Reactive oxygen species and senescence modulatory effects of rice bran extract on 4T1 and NIH-3T3 cells co-treatment with doxorubicin
Ummi Maryam Zulfin, Ave Rahman, Mila Hanifa, Rohmad Yudi Utomo,2, Sari Haryanti, Edy Meiyanto,4✉
1Cancer Chemoprevention Research Center, Faculty of Pharmacy, Universitas Gadjah Mada (UGM), Sekip Utara, Yogyakarta 55281, Indonesia
2Laboratory of Medicinal Chemistry, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, UGM, Sekip Utara, Yogyakarta 55281,Indonesia
3Medicinal Plant and Traditional Medicinal Research and Development Centre, Ministry of Health, Republic of Indonesia
4Laboratory of Macromolecular Engineering, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, UGM, Sekip Utara, Yogyakarta 55281,Indonesia
ABSTRACT
KEYWORDS: Rice bran extract; ROS; Senescence; Doxorubicin;4T1; NIH-3T3
1. Introduction
Doxorubicin (Dox) is an anthracycline class of chemotherapy drugs used as first-line therapy for various cancers, including those of the breast [especially triple-negative breast cancer cells(TNBC)], blood, and liver[1]. Dox can kill cancer cells through DNA intercalation mechanism, disruption of DNA repair mediated by topoisomerase Ⅱ, and the formation of free radicals and damage to cellular membranes, DNA, and proteins[2]. However, the use of anthracycline anti-cancer can increase the level of free radical production in cells[3]. Meanwhile, the reductive activation of Dox to semiquinone triggers the formation of superoxide by the reduction of one electron of oxygen, where the breakdown of superoxide produces HO[4]. Therefore, the use of Dox as a chemotherapeutic agent needs to be accompanied by the use of a synergistic agent that can reduce the dose[3].
Related to the induction effect of intracellular reactive oxygen species (ROS), one of the side effects of Dox is the induction of cell senescence, which occurs not only in cancer cells but also in normal cells[5]. Dox can increase intracellular ROS levels and stimulate senescence in non-cancer NIH-3T3 fibroblast cells[3].Fibroblast cells confer skin elasticity by synthesizing collagen as the main component of connective tissue[6]. Therefore, compounds that can reduce cell senescence with Dox in fibroblast cell models must be explored to develop anti-aging agents. High ROS production is correlated with increased senescence and apoptosis in cancer cells[7].Therefore, this phenomenon is often used as a basis for developing anti-cancer targets[8]. The mechanisms of oxidants and antioxidants have different pathways and can simultaneously occur; thus, this relationship can also be used as a target in the development of cytoprotective agents through decreasing ROS levels and inhibiting senescence[3,9]. Glutathione-S-transferase (GST) is a pivotal enzyme to neutralize intracellular ROS that is usually expressed in cancer cells, including 4T1 cells[10].
Compounds with antioxidant properties, such as vitamin E, are agents that can modulate intracellular ROS levels and may interact with ROS metabolizing enzymes[8]. Vitamin E is a fat-soluble component that originates from food and has many vital roles in the body because of its antioxidant activity[11]. Tocotrienol (TTE),a member of the vitamin E family, is a natural compound that can be found in several vegetable oils, wheat germ, and several types of nuts and seeds[12]. TTE reduces senescence in normal cells[13]. It also exerts a cytoprotective effect on cell death induced by glutamate through its antioxidant mechanism[14]. TTE is abundant in rice bran in the form of fat-soluble components (oil)[15]. Rice bran is one of the byproducts of cheap rice milling and has been used more often as animal feed.
Rice bran acts as an anti-cancer agent through anti-inflammatory pathways, cell cycle inhibition, cell apoptosis, and increasing the effectiveness of chemotherapeutic compounds[16]. The dominant active compound, namely, the vitamin E derivative TTE, affects the physiological condition of cancer cells by increasing the apoptosis of the caspase 3 pathway and inhibiting the G/Gphase cell cycle in gastric adenocarcinoma cells SGC-7901[17]. In addition,arabinoxylan rice bran compounds increase the effectiveness of paclitaxel against MCF-7 breast cancer cells[18]. A previous study evaluated the efficacy of TTE compounds in breast cancer MCF-7/Adr and T47D cells[19]. Considering the activity of these synthetic compounds, further studies should assess the activity of rice bran extracts (RBE) containing vitamin E complex compounds in malignant breast cancer, such as in triple-negative subtype breast cancer. This study aimed to explore the potential of rice bran in the form of fat-soluble components as a co-chemotherapeutic and cytoprotective (anti-aging) agent. We employed the 4T1 cells as the representative of TNBC cells characterized by highly proliferative and metastatic properties[20]and NIH-3T3 cell line served as a representative of non-cancer cells[21]. Since cell cycle arrest and apoptosis are important markers of the cytotoxic effect of anti-cancer agents[22]which are closely correlated to an abrogation of cell cycle progression permanently through cellular senescence[23]and ROS generation, we investigated the effect of RBE combined with Dox on 4T1 cells via determination of cell cycle arrest and apoptosis in this study.
