescents suffer from one or more mental or behavioural problems[2]. Mishra et al. showed that developing attentive self-focus with the breath as an anchor had wide-ranging positive effects on the important functional brain networks, cognition, and real-life behaviours in adolescents with a history of adversity[10].
Many herbal drugs have been investigated for their nootropic and neuroprotective effects on different CNS disorders. Ayurvedic practitioners regard Celastrus paniculatus (C. paniculatus) as the “intellect plant” and use it to improve IQ of mentally retarded children. Oil, ethanolic, methanolic and aqueous extracts of the seeds showed dose-dependent protection against hydrogen peroxide and glutamateinduced toxicities in forebrain neuronal cells of rats[11]. Chronic administration of seed oil caused decreases in the turnover of NE, DA, and 5-HT and their respective metabolites in the brain and urine of albino rats. It reversed the scopolamine-induced task performance deficits and improved memory process in rats[12]. Both the seed oil and petroleum ether extract showed an anti-anxiety effect in rats. Further, the oil and its different extracts showed sedative, anticonvulsant, tranquillizing, cognition-enhancing, and anti-inflammatory activities in different animal models[13]. The literature supported the neuroprotective effect of C. paniculatus oil in chronic aluminium-induced corticohippocampal neurodegeneration, scopolamine-induced amnesia, and sodium nitrite-induced amnesia models using C. paniculatus oil alone or in combination with ghee[14]. These studies primarily focused on behavioural tests and lacked any data on its effectiveness on ADHD. Thus, the present study was undertaken to evaluate neuropharmacological activity of C. paniculatus oil and to investigate the downstream signalling pathways of the treatment of ADHD induced by social isolation and lead acetate, respectively.
2. Materials and methods
2.1. Chemicals and drugs
C. paniculatus oil was bought from Lalubhai Vrijlal Gandhi, Ahmedabad, Gujarat, India. Authentication was done by Department of Pharmacognosy, Institute of Pharmacy, Nirma University, Ahmedabad and a voucher specimen was deposited (NIP/PCOL/2016/02). All the chemicals used were of analytical grade and were obtained from Sigma Aldrich, India. Kits for biochemical estimation of interleukin-6 (IL6), NF-κB, nerve growth factor (NGF), tumour necrosis factor-α (TNF-α) were purchased from Labex Corporation, Mumbai, India.
2.2. Phytochemical analysis of C. paniculatus oil
C. paniculatus oil was screened for the presence of carbohydrates, reducing sugars, tannins, proteins, steroids, saponins, phenolic compounds, and alkaloids[15].
2.3. Experimental animals and protocol
Healthy male Sprague Dawley rats of 1 month age (weighing 75-150 g) were chosen and maintained under the well-controlled condition of temperature (22 ± 2) ℃, humidity (55 ± 5)% and a 12 h/12 h light-dark cycle. Standard laboratory rat chow and UV filtered water were provided ad libitum.
The rats were randomly divided into eight groups of eight animals each i.e. NC: Normal control, NP: Normal control treated with lead acetate (1 000 ppm)[15], NPS: Normal control animals treated with lead acetate (1 000 ppm) + atomoxetine (70 mg/kg), NPC: Normal control animals treated with lead acetate (1 000 ppm) + C. paniculatus oil (2 g/kg)[16], SI: Social isolation, SP: Socially isolated animals treated with lead acetate (1 000 ppm), SPS: Socially isolated animals treated with lead acetate (1 000 ppm) + atomoxetine (70 mg/kg)[17], SPC: Socially isolated animals treated with lead acetate (1 000 ppm) + C. paniculatus oil (2 g/kg). The rats were trained for behavioural paradigms i.e. Y-maze, novel object preference, residentintruder aggression tests and fear conditioning test. The animals were caged according to the group. Rats in the normal control group were group-housed; while socially isolated animals were caged as 1 animal/cage. Except for the NC and SI groups, all the animals were administered lead acetate (1 000 ppm) in drinking water for 2 months. From the 9th week, all the animals were given their respective treatments for the next 2 weeks. At the end of the 10th week, the animals were subjected to behavioural paradigms. Blood was collected in heparinised centrifuge tubes from the retro-orbital plexus under light ether anaesthesia. Plasma was separated and stored at -80 ℃ for further estimations. The animals were sacrificed and brains were isolated, placed into ice-cold saline and processed for biogenic amine estimation, immunohistochemical assay and histopathological examination.
2.4. Behavioural paradigms
2.4.1. Novel object preference test
The rats were assessed for free-choice novel object preference. Experiments lasted for 3 d and were divided into three phases: habituation, familiarization, and novel object preference. During the habituation phase, rats were allowed to explore the empty box for 5 min followed by exploring two identical objects for 3 min on the second day. One of the objects was replaced with a new object on the third day and novel object exploration was recorded for one minute if the centre of the rat’s head was oriented within 45° of the objects and within 4 cm of it. Exploratory time spent on novel objects was calculated by subtracting the exploration time devoted to the familiar object from exploration time devoted to the novel object. Discrimination index is a ratio of difference in exploration time for familiar object, to that of the total amount of exploration of the novel and familiar objects[18].
