Lentinula edodes extract inhibits matrix metalloproteinase expression and increases typeⅠprocollagen expression via the p38 MAPK/c-Fos signaling pathway in ultraviolet A and B-irradiated HaCaT keratinocytes
Jung Im Lee, Jung Hwan Oh, Fatih Karadeniz, So Young Park, Hye Ran Kim, Hyun Jin Jo, Kyung Im Jung,Byung-Jin Jeon, Chang-Suk Kong,✉
1Marine Biotechnology Center for Pharmaceuticals and Foods, Silla University, Busan 46958, Korea
2Department of Food and Nutrition, College of Medical and Life Sciences, Silla University, Busan 46958, Korea
3Ocean Fisheries and Bio Center, Busan technopark, Busan 46048, Korea
ABSTRACT
KEYWORDS: Lentinula edodes; Ultraviolet; Matrix metalloproteinases; TypeⅠprocollagen; HaCaT
1. Introduction
Solar ultraviolet (UV) radiation is the primary cause of various harmful effects like oxidative damage, inflammation,immunosuppression, and premature skin aging and DNA damage[1].Solar UV radiation is composed of UVA (320-400 nm), UVB (280-320 nm), and UVC (200-280 nm). Although the UVC is the most harmful of all for the skin, it is mostly filtered while passing the ozone layer and has little to no effect on human skin. UVB constitutes only 5% of the UV radiation that reaches to human skin but is considered significantly harmful and toxic to the skin. UVB's ability to reach the epidermis layer causes direct DNA mutations which can lead to apoptosis or other complications[2]. UVA is considered less toxic than UVB in terms of cellular damage, but it is at least 20 times more abundant. Hence, more UVA penetrates deeper than the epidermis and reaches the dermis layer of the skin where it causes indirect DNA damage. In addition, UV radiation results in excessive generation of intracellular reactive oxygen species (ROS) and inflammatory cytokines, as well as matrix metalloproteinases (MMPs) expression, all of which contribute to skin damage.
MMPs digest the extracellular matrix (ECM) proteins, consisting of collagen, fibronectin, elastin, and proteoglycans. There are more than 28 types of MMPs classified into 5 groups by their structure, location,and substrates[3]. MMP-1 is a collagenase that can degrade native typeⅠand type Ⅲ collagen. MMP-9 and MMP-2 are gelatinases that cleave degraded collagen into gelatin and other small peptides[4]. A recent study showed that extensive degradation of ECM components through UV-stimulated activities of MMP-1 and MMP-9 contributes substantially to the formation of wrinkles via connective tissue damage[5].
Regulation of MMP-1 and MMP-9 expressions on transcriptional level is partly modulated by mitogen-activated protein kinase(MAPK), which stimulates the formation of activator protein 1(AP-1) via phosphorylation of its sub-domains, c-Fos and c-Jun[6,7].Phosphorylation of AP-1 promotes its nuclear translocation and triggers its DNA binding affinity which further activates the expression of MMPs[8,9].
Mushrooms have been a part of traditional medicine for ages and are rich sources of dietary fiber, minerals, and vitamins in addition to lowfat content[10]. Lentinula edodes (L. edodes) presently is one of the most sought-after mushrooms and therefore, widely cultivated. Its bioactive secondary metabolites include lentinan, beta-glucans, eritadenine,ergosterol, and polyphenols, all of which are reported for various health beneficial effects such as antitumor, immunomodulation, antimicrobial,antiviral, antibacterial, antifungal, antioxidant, and liver protection[11-14].Some studies reported that beta-glucans and polyphenols inhibit MMP-1 expression. It was also shown that the same polyphenols further exert anti-aging properties by stimulating typeⅠcollagen expression in human keratinocytes under simulated photoaging conditions[4,15]. Considering that L. edodes is rich in beta-glucan and polyphenols, L. edodes extract is expected to suppress the MMP-1 expression while at the same time enhancing collagen expression. However, it remains unknown whether L. edodes extract can prevent skin photoaging in HaCaT keratinocytes.Therefore, in this study, the attenuating effect of L. edodes extract on UVA and UVB-mediated damages in MMP and collagen production of keratinocytes was assessed.