2. Materials and methods
2.1. Sample preparation
Rice bran that originated from white rice (Oryza sativa) was obtained from a rice milling in Solo, Indonesia. The identification was performed in the Faculty of Pharmacy, Universitas Gadjah Mada, Indonesia. Approximately 75 g of the rice bran was extracted by maceration using n-hexane (1:3) as the solvent. Remaceration was carried out after 2 d, and the produced macerate was evaporated to obtain the oil extract yield.
2.2. Cell culture
4T1 and NIH-3T3 cell lines were provided by Nara Institute of Science and Technology, Japan. DMEM medium (Sigma) was used to maintain 4T1 and NIH-3T3 cells. DMEM culture was supplemented with sodium bicarbonate (Sigma), HEPES (Sigma),1% penicillin-streptomycin (Gibco), and 10% v/v FBS (Sigma).
2.3. Metabolites profiling
Metabolite profiling of RBE was carried out by high-performance liquid chromatography (HPLC) (Hitachi Japan) with a reversedphase C18 column and acetonitrile:water (80:20) as the mobile phase. A 20 µL aliquot of the sample was injected with a flow rate of 1 mL/min and detected by UV-Vis. Vitamin E (EverE) was used as a reference.
2.4. Cytotoxic assay
4T1 and NIH-3T3 cells with a density of 2 500 and 1×10cells/well, respectively, were grown onto 96-well plates and then treated with various doses of RBE (10-500 µg/mL) or Dox (Sigma)(10-1 000 nM) or both at the selected concentrations. After being incubated for 24 h, the cells were washed in phosphate buffer saline(PBS), and MTT reagent (Biobasic) was added into each well. After around 4 h, formazan crystals formed, a stopper solution (0.01 M HCl containing 10% SDS) was added, and the plate was incubated overnight in a dark place. The absorbance of the plate was obtained on an ELISA reader at λ 595 nm (BioRad). The absorbance data were converted into percent of cell viability to calculate the ICand combination index (CI) values.
We calculated the CI values based on the formula[24]:
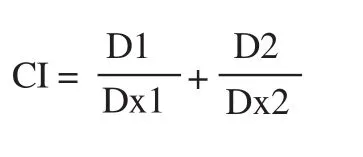
Dx1 and Dx2 are the concentrations of each agent which cause x% effect, while D1 and D2 are the concentration of the agents used in the combination treatment. The classification of CI values is: CI<1,=1, and >1 indicates synergism, additive effect and antagonism,respectively.
2.5. Cell cycle analysis
4T1 cells (2×10cells/well) were grown onto a six-well plate. The cells were treated with RBE or Dox (Sigma) or both at selected concentrations following 24 h of treatment. Cells were harvested by spin centrifugation. The collected cell pellets were then washed with a buffer solution as described by the manufacturer (BD CycletestPlus DNA Kit; BD Biosciences, San Jose, USA). The treated cell suspension was measured using an Accuri C6 (BD Bioscience) flow cytometer. Data were analyzed using BD C6 Software.
2.6. Apoptosis assay
A flow cytometry-based assay using annexin Ⅴ-PI staining was used for apoptosis analysis. 4T1 cells (2×10cells/well) were grown onto a six-well plate. The cells were treated with RBE or Dox (Sigma) or both at selected concentrations following 24 h of treatment. The cells were then harvested and proceeded to be centrifuged. The Annexin-Ⅴ-FLUOS staining kit (Roche) was used to stain the cells for 10 min incubation. The treated cell suspension was measured with an Accuri C6 (BD Bioscience) flow cytometer.Data were analyzed using BD C6 Software.
2.7. ROS assay
4T1 and NIH-3T3 cells (5×10cells/well) were grown on a 24-well plate. The medium was removed, and then the cells were washed with PBS and added with 200 µL of trypsin-EDTA 0.25% (Gibco).Trypsin was removed, and 500 µL of 1× supplemented buffer (PBS containing fetal bovine serum 10%) was added. Suspended cells were collected into a dark microtube, stained with 25 µM DCFDA,and then incubated for 30 min in a 37 ℃ CO5% incubator. The cells were treated with RBE or Dox (Sigma) or both at selected concentrations for 4 h. Dox was used as a positive control. The measurement of ROS level from at least 20 000 cells was done using a BD Accuri C6 flow cytometer (BD Bioscience) at 485 nm excitation wavelength and 535 nm emission wavelength.
2.8. Senescence assay
4T1 and NIH-3T3 cells (1.2×10cells/well) were grown on a sixwell plate, and treated with RBE or Dox (Sigma) or both at selected concentration overnight. The cells were then washed with 1×PBS. A fixation solution containing 2% formaldehyde was added, and then the cells were incubated for 20 min at 25 ℃. The cells were washed with 1×PBS, stained with X-Gal solution, and then incubated at 37℃. Observations were made using a microscope (CKX-41 Olympus)at 200× magnification. The green color of the cells indicated senescence.
2.9. Glutathione-S-transferase (GST) activity assay
A cytosolic fraction containing GST was prepared from chicken liver. Centrifugation was carried out to help cell lysis to facilitate GST isolation. A total of 250 µL homogenate was added to 300 µL of loading buffer. Furthermore, centrifugation and determination of GST enzyme activity were carried out. In 30 µL cell lysates, 920µL of phosphate buffer (0.1 M, pH 6), 20 µL of glutathione (GSH),20 µL of 1-chloro-2,4-dinitrobenzene (CDNB), and 10 µL of RBE in dimethyl sulfoxide were added. The mixture was observed with a spectrophotometer at λ = 340 from minute 0 to minute 3 after addition of CDNB.