2.4.2. Modified Y-maze test
A modified version of the Y-maze test was conducted on the 10th week. Sample trial was conducted wherein one arm of the Y-maze was closed, the rat was placed in open arm with its head facing towards the periphery of the maze and was allowed to move freely for 5 min. In the test trials, the animals were allowed to explore all the open arms for 5 min. The time spent on the new arm that had been closed in the sample trials was recorded[19].
2.4.3. Fear conditioning test
On the 10th week, the animals were allowed to explore freely for 4 min in fear conditioning chamber, which consisted of a transparent acrylic chamber (30 cm×30 cm×30 cm) and a stainless-steel grid floor equipped with an electric shock generator. In the training trials, the animal received an acoustic tone (2.9 kHz, 20 s, 80 dB) coterminated with electric foot shocks (0.8 mA, 2 s) for 5 times with a one-minute interval. One min after the final foot shock delivery, the rats were returned to their home cage[11]. The test trials were conducted 24 h after the training trials to measure freezing responses to the context and auditory tone. The rats were placed in the same chamber to provide the contextual stimuli and allowed to move freely for 3 min. Behavioural responses were analysed to measure freezing behaviour as an index of contextual memory. After the contextual recording, the rats were exposed to the tone for 2 min and freezing behaviour was recorded as auditory dependent fear memory. Freezing was defined as the absence of any movement except for those related to respiration[19].
2.4.4. Resident-intruder aggression test
Aggressive behaviour of rats was analysed at the end of the 10th week. Briefly, an intruder rat of the same gender was placed in a resident home cage (24 cm×17 cm×12 cm) and resident-intruder interactions were video recorded for 20 min. The aggressive behaviour of resident rat was characterized by an initial pattern of exploratory activity around the intruder, which was followed by rearing and tail rattle, accompanied in a few seconds by wrestling and/or a violent biting attack. The total duration of these attacks and/or wrestling during the 20 min observation period was measured[19].
2.5. Biochemical parameters
IL-6, NGF, nuclear factor NF-κB, and TNF-α were quantitatively estimated in plasma using enzyme-linked immunosorbent assay (ELISA) kits by spectrophotometric measurement.
For determination of biogenic amines, homogenization of frozen brains was carried out in an extraction medium using an electrical homogenizer. The neurochemical analysis was done to assess concentrations of 5-HT, DA, and NE using Jacobowitz Method. The tissue was homogenized in butanol and 0.01 mol/L HCl and centrifuged. The butanol supernatant was extracted with phosphate buffer for NE and DA estimation, and HCl extraction yielded samples for 5-HT estimation. The estimation of NE and DA was done by adding versene, iodine, alkaline sodium sulphite and 5 mol/L acetic acid to phosphate. The fluorescence was read at excitation 385 nm and emission 485 nm. Fluorophore for 5-HT was developed using ortho-phthalaldehyde and concentrated HCl. The 5-HT fluorescence was read at excitation 360 nm and emission 470 nm[20].
2.6. Oxidative stress parameters
The brain was homogenized in phosphate buffer saline using a homogenizer to prepare 10% homogenate and centrifuged at 4 000 g in the refrigerated centrifuge for 30 min. The supernatant was taken as aliquots for the estimation of the biochemical parameters.
Brain tissue lipoperoxidation (LPO) estimation was performed using the TBARS assay. The colorimetric reaction of the LPO product malondialdehyde (MDA) with thiobarbituric acid was quantified. The concentration of thiobarbituric acid reactive substances was measured at 532 nm using a standard curve of MDA, and the results were expressed as nmol MDA/mg protein[21]. Superoxide dismutase (SOD) is an enzyme which catalyses the mutation of superoxide radical to form hydrogen peroxide and oxygen. The assay for the determination of indirect SOD activity is based on the inhibition of reaction between superoxide radical with adrenaline[21]. For reduced glutathione estimation, tissue non-protein sulfhydryl groups were quantified after mixing the homogenate with 10% trichloroacetic acid (1:1, v/v), followed by centrifugation as described by Ellman. Cysteine was used for the preparation of a standard curve[21]. Catalase (CAT) activity was determined by measuring the decrease in hydrogen peroxide (HO) absorption at 32 ℃. The method is based on the consumption of HOby CAT and loss of absorbance at 240 nm[22].
2.7. Immunohistochemistry
The brain tissues were isolated and stored in buffered formaldehyde for 24 h at 4 ℃. The fixed brains were sliced coronally at 25 µm using a vibrating microtome (Biochrom, India). For a primary antibody (Synaptophysin 1:200), three to five consecutive sections from each brain were used. The sections were further incubated with biotin-labelled secondary antibodies for 1 h at 37 ℃ and visualized with 3, 3'-diaminobenzidine as brown in colour. The images were observed using a microscope (Olympus CKX41, Japan)[23].
2.8. Histopathological examination of brain
Histopathologic assessment was performed on the rat brain specimens. Brains were immediately fixed in 10% phosphate buffered formaldehyde, embedded in paraffin, and 5 µm longitudinal sections were performed. The sections were stained with hematoxylin and eosin (H&E) and examined microscopically[24].