2. Materials and methods
2.1. Plant material and extraction
L. edodes grown in smart farms (OLCHI-Smartpharm, Busan,Korea) was used for this study. L. edodes was kindly provided by Busan Technopark. L. edodes was sliced and then dried for 15 h in 65 ℃ heating dryer (Jeil Machine Co. Ltd Ichen, Korea). The dried slices of L. edodes (25 g) were added in 250 mL of 80% ethanol and extracted for 4 h at 80 ℃ using reflux apparatus. Extraction was repeated two times using the same method. Extract from 80% ethanol was concentrated in vacuo with a rotary evaporator (EYELA N-10000, Rikakikai Co., Tokyo, Japan). L. edodes ethanol extract(LEE) was obtained and was kept in a refrigerator (—20 ℃) until further experiments.
2.2. Total phenolic content quantification
The phenolic compound content of LEE was detected by a modified Folin-Ciocalteu method[16]. One hundred microliters of the extract solution (5 mg/mL) and 500 µL 1 mol/L Folin-Ciocalteu's phenol reagent were mixed, and 400 µL of 2.5% sodium carbonate(NaCO) was added. Absorbance values were determined at 760 nm wavelength by a spectrophotometer (Tecan Austria GmbH, Austria)following a 1.5 h incubation in dark at room temperature. The total phenolic content of LEE was quantified with the help of a standard curve prepared with the same procedure using the gallic acid samples with known concentrations (50-250 µg/mL). Hence, the total phenol content of LEE was given as gallic acid equivalent (GAE; mg) per dry weight (DW; g) of LEE.
2.3. Beta-glucan content quantification
The total 1,3-1,6-β-glucan content of LEE was analysed with a commercial mixed linkage β-Glucan Assay Kit (Cat. no. K-BGLU;Megazyme Ltd., Bray, Wicklow County, Ireland) following the directions of the kit. Briefly, the total β-glucan content of LEE was determined by subtracting the α-glucan/sucrose contents from the total glucan contents calculated according to the kit's instructions.
2.4. Cell culture
Immortalized human HaCaT keratinocytes were obtained from CLS-Cell Line Service (300493, Eppelheim, Germany). HaCaT keratinocytes were fed Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum and 100 units/mL penicillin/streptomycin at 37 ℃ in 5% COincubator. Cells were fed fresh medium every two days.
2.5. UVA and UVB irradiation of HaCaT keratinocytes
HaCaT cells were exposed to UVA and UVB via Bio-Sun biological irradiation system (VilberLourmat, Marine, France)installed with UVA and UVB lamps. HaCaT cells were plated at the density of 1×10cells/well and grown to confluence. Plates were irradiated by UVA (10 J/cm) and UVB (20 mJ/cm), separately.After UV exposure was completed, keratinocytes were fed Dulbecco's Modified Eagle Medium without fetal bovine serum with/without sample for 24 h.
2.6. Cell cytotoxicity
The cytotoxicity effect of LEE extract in HaCaT keratinocytes was examined by MTT assay with a previously reported procedure[17].Briefly, keratinocytes in 96-well plates were treated with LEE (0,10, 25, 50, and 100 µg/mL) and incubated for 24 h. Next, the MTT solution (1 mg/mL) was added to the wells after the media were aspirated. The formed formazan crystals were dissolved with DMSO and were measured at 540 nm wavelength with a microplate reader(GENios FL; Tecan Austria GmbH, Grodig, Austria). The viability level of the HaCaT keratinocytes was determined compared with the untreated control group. The photoprotective effect of LEE in UV-irradiated HaCaT keratinocytes was also determined by its effect on cell viability. The cells in 12-well plates were irradiated by UVA(10 J/cm) and UVB (20 mJ/cm), separately. Following irradiation,cells were fed with serum-free media containing LEE (0, 10, 25, and 50 µg/mL). The viability of UV-irradiated keratinocytes after 24 h was evaluated by MTT assay as described above and calculated as a percentage compared with the untreated group.