2.10. Molecular docking
Molecular interaction between TTE with GST-pi was simulated by molecular docking following a previous method[25]. The default setting was applied MOE 2010.10 (Licensed of Faculty of Pharmacy UGM) for molecular docking simulation. The structure of TTE was subjected to geometry optimization using MMFF94x force field and conformational search in MOE. The crystal structure GST-pi in complex with natural ligand GSH (PDB ID 3SCH) was downloaded from Protein Data Bank. Compound with the lowest docking score was collected, and the binding interaction was analyzed.
2.11. Statistical analysis
All data with triplicate measurement were analyzed through oneway ANOVA followed by Bonferroni post-hoc test using SPSS v.16.Data are expressed as mean ± SD of three independent experiments.Statistically significant differences were considered at P < 0.05.
3. Results
3.1. Extraction and characterization of RBE
Rice bran was successfully extracted with n-hexane to obtain about 18.42% w/w yield of oil extract (RBE) (approximately 17 mL of oil)characterized by liquid with yellow-brown color and rice bran aroma.HPLC analysis of the oil was then conducted to characterize the separating profiles (peaks) in comparison with commercial vitamin E (Supplementary Figure). The chromatograms of both samples showed a slight difference in the retention time of the main peak.The retention time of vitamin E was achieved at 1.5 min, whereas that of RBE was at 1.99 min. The difference in peak position could be caused by the difference in the compound's polarity, suggesting that RBE may contain a less polar compound than vitamin E(tocopherol). This compound was predicted to be TTE.
3.2. Cytotoxic effect of RBE
The cytotoxic activity of RBE alone or in combination with Dox against cancer cells was evaluated. We found that RBE did not decrease cell viability significantly at the concentration up to 250µg/mL on both cells, confirming that RBE did not have a cytotoxic effect on both cells (Figure 1A). Cytotoxic assay of Dox revealed an ICvalue of 552 nM (Figure 1B). However, RBE at the nontoxic concentration (100 and 200 µg/mL) significantly promoted (P< 0.05) the reduction of 4T1 cell viability by Dox treatment (Figure 1C). Interestingly, co-treatments of RBE at 100 and 200 µg/mL with Dox showed CI values of 0.70 and 0.58, respectively. These CI values less than 1 indicate that the co-treatments have synergistic properties. These synergistic effects might be affected by different molecular events of each component that lead to the changes in the physiological phenomena.
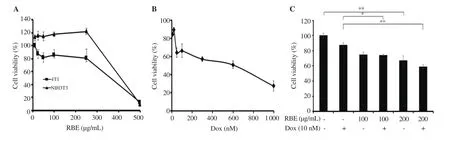
Figure 1. Cytotoxic effect of RBE on 4T1 and NIH-3T3 cells. Cytotoxic effect was evaluated using MTT assay. (A) The effect of RBE on cell viability of 4T1 and NIH-3T3 cells. (B) The cytotoxic profile of Dox in 4T1 cells. (C) The viability of 4T1 cells treated with RBE alone and in combination with Dox (n=3).Data are expressed as mean ± SD of three independent experiments. *P < 0.05, **P<0.01. RBE: rice bran extract, Dox: doxorubicin.
3.3. Flow cytometry for cell cycle and apoptosis profiling
The single treatment of RBE showed no significant effect as confirmed by similar profiles to the untreated cells. Dox alone at a low concentration (10 nM) did not affect cell viability but changed the cell cycle profile with increased cell accumulation at G/M (P <0.01).
The combination treatment increased cell accumulation at the sub-Gphase compared with Dox treatment alone (Figure 2A), which indicates the increased apoptotic cells. Moreover, 200 µg/mL RBE showed the more significant effect than 100 µg/mL RBE. Flow cytometry analysis based on annexin Ⅴ/PI staining confirmed that combination treatments of RBE (in both concentrations) and Dox increased apoptosis significantly (P < 0.01) (Figure 2B).