2.9. Statistical analysis
GraphPad Prism 5 was used. The results were expressed as mean ± SEM. One-way ANOVA followed by Bonferroni's multiple comparison test was applied. P<0.05 was considered to be statistically significant.
2.10. Ethical statement
The protocol of the experiment was approved by Institutional Animal Ethics Committee of Nirma University, Ahmedabad as per the guidance of Committee for the Purpose of Control and Supervision of Experiments on Animal, Ministry of Social Justice and Empowerment, Government of India with protocol number IP/PCOL/MPH/18/002 on 4th January 2016.
3. Results
3.1. Pharmacognostic analysis
The oil of C. paniculatus showed the presence of carbohydrates, steroids, saponins, and alkaloids.
3.2. Behavioural tests
3.2.1. Novel object preference test
The exploration time of the novel object was increased in the treatment groups except the NPC group as compared with the NP, SI and SP group. The discrimination index showed that all animals could easily discriminate between novel and known object and the SPC group showed significant (P<0.05) improvement as compared to Day 1 (Table 1).
3.2.2. Y-maze test
The percentage of time spent in the new arm of Y-Maze was decreased in the treatment groups as compared with the NP, SI and SP groups, respectively. The results of the NPS, SPS, and SPC groups were comparable to normal control animals, while the NPC group showed a significant decrease compared with the NP group.
3.2.3. Fear conditioning test
The freezing behaviour under contextual fear conditioning was more prominent in the normal control and the treatment groups as compared with the NP, SI and SP groups, although the difference was insignificant. The freezing behaviour under conditioned fear conditioning was similar to contextual fear conditioning (Table 1).
3.2.4. Resident-intruder aggressive test
Animals in the SI group was more aggressive as compared with the NC group. Lead acetate administration (NP and SP) aggravated the aggressiveness in animals. Atomoxetine (NPS and SPS groups) and C. paniculatus oil (NPC and SPC groups) alleviated the aggressiveness (Table 2).
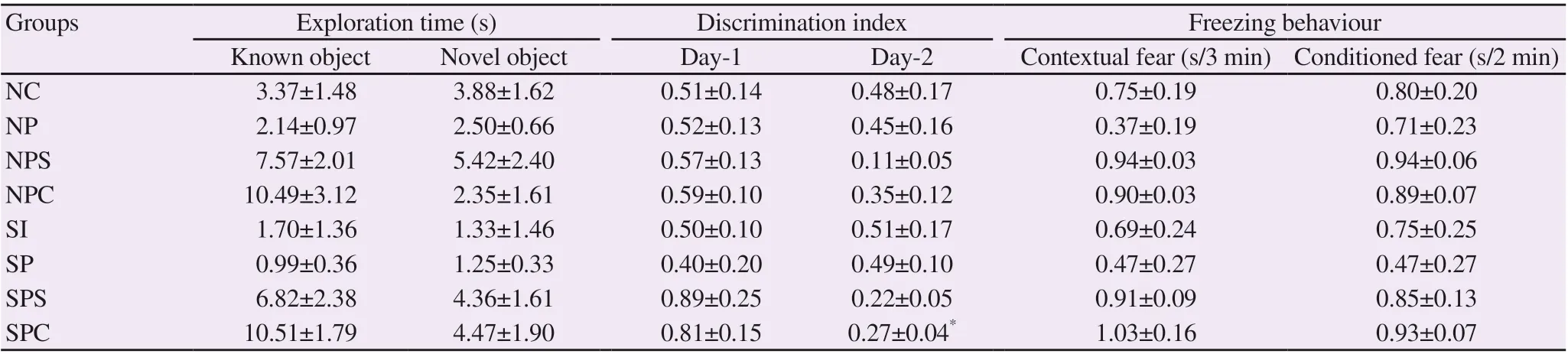
Table 1. Effect of Celastrus paniculatus oil on behavioural paradigms.
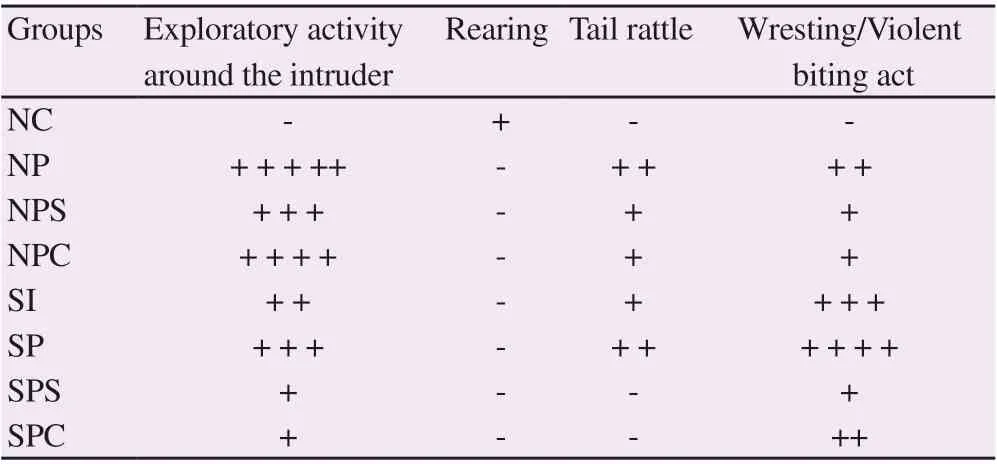
Table 2. Effect of Celastrus paniculatus oil on resident intruder aggressive behaviour.