2.7. Intracellular DCFH-DA scavenging assay
The generation of intracellular ROS after UV irradiation was estimated using DCF-DA method[18]. The keratinocytes were plated in 96-well black plates. The cells were stained with DCFH-DA (20µM) in PBS for 20 min at 37 ℃. Next, LEE was introduced to the cells and then cells were exposed to UVA (10 J/cm) and UVB (20 mJ/cm), separately after 1 h. After 120 min, the generation of DCF in cells was measured at the 495 nm excitation (Ex) and 539 nm emission (Em) wavelengths using a fluorescence microplate reader(Infinite 200 Pro; Tecan, Mannedorf, Switzerland). Retinoic acid was used as positive control. Intracellular ROS scavenging effects were calculated as a relative percentage compared to DCF fluorescence intensity of UV-irradiated untreated group.
2.8. Determination of MMP-1 and typeⅠα1 procollagen by enzyme-linked immunosorbent assay (ELISA)
The UVA (10 J/cm) and UVB (20 mJ/cm)-mediated changes in the production of MMP-1 and typeⅠα1 procollagen were determined in HaCaT keratinocyte culture medium by ELISA. Cells were plated in 12 well plates and incubated for 24 h before UVA(10 J/cm) and UVB (20 mJ/cm) exposure, separately. The UVA-and UVB-irradiated cells were treated with LEE (10, 25, and 50 µg/mL) and incubated for another 24 h. The culture media were then quantified for their MMP-1 and human procollagenⅠα1 levels using commercial ELISA kits (for MMP-1 cat no. DY901B, for human procollagenⅠα1 cat no. DY6220-05; R&D Systems, Minneapolis,MN, USA). Retinoic acid was used as positive control.
2.9. Determination of MMP-1, MMP-9 and typeⅠprocollagen by reverse transcription-polymerase chain reaction (RT-PCR) assay
HaCaT keratinocytes were plated in 6-well plates (1.5×10cells/well) and subjected to UVA (10 J/cm) and UVB (20 mJ/cm)irradiation. Following irradiation, cells were treated with LEE for 24 h at 37 ℃. Total RNA was isolated from cells with AccuPrepUniversal RNA Extraction Kit (Bioneer Corp. Daejeon, Republic of Korea). The cDNA synthesis from total RNA was performed using Cell ScriptTM cDNA master Mix (Cellsafe, Gyeonggi-do,Republic of Korea). The thermocycler T100 (Bio-Rad Laboratories,Inc., Hercules, CA, USA) was used for reverse transcription with the following temperature protocol: 42 ℃ for 60 min and 72 ℃ for 5 min. Subsequently, PCR was performed using the following primers:MMP-1, forward 5‘-TCT GAC GTT GAT CCC AGA GAG CAG-3',reverse 5‘-CAG GGT GAC ACC AGT GAC TGC AC-3'; MMP-9,forward 5'-CAC TGT CCA CCC CTC AGA GC3' and reverse 5'-CAC TTG TCG GCG ATA AGG-3'; typeⅠprocollagen, forward 5'-CTC GAG GTG GAC ACC ACC CT-3', reverse 5'-CAG CTG GAT GGC CAC ATC GG-3';β
-actin, forward 5'-AGA TCA AGA TCA TTG CTC CTC CTG-3', reverse 5'-CAA GAA AGG GTG TAA CGC AAC TAA G-3'. The following steps were used for PCR in T100 thermocycler (Bio-Rad Laboratories, Inc.): 30 cycles of 95 ℃for 45 s, 60 ℃ for 1 min, and 72 ℃ for 45 s. The final PCR products were separated by electrophoresis for 30 min at 100 V on a 1.5% agarose gel. Following staining with 1 mg/mL ethidium bromide,gels were imaged under a UV light using a CAS-400SM Davinch-Chemi Imager (Davinch-K, Seoul, Republic of Korea). Retinoic acid was used as positive control.2.10. MMP, MAPK and AP-1 Western blotting assay