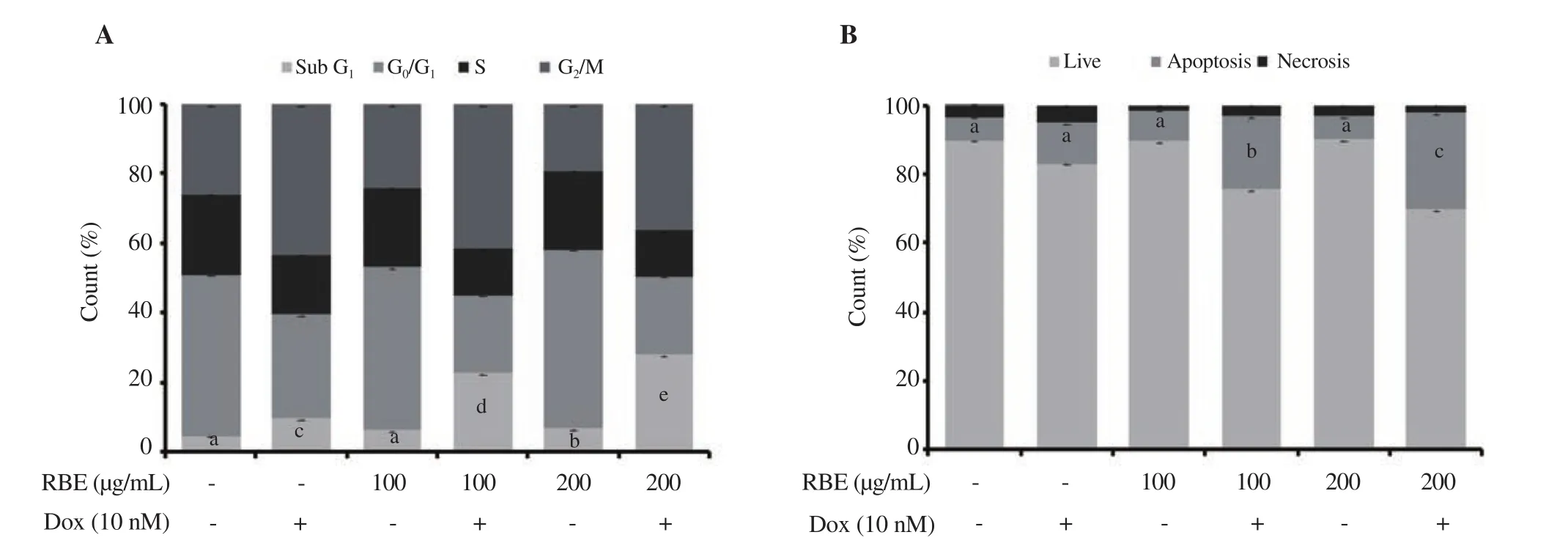
Figure 2. Cell cycle distribution and apoptotic effects of RBE alone and in combination with Dox. 4T1 cultured cells were treated with RBE 100 and 200 µg/mL and in combination with Dox at 10 nM for 24 h and subjected to cell cycle analysis by propidium iodide (PI) staining as well as annexin Ⅴ/PI staining by flow cytometry (n = 3). (A) Cell cycle distribution profile. (B) Apoptotic cell profile. Data are expressed as mean ± SD of three independent experiments.Different letters (a-e) represent significant difference, P<0.01.
3.4. Effect of RBE on senescence of 4T1 cells
To confirm that senescence effect correlated with cell cycle and apoptosis modulatory effects, we carried out SA-β-Gal assay. We used a sub-cytotoxic dose of Dox (<IC) to avoid the apoptosis evidence, allowing us to observe the senescent cells marked by SA-β-Gal staining. By this setting, we could find the cell shape without any change in morphology, and detect differences in SA-β-Gal-positive cell profiles with respect to the senescent cells (Figure 3A).Dox significantly increased the number of senescent cells compared with the untreated cells (P < 0.01), but RBE exerted no effect on cell senescence. Interestingly, RBE enhanced the senescence effect of Dox treatment on 4T1 cells.7). By contrast, RBE treatment with Dox significantly (P<0.01)decreased SA-β-Gal-positive cells [(0.30±0.15)% for RBE 100µg/mL and (0.10±0.14)% for RBE 200 µg/mL in combination with Dox], which were lower than those of the untreated cells[(0.40±0.07)%]. This result demonstrated that RBE can prevent senescence caused by Dox.
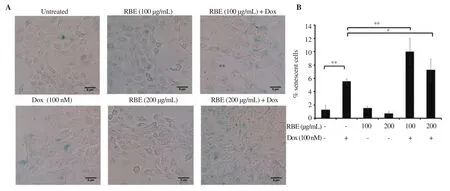
Figure 3. Induction of senescence following RBE treatment in 4T1 cells. Senescent cells were analyzed using the SA-β-galactosidase staining assay. (A) The morphology of cells was observed after 24 h staining under an inverted microscope. (B) The percentage of senescent cells was determined after treatment. Data are expressed as mean ± SD of three independent experiments. *P < 0.05, **P<0.01.
3.5. Effect of RBE on the ROS generation of 4T1 cells
We then evaluated the increase in the senescence effect of RBE concomitant with ROS production in the 4T1 cells. In this experiment, we used Dox at 100 nM and performed the observation after 4 h of treatment to avoid the cytotoxic effect at a longer time.The flow cytogram of ROS profiles exhibited the same pattern except for Dox. Dox increased the ROS level in 4T1 cells, whereas RBE did not cause significant changes in the ROS level (Figure 4A-4B). This result indicated that RBE does not affect the ROS generation in 4T1 cells induced by Dox treatment. This phenomenon seems irrespective of the senescence effect in this same cell, which should be clarified on the healthy or non-cancer cells to evaluate its cytotoxicity. However, the nonfunctional antioxidant property of RBE should also be confirmed accordingly, considering that TTE may also interact with ROS metabolizing enzymes.