3.3. Effect of treatment on neuroinflammatory parameters
IL-6 levels were increased in the NP and SP groups, which were decreased by atomoxetine and C. paniculatus oil (Figure 1A). NGF levels were higher in treatment groups as compared with the NP, SI and SP groups, which were higher than in the NC group (Figure 1B). NF-κB, and TNF-α levels were significantly increased in the NP, SI, and SP groups as compared with the NC group (P<0.05), which were decreased in the treatment groups (Figure 1C & 1D).
3.4. Effect of treatment on biogenic amine concentrations
5-HT concentration was reduced in the SI group, while lead acetate increased significantly 5-HT (P<0.05) in the NP group as compared with the NC group. Atomoxetine and C. paniculatus oil restored the 5-HT levels (Figure 2A). DA content was increased in the NP and SP groups which was decreased by the treatments (Figure 2B); while NE content was decreased in the NP, SI and SP group as compared with the NC group, which was increased by atomoxetine and C. paniculatus oil (Figure 2C).
3.5. Effect of treatment on oxidative stress parameters
SOD levels were decreased in the NP, SI and SP groups as compared to the NC group. However, SOD levels were increased in the SPS and SPC groups but decreased in the NPS and NPC groups (Figure 3B). MDA levels were increased in the NP group as compared with the NC group but decreased in the SI and SP groups. The NPC group showed a significant decrease as compared with the NP group (P<0.001). In addition, the SPC group significantly (P<0.05) reduced the MDA level as compared with the SI Group (Figure 3A). Glutathione levels were decreased in the NP, SI, and SP group as compared with the NC group and the treatment groups increased the levels, but all differences were not significant (Figure 3C). CAT levels were increased in the NP, SI, SP, NPS, NPC, SPS and SPC groups compared with the NC group (Figure 3D).
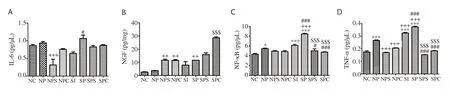
Figure 1. Effect of treatment on neuroinflammatory parameters. A: interleukin-6 (IL-6) level, B: nerve growth factor (NGF) level, C: nuclear factor-κB (NFκB) level, D: tumour necrosis factor (TNF)-α level. Values are expressed as mean ± SEM. Compared with the NC group, *P<0.05, ***P<0.001; Compared with the NP group, ++P<0.01, +++P<0.001; Compared with the SI group, #P<0.05, ###P<0.001; Compared with the SP, $$$P<0.001. NC: normal control, NP: normal control treated with lead acetate, NPS: normal control animals treated with lead acetate & atomoxetine, NPC: normal control animals treated with lead acetate and Celastrus paniculatus oil, SI: social isolated, SP: social isolated animals treated with lead acetate, SPS: social isolated animals treated with lead acetate and atomoxetine, SPC: social isolated animals treated with lead acetate and Celastrus paniculatus oil.
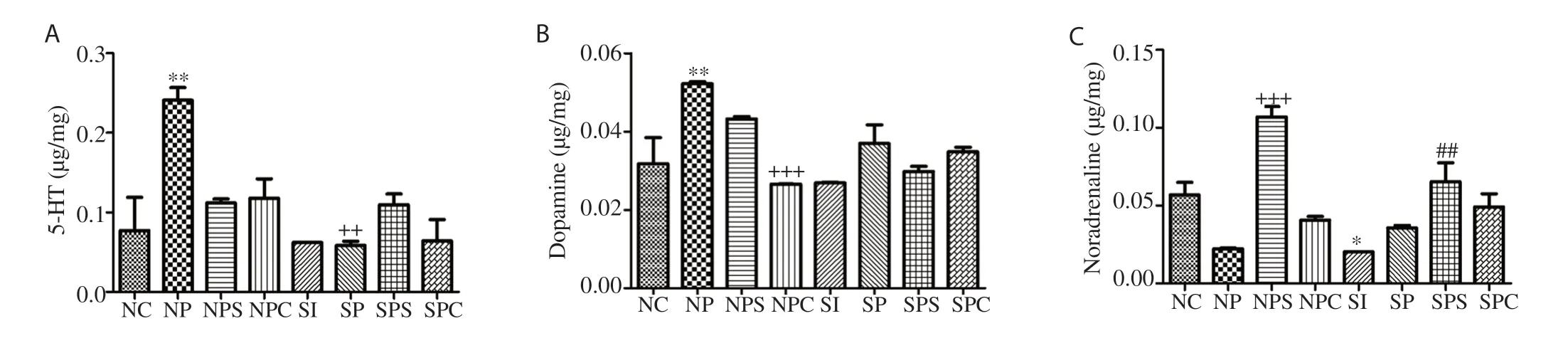
Figure 2. Effect of treatment on biogenic amine concentrations. A: 5-hydroxytryptamine (5-HT), B: dopamine, C: noradrenaline. Values are expressed as mean ± SEM. Compared with the NC group, *P<0.05, **P<0.01; Compared with the NP group, ++P<0.01, +++P<0.001; Compared with the SI group, ##P<0.01.