Detection of proteins in HaCaT keratinocytes was carried out following standard Western blot procedures described earlier[17].Briefly, UV (UVA, 10 J/cm; UVB, 20 mJ/cm)-irradiated HaCaT cells were treated with or without LEE for 24 h. The cells were lysed with vigorous pipetting using RIPA lysis buffer (Sigma-Aldrich Corp., St. Louis, MO, USA). Protein contents were determined by a commercial BCA protein assay kit (Thermo Fisher Scientific,Rockford, IL, USA). Following protein content determination, a 20µg protein was aliquoted from each group and separated by SDS-PAGE. Separated proteins were transferred from the SDS-PAGE gel to a PVDF membrane (Amersham, GE Healthcare, Little Chalfont,UK) from gels. Membranes were kept in 5% skimmed milk for blocking which was followed by Western blotting assay (overnight at 4 ℃) with antibodies against specific proteins. Blotted proteins were hybridized with HPR-conjugated secondary antibodies at room temperature for 1 h. Detection of stained proteins was carried out by WestGlowFEMTO chemiluminescent substrate (Biomax, Seoul,Republic of Korea) and observed with CAS-400SM Davinch-Chemi imager (Davinch-K, Seoul, Republic of Korea). Antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA)and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2.11. Statistical analysis
The numerical results were expressed as mean of three different runs ± SD if applicable. Statistically significant differences between the means were determined by ANOVA coupled with Duncan's posthoc test (SAS v9.1, SAS Institute Inc., Cary, NC, USA) at P<0.05 level.
3. Results
3.1. Total phenolic and beta-glucan contents of LEE
To investigate active components of LEE, total polyphenols contents and beta-glucan contents were determined. As a result, total phenolic contents were (18.03 ± 0.23) mg GAE/g DW and betaglucan contents were (125.50 ± 6.18) mg/g.
3.2. Cell cytotoxicity and phototoxicity
Any toxic effect of LEE on HaCaT keratinocytes was determined by MTT assay. The effect of LEE on HaCaT cell viability was represented as a percentage compared with the viability of LEE-untreated control. LEE did not exert any significant toxicity at the concentrations lower than 50 µg/mL (Figure 1A). Based on the results, further experiments were conducted at concentrations below 50 µg/mL. UVA and UVB irradiation hindered the proliferation of the cells which was reverted by LEE treatment (Figure 1B and 1C).LEE treated groups showed a higher cell amount compared to the untreated irradiated group.

Figure 1. Cytotoxic and photoprotective effects of Lentinula edodes ethanol extract (LEE) on HaCaT keratinocyte using the MTT assay. (A) The cytotoxicity of LEE on the viability of HaCaT keratinocytes. (B) Effect of LEE on the viability of ultraviolet (UV)A and UVB-irradiated HaCaT keratinocytes,respectively. HaCaT keratinocytes were irradiated with 10 J/cm2 and 20 mJ/cm2 of UVA and UVB. a-bMeans with different letters are significantly different at P<0.05.
3.3. Effect of LEE on ROS generation in UVA and UVB-irradiated HaCaT keratinocytes
ROS scavenging ability of LEE was examined using oxidative stress-sensitive fluorescence dye DCF-DA. Treatment with LEE significantly lessened the DCF fluorescence intensity in a dosedependent manner in UV-irradiated keratinocytes (Figure 2). ROS scavenging rates of LEE were 11.36, 19.50, and 31.27% at 10, 25,and 50 µg/mL compared with the only UV-irradiated control at 120 min.

Figure 2. Effect of LEE on intracellular reactive oxygen species (ROS) generation in UVA (A)- and UVB (B)-irradiated HaCaT keratinocytes. HaCaT keratinocytes were irradiated with 10 J/cm2 and 20 mJ/cm2 of UVA and UVB after pre-treatment with/without LEE for 1 h. Intracellular ROS generation was quantified by the fluorescence intensity of DCF. The positive control was retinoic acid. a-fMeans with different letters are significantly different at P<0.05 level.