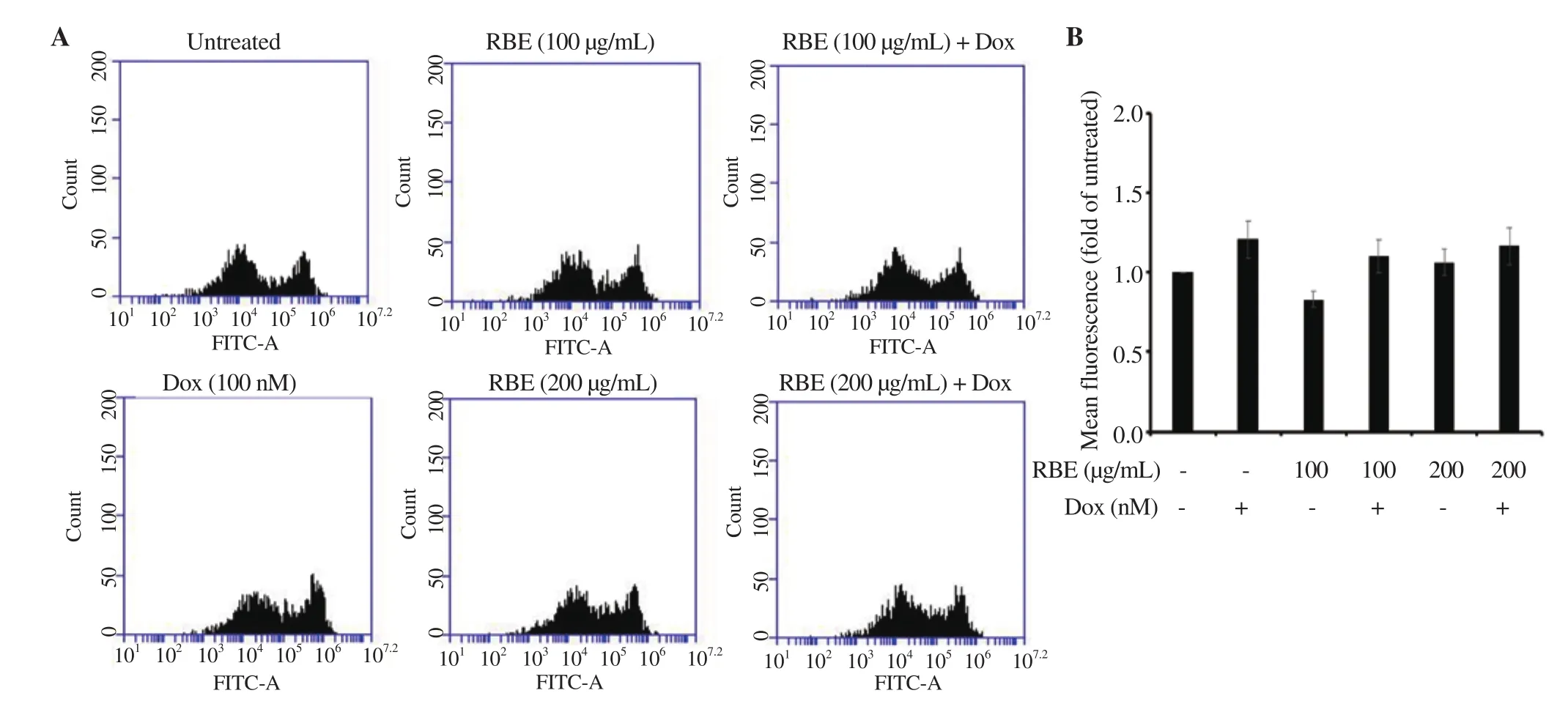
Figure 4. Induction of ROS following RBE treatment in 4T1 cells. Intracellular ROS level upon RBE treatment alone and in combination with Dox. 4T1 cultured cells were treated with RBE 100 and 200 µg/mL and in combination with Dox at 100 nM for 4 h and subjected to ROS detection with DCFDA staining using flow cytometry (n = 3). (A) Flow cytogram of ROS level in 4T1 cells. (B) ROS level profile of 4T1 cells. Data are expressed as mean ± SD of three independent experiments.
3.6. Inhibitory property of RBE against GST
We then evaluated whether or not TTE, the main constituent of rice bran oil, possesses the property as a GST inhibitor. Accordingly,we confirmed that RBE inhibited GST activity in a dose-dependent manner (P < 0.01) (Figure 5A). We could also predict the interaction of TTE with GST at the GSH binding site through molecular docking. Docking scores showed that the interaction force between TTE and GST (—10.09) was lower than that between GSH and GST(—12.26), but it may still be able to compete on the same amino acid residues (Figure 5B). This result indicates that the TTE interaction with GST in RBE contributes to the effect of not decreasing ROS and increasing senescence in 4T1 cells.