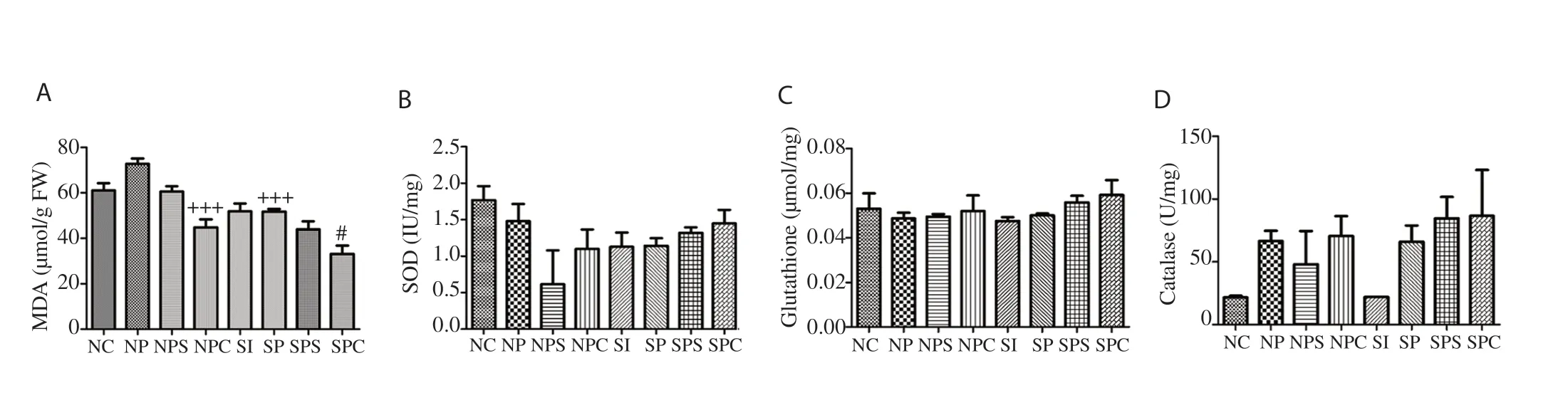
Figure 3. Effect of treatment on oxidative stress parameters. A: malondialdehyde (MDA), B: superoxide dismutase (SOD), C: glutathione, D: catalase. Values are expressed as mean ± SEM. Compared with the NP group, +++P<0.001; Compared with the SI group, #P<0.05.
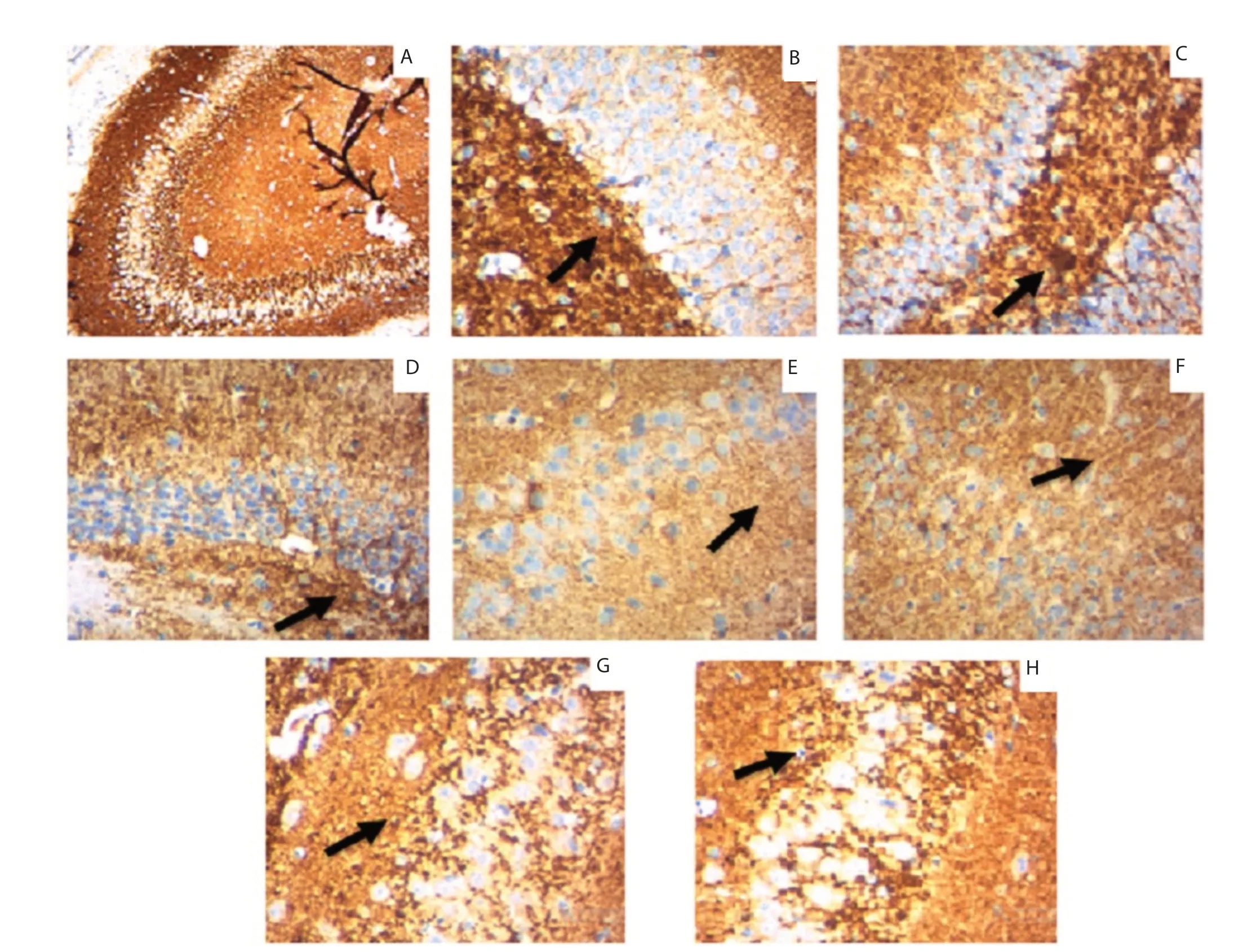
Figure 4. Synaptic plasticity was assessed by synaptophysin expression in the hippocampus of rats by immunohistochemistry. A: Sections show normal synaptophysin expression. B, E, F: Sections show reduced expression of synaptophysin which is represented by weak brown stain (shown by arrows) intensity (borderline to weak) in cells. C, D, G, H: Sections show strong staining (shown by arrows) intensity (moderate to strong) in cells. (Magnification 10×). A: normal control; B: normal control treated with lead acetate; C: normal control animals treated with lead acetate and atomoxetine; D: normal control animals treated with lead acetate and Celastrus paniculatus oil; E: social isolated; F: socially isolated animals treated with lead acetate; G: socially isolated animals treated with lead acetate and atomoxetine; H: socially isolated animals treated with lead acetate and Celastrus paniculatus oil.
3.6. Immunohistochemical analysis
Synaptophysin expression was evaluated by the intensity of brown staining in immunohistochemical analysis. The result showed that the SI and SP group showed less expression of synaptophysin represented by weak brown stain intensity (borderline to weak) in cells. However, there was strong staining intensity (moderate to strong) found in both atomoxetine and C. paniculatus oil treated groups indicating increased expression of synaptophysin after treatment (Figure 4).
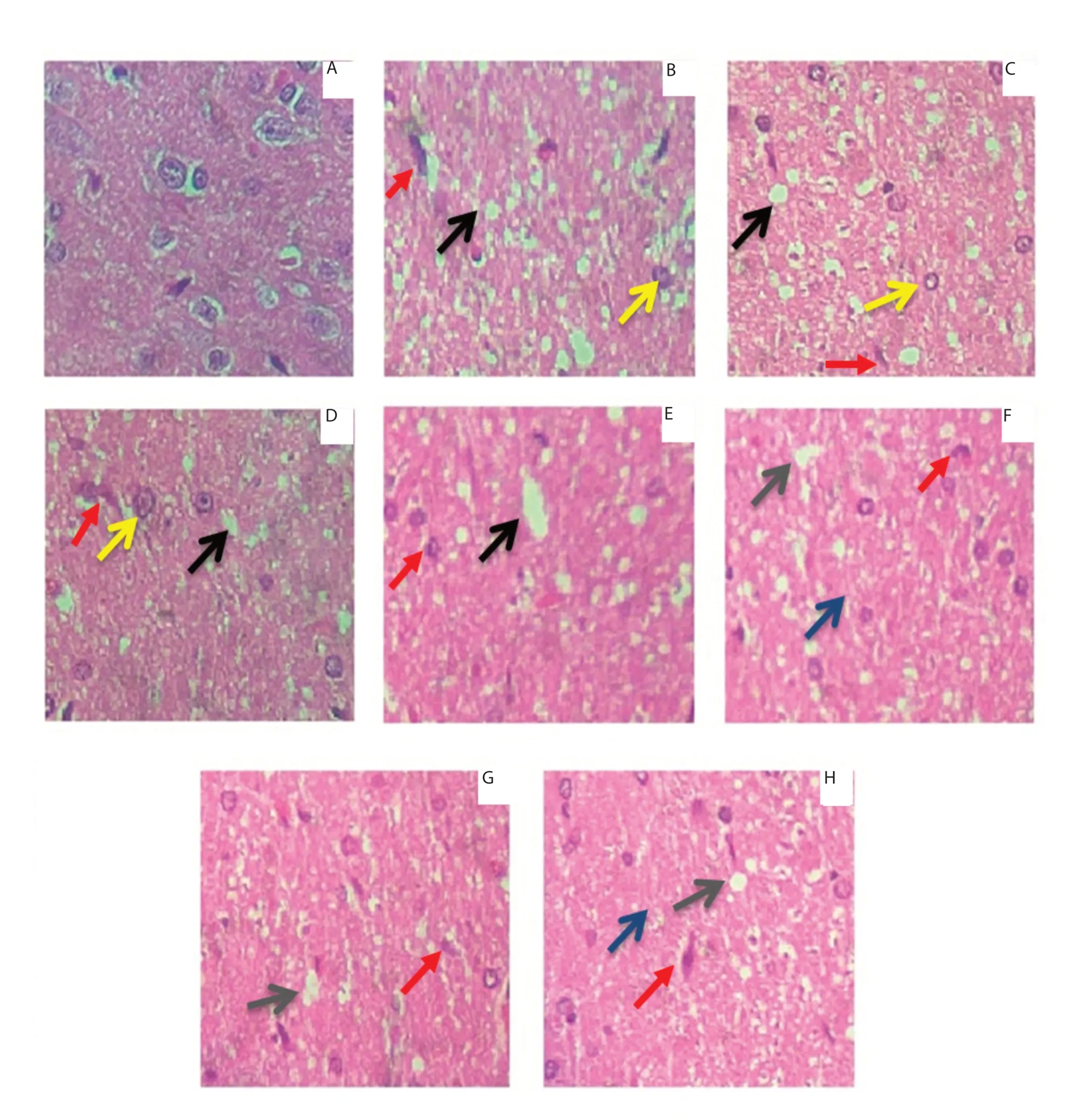
Figure 5. Histopathological examination results. Neuronal damage was assessed by H&E staining (Magnification: 10×). A: normal histo-architecture; B: significant reactive gliosis (red arrows), mild degeneration (black arrows) and vascular proliferation (yellow arrow); C, D: decreased reactive gliosis (red arrows), mild degeneration (black arrows) and vascular proliferation (yellow arrow); E: reactive gliosis (red arrows) and mild degeneration (black arrows); F: reactive gliosis (red arrows), spongiotic degeneration (grey arrows) and inflammatory infiltration (blue arrows); G, H: decreased reactive gliosis (red arrows), spongiotic degeneration (grey arrows) and inflammatory infiltration (blue arrows). A: normal control; B: normal control treated with lead acetate; C: normal control animals treated with lead acetate & atomoxetine; D: normal control animals treated with lead acetate & Celastrus paniculatus oil; E: social isolated; F: socially isolated animals treated with lead acetate; G: socially isolated animals treated with lead acetate & atomoxetine; H: socially isolated animals treated with lead acetate & Celastrus paniculatus oil.
3.7. Histopathological examination
Rats in the NP and SI group showed reactive gliosis and mild degeneration; while rats in the SP group showed reactive gliosis, spongiotic degeneration, and inflammatory infiltration. The treatment with C. paniculatus oil and standard drug improved these changes, with fewer reactive gliosis, degeneration and inflammatory infiltration (Figure 5).
4. Discussion
ADHD is characterized by impaired attention, impulsivity, and hyperactivity in children. These symptoms are usually maintained in adults. During adolescence, prefrontal cortex develops connectivity with other brain regions to engage executive functions such as latent inhibition, attention and inhibitory control[25]. It is well established that ADHD patients exhibit impairments across a range of cognitive abilities, such as learning performance (i.e., latent inhibition), executive functions, novelty-seeking and exploratory activity, and short-term memory[26]. Social isolation in an early age i.e. childhood causes developmental abnormalities together with increased aggressiveness, hyperactivity, and impulsiveness. Lead has been known as environmental neurotoxicant responsible for impairment in brain development and resultant impaired intelligence, attention, and behavioural problems. High lead exposure during the developmental stage is known to cause aggravation of ADHD symptoms[27].