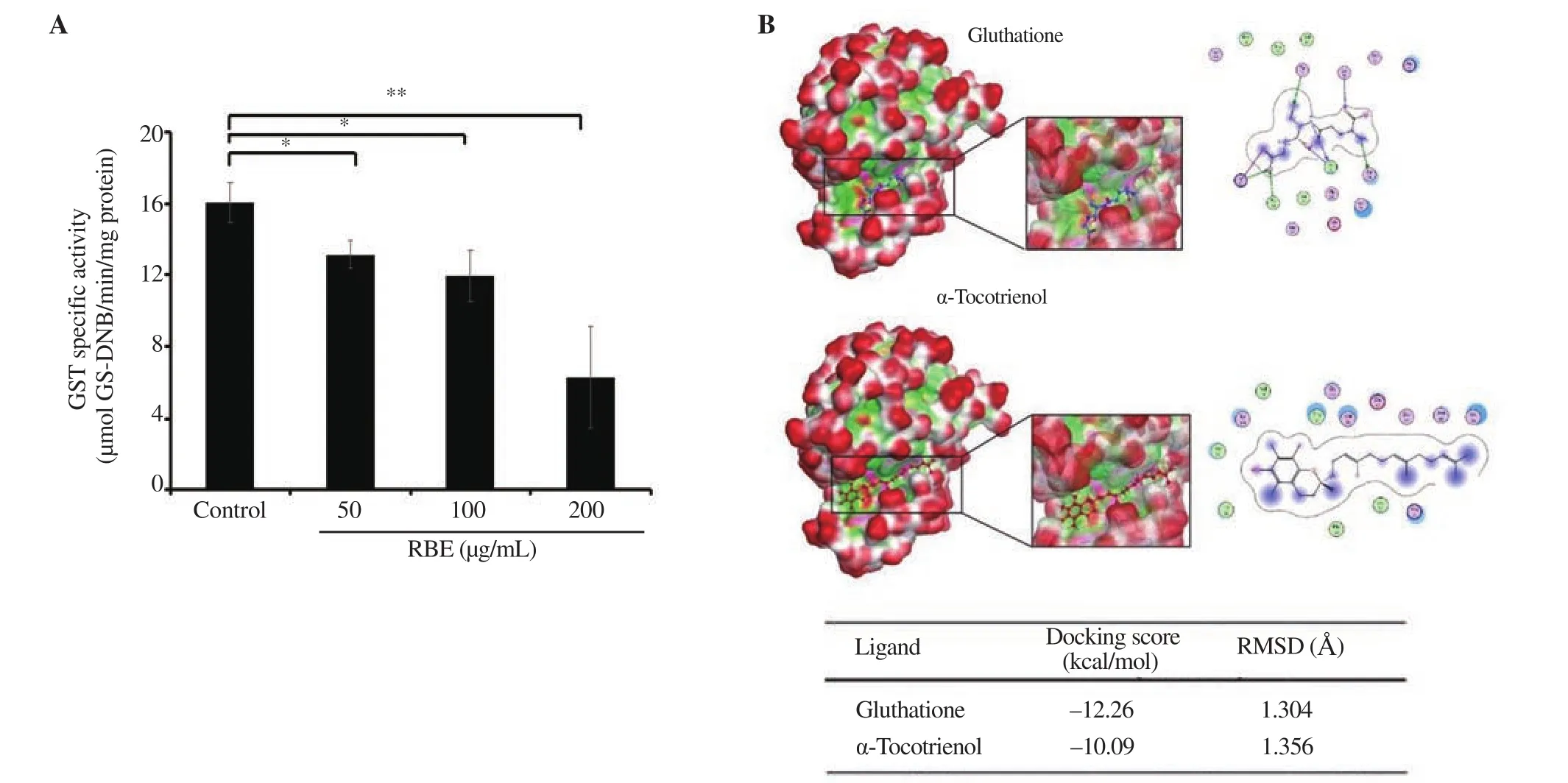
Figure 5. Inhibition effect of RBE on GST activity. GST activity was measured using cytosolic fractions obtained from a chicken liver at concentrations of 50,100, and 200 µg/mL. Molecular docking was performed on α-tocotrienol at the glutathione binding site. (A) GST activity profile following RBE treatment. (B)Interaction and docking score between glutathione and α-tocotrienol proteins. GST: glutathione-S-transferase, GS-DNB: glutathione-S-dinitrobenzene. Data are expressed as mean ± SD of three independent experiments. *P<0.05 **P<0.01.
3.7. Effect of RBE on the ROS generation of NIH-3T3 cells
We further assessed the ROS generation in non-cancer cells NIH-3T3 under the treatment of RBE and Dox. In this treatment, Dox significantly increased intracellular ROS (Figure 6A-6B), whereas RBE at the concentration of 200 µg/mL showed no change compared with the the control. Treatment of RBE impeded the ROS induction of Dox. This phenomenon confirms the inhibitory effect of RBE on Dox-induced ROS generation.
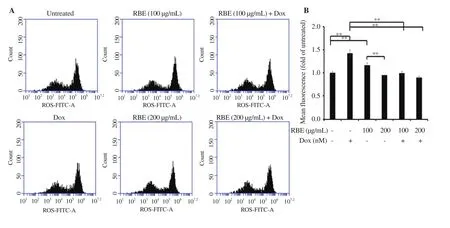
Figure 6. Induction of ROS following RBE treatment in NIH-3T3 cells. Intracellular ROS level upon RBE treatment alone and in combination with Dox by DCFDA staining assay. NIH-3T3 cultured cells were treated with RBE at 100 and 200 µg/mL and in combination with Dox at 10 nM for 4 h and subjected to ROS detection with DCFDA staining using flow cytometry (n = 3). (A) Flow cytogram of ROS signal distribution. (B) ROS level profiles. Data are expressed as mean ± SD of three independent experiments. **P<0.01.
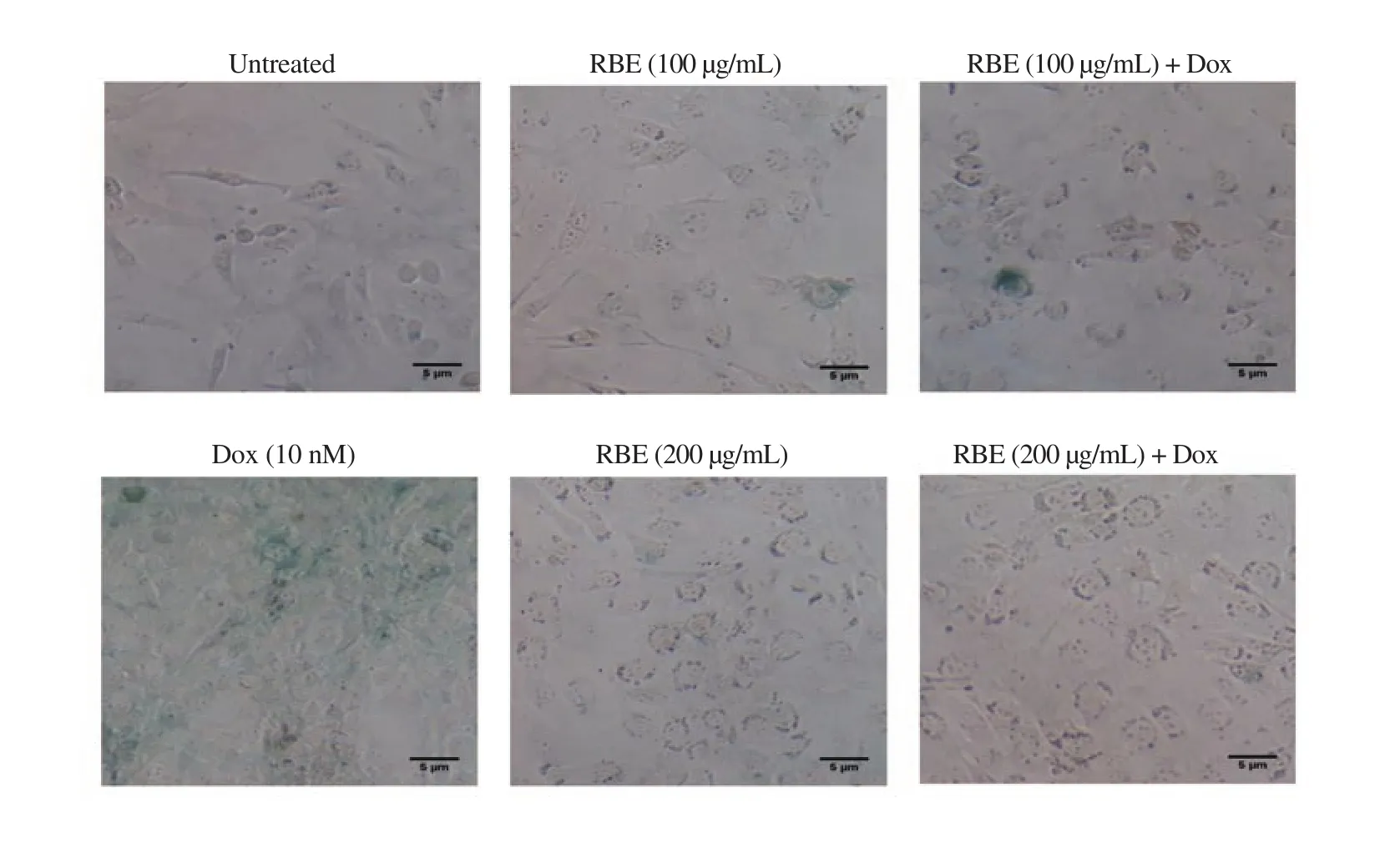
Figure 7. Inhibition of senescence following RBE treatment in NIH-3T3 cells. Senescent cells were analyzed using the SA-β-galactosidase staining assay. The morphology of cells was observed after 72 h staining under an inverted microscope with a magnification of 200×.
3.8. Effect of RBE on senescence of NIH-3T3 cells
Dox exerted a similar effect (i.e., slightly increased) on ROS generation on both 4T1 (cancer) and NIH-3T3 (non-cancer) cells,as shown by a significantly (P<0.01) high population of green color cells (22.10±3.16)% that represented the senescent cells (Figure
4. Discussion
This research evaluated the potential of RBE (Oryza sativa L.) as a co-chemotherapeutic agent with Dox on 4T1 TNBC cancer cells as well as its adverse effects, specifically senescence on fibroblast cells. Dox was used as a chemotherapeutic agent model in this study because of its general mechanism in inhibiting the growth of TNBC cells but with side effects, including senescence acceleration of normal cells and resistance problems in patients[5,26]. These side effects are recognized mainly because Dox increases intracellular ROS and triggers senescence in exposed cells[27,28]. Because of its general and non-selective mechanism, the use of Dox is also at risk for normal cells, especially fibroblast cells[29,30]. Therefore, this study analyzed the effects of RBE and Dox co-treatment on NIH-3T3 cells as a model of non-cancer fibroblast cells.