In the present study, social isolation and lead acetate were used to establish ADHD rat model. Socially isolated animals have been previously compared with the normal animals wherein the researchers found that the animals were more aggressive and restless. In accordance with the other studies, our study also found that social isolation causes ADHD like symptoms, which are worsened by lead toxicity. These animals were enthusiastic, restless, impulsive, aggressive and were difficult to handle. These observations are backed by the pre-clinical testing data[15].
Previous studies showed that behaviour in the Y-maze test was altered in a spontaneously hypertensive rat model of ADHD[18,24]. Our study showed that the percentage of time spent on the new arm of Y-maze was increased in the NP and SP groups as compared with the NC and SI groups, which showed hyperactivity and risk-taking behaviour. Atomoxetine and test drug C. paniculatus decreased the time, showing the beneficial role of C. paniculatus in treating ADHD. The exploration time of the novel object was increased in the treatment groups as compared with the NP, SI, and SP groups. It has been reported that social isolation induces deficit of latent learning performance, which can be measured by fear conditioning test and discrimination index[25]. The discrimination index was significantly decreased in the treatment groups as compared to the NP, SI, and SP groups, which indicated that the hyperactivity in novel setting was under control because of the treatment with atomoxetine and C. paniculatus oil. Fear conditioning test illustrated that the freezing behaviour in contextual and conditioned fear conditioning test was more prominent in the treatment groups as compared with the NP, SI, and SP groups, indicating better long-term memory function. Resident intruder aggressive test showed that rats in the NP, SP, and SI groups were more aggressive as compared with the treatment groups and the normal control group. The behavioural tests showed that social isolation and lead toxicity causes symptoms similar to ADHD i.e. hyperactivity, impulsiveness, and aggressiveness. Treatment with C. paniculatus oil could alleviate these symptoms.