Although RBE up to a concentration of 200 µg/mL did not show cytotoxic effects on 4T1 cells, it significantly increased the cytotoxic effects of Dox. This increased cytotoxic effect seemed to be related to the effect of senescence induction in cells, which is believed to be related to cell cycle arrest and apoptosis. The ability of natural ingredients to enhance cytotoxic effects through increased cell senescence is also found in galangal[3]extracts and curcumin[8].These agents exert these effects by inhibiting the activity of antioxidant enzymes, such as GST[8]. RBE also had the same mechanism, as shown by the ability of TTE (the main constituent of RBE) to interact with GST, and decreased GST enzyme activity.However, further investigations are still needed to explore the possibility of inhibition of other antioxidant enzymes.
The cytotoxic effect against 4T1 cells showed that RBE has the potential to be further developed as a co-chemotherapeutic agent with Dox to overcome TNBC cancers. This research is limited to the TNBC cell model originating from mice. Thus, it still needs to be investigated with TNBC breast cancer models from humans, such as MDA-MB-254 cells, for further development. However, the 4T1 cells used in this study also easily metastasize; thus, the effect of RBE in combination with Dox not only on the proliferation but also on the migration ability of cells needs to be observed[20]. This will be an interesting challenge for further research.
The ability of RBE to strengthen apoptotic effects through increased senescence and intracellular ROS production in cancer cells needs to be evaluated for their effects on normal cells. Dox increases ROS production through oxidation-reduction reactions that convert compounds into free radicals in cells[31]. DCFDA staining assay showed that RBE significantly reduced the ROS levels induced by the positive control Dox in NIH-3T3 cells, which may be attributed to TTE contained in RBE with a radical scavenging property. These results are consistent with those of previous studies, which showed that TTEs have better radical scavenging ability than tocopherols[32].The radical scavenging activity is indispensable for fibroblast cells,which maintain skin elasticity. In this study, Dox was exposed to fibroblast cells as a model of chemical exposure to skin damage through increased radicals. Thus, the results of this study can be used as a basis for further development of rice bran in preventing skin damage caused by free radical compounds by UV light[33]. The results of this study also agree with previous studies, which showed that TTE could prevent the appearance of wrinkles caused by exposure to UV light[34].
Moreover, this study also showed that RBE provided different senescence effects between cancer and non-cancer cells. RBE tended to have a synergistic effect in increasing the incidence of senescence with Dox in cancer cells. Conversely, RBE decreased the senescence effects of Dox in non-cancer cells. Senescence is one of the hallmarks of aging, characterized by increased activity of SA-β-Gal. In this study, Dox increased cell senescence possibly through increased ROS generation. Our results confirmed that RBE also reduced the incidence of senescence caused by Dox with a sharp decrease in ROS generation in non cancer cells. These results are in accordance with previous results that TTEs can reduce SA-β-Gal activity[35], suggesting that rice bran oil can be used as a cochemotherapeutic agent that is safe for healthy cells.
In this study, NIH-3T3 represents a healthy fibroblast cell line.This cell line has often been used as a model of skin-supporting cells, which can be induced to become senescent cells. Naturally,fibroblast cells are also susceptible to physical and chemical agents that can affect cellular physiology, which can cause signs of aging,such as increased activity of SA-β-Gal, MMP expression, wrinkles,and collagen degradation[36]. Thus, RBE has the potential to be developed as an anti-aging agent for skincare.
Overall, the results of this study show the potential of rice bran not only as a co-chemotherapeutic agent but also as a natural ingredient to prevent premature aging associated with ROS and cellular aging. These potential effects are most likely due to the relatively high content of TTEs in rice bran. TTE is a non-polar substance which, in subsequent developments, is necessary to be characterized as a compound in the form of oil. However, this research is still limited to two aspects related to aging, namely, ROS and senescence. Therefore, further research is needed to observe other aging characteristics, such as MMP expression, wrinkles,and collagen degradation, to strengthen further the results of this study. Considering that this material is obtained in the form of oil,its development needs to be considered as a preparation that can be applied easily, such as a serum, nanoemulsion, cream, and others.
This study can be used as a basis for developing rice bran as a cochemotherapeutic agent for patients with TNBC through increased Dox cytotoxicity related to the effects of ROS and apoptosis induction on cancer cells. Rice bran also has the potential to overcome the side effects of Dox in normal cells or to counteract aging caused by oxidative stress. This potential needs to be explored further by examining other markers related to cytotoxicity and aging.
Conflict of interest statement
We declare that we do not have conflict of interest.
Acknowledgments
This work is mainly supported by RTA program of Universitas Gadjah Mada 2020.
Authors’ contributions
EM designed the study. UMZ, AR, MH, RYU, and SH carried out the laboratory works. UMZ and AR analyzed the data. EM and UMZ prepared the manuscript. EM wrote the manuscript. All authors read and approved the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年4期
Asian Pacific Journal of Tropical Biomedicine2021年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cannabinoid CB2 receptors and spinal microglia are implicated in tingenonemediated antinociception in mice
- Morin attenuates L-arginine induced acute pancreatitis in rats by downregulating myeloperoxidase and lipid peroxidation
- Natural compounds as potential inhibitors of SARS-CoV-2 main protease: An insilico study
- Lentinula edodes extract inhibits matrix metalloproteinase expression and increases typeⅠprocollagen expression via the p38 MAPK/c-Fos signaling pathway in ultraviolet A and B-irradiated HaCaT keratinocytes