Social isolation and lead toxicity induce free radical generation and thus, in turn, increase oxidative stress[26,27]. The present study showed a decrease in SOD and reduced glutathione levels in the NP, SI, and SP groups. Catalase level was found to be increased in the NP and SP groups. MDA levels were elevated in the NP group and decreased in the SI and SP groups. The treatment groups showed increased SOD, reduced glutathione and catalase levels as well as decreased MDA levels. Thus, due to anti-oxidant potential, C. paniculatus might be useful in the treatment of ADHD.
The bioamines i.e. DA, NE and 5-HT are implicated in the pathophysiology of ADHD[26]. DA is responsible for rewardmotivated behaviour. NE is responsible for maintaining alertness, increasing focus and sustaining effort, thought, and motivation[28]. 5-HT is related to hyperactivity[29]. The clinical data shows that ADHD patients have decreased DA and NE contents and thus the drugs available for the treatment are either noradrenaline reuptake inhibitors or dopamine agonists[30,31]. The present study showed that DA concentration was decreased in the SI group and increased in NP and SP groups as compared with the NC group. The NPC group showed a decrease in DA concentration, which was comparable to the NC group. NE levels in the NP, SI, and SP group were decreased as compared with the NC group. The treatments group showed the comparable result to the normal control group. 5-HT concentration was increased in the NP group while all the other groups had similar levels of 5-HT. These results suggest that C. paniculatus might be useful in relieving anxiety, hyperactivity, reward-motivated behaviour and ameliorating the symptoms of ADHD by alternating neurotransmitter levels.
NGF is found to be increased in ADHD subjects as compared with normal control subjects[32]. NGF levels are increased in response to inflammation and oxidative stress. Our study showed a significant increase in the NGF levels in the treatment groups. Metal toxicity produces free radicals and in turn causes oxidative stress, which induces inflammation and thus IL-6 production[33]. Social isolation alters neroinflammatory response which can lead to decrease in IL-6 concentrations. IL-6 levels were found to be increased in lead acetate administered groups (NP and SP) which was reduced by the treatments. IL-6 levels are lower in ADHD subjects than in normal control animals. In our study, IL-6 levels were decreased by atomoxetine and C. paniculatus oil. NF-κB plays an important role in the pathogenesis of inflammation-associated neurodegeneration[34]. In our study, NF-κB was increased in the NP, SI, and SP groups indicating inflammation and neuronal damage due to stress and lead acetate toxicity. It was reduced by C. paniculatus oil treatment. Previous reports showed that an increase in cytokines levels is associated with symptoms and brain functions in ADHD patients[35]. In our study, TNF-α was increased in the NP, SI, and SP groups and C. paniculatus could decrease TNF-α. Thus, the neuroprotective effect of C. paniculatus oil may be attributed to its anti-inflammatory potential.
Neurotransmitter dysfunction is the recognized etiology of ADHD and hence proteins encoding proteins involved in the vesicular release can serve as attractive candidates for ADHD genetic studies. Synaptophysin plays an important role in synaptic plasticity and vesicular regulation[36-38]. Literature has conflicting opinions on whether social isolation has an effect on synaptophysin expression. Our study shows that lead toxicity alters synaptophysin expression. Synaptophysin expression was decreased in the NP, SI, and SP group and was restored almost to normal level in the treatment groups.
Social isolation aggravates the severity of brain injury and its subsequent memory deficits by increasing subcortical cell injury and cell death. Metal toxicity or lead toxicity affects the normal histological structure of the brain, which can lead to disturbances of brain function and aggravate defects due to social isolation[39,40]. The C. paniculatus oil restored the disturbed histological architecture of the brain.
Our study shows anti-oxidant, anti-inflammatory and neurotransmitter balancing properties of C. paniculatus oil. In addition, C. paniculatus oil ameliorates the synaptic plasticity and confers neuroprotective effect. It can be used for the treatment of ADHD.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
We are thankful to The Gujarat Council on Science and Technology (GUJCOST) for providing financial support in form of minor research project (GUJCOST/MRP/2014-15/2592).
Authors’ contributions
KGF contributed to the background literature review, animal studies, data acquisition, and manuscript preparation. SSP contributed to data analysis, drafting, and revision of the manuscript. JSS contributed to the conception, design of the study, supervision of the work, and critical revision of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年3期
Asian Pacific Journal of Tropical Biomedicine2021年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Phytochemical analysis of Berberis lyceum methanolic extract and its antiviral activity through the restoration of MAPK signaling pathway modulated by HCV NS5A
- Effect of Thunbergia laurifolia water extracts on hepatic insulin resistance in high-fat diet-induced obese mice
- p-Coumaric acid alleviates adriamycin-induced hepatotoxicity in rats
- Apoptotic and cytostatic actions of maslinic acid in colorectal cancer cells through possible IKK-β inhibition