3.4. Effects of LEE on MMP-1 and typeⅠα1 collagen production in UVA- and UVB-irradiated keratinocytes
The UVA- and UVB-induced alterations in the production of MMP-1 and typeⅠα1 procollagen production and the effect of LEE against it were confirmed by ELISA. UVA and UVB irradiation of 10 J/cmand 20 mJ/cm, respectively, elevated MMP-1 secretion from UV-irradiated keratinocytes whereas typeⅠα1 collagen secretion was diminished (Figure 3). When treated with LEE(10, 25, and 50 µg/mL), the secretion of MMP-1 was reduced in a concentration-dependent manner in UVA- and UVB-irradiated HaCaT keratinocytes, while the secretion of the typeⅠα1 collagen was increased.

Figure 3. Effect of LEE on the secretion of matrix metalloproteinase (MMP)-1 and procollagenⅠα1 in UVA- and UVB-irradiated HaCaT keratinocytes.HaCaT keratinocytes were irradiated with 10 J/cm2 and 20 mJ/cm2 of UVA and UVB and treated with/without LEE. After incubation for 24 h, the amount of UV-induced MMP-1 and typeⅠα1 procollagen was measured by ELISA. The positive control was retinoic acid. a-eMeans with different letters are significantly different at P<0.05 level. A: MMP-1 in UVA-irradiated HaCaT keratinocytes; B: MMP-1 in UVB-irradiated HaCaT keratinocytes; C:procollagenⅠα1 in UVA-irradiated HaCaT keratinocytes; D: procollagenⅠα1 in UVB-irradiated HaCaT keratinocytes.
3.5. Effects of LEE on mRNA and protein expressions of MMPs and collagen in UVA-irradiated keratinocytes
The UVA (10 J/cm)-induced changes in the expressions of MMP-1, MMP-9, and typeⅠprocollagen and its possible attenuation by LEE treatment were determined at mRNA and protein levels.When HaCaT keratinocytes were exposed to UVA, the mRNA and protein expressions of MMP-1 and MMP-9 were increased whereas a concurrent decrease in typeⅠprocollagen expression was observed. The introduction of LEE dose-dependently attenuated the UVA-mediated changes in mRNA (Figure 4A) and protein (Figure 4B) levels of MMP-1, MMP-9, and typeⅠprocollagen.

Figure 4. Effect of LEE on the MMP-1, MMP-9, and typeⅠprocollagen expression in UVA-irradiated HaCaT keratinocytes. HaCaT keratinocytes were irradiated with 10 J/cm2 of UVA and treated with/without LEE for 24 h. The mRNA and protein levels were determined by RT-PCR (A) and Western blotting assay (B). The positive control was retinoic acid. β-actin was used as the loading control. a-eMeans with different letters are significantly different at P<0.05 level.
3.6. Effect of LEE on UVA-induced MAPK and AP-1 pathway activation
To determine the effect of LEE on the UVA-induced stimulation of MAPK pathway, the phosphorylation of extracellular signalregulated kinase (ERK), c-Jun N-terminal kinases (JNK), and p38 were investigated by observing total and phosphorylated (phospho-)protein levels. UVA irradiation in HaCaT keratinocytes increased the phosphorylation levels of MAPK signaling components,such as ERK, JNK, and p38 protein. LEE treatment (50 µg/mL)significantly decreased the phosphorylated p38 levels. However,the phosphorylation levels of ERK and JNK (Figure 5A) were not affected by LEE treatment. AP-1, a dimeric transcription factor,activates the expression of several genes such as MMP-1, MMP-3,and MMP-9. AP-1 activity is controlled via activation of MAPKs.UV irradiation increased the phosphorylated levels of AP-1 subunits;c-Jun and c-Fos. LEE suppressed the c-Fos phosphorylation but did not affect the c-Jun phosphorylation (Figure 5B).

Figure 5. Effect of LEE on the phosphorylation of MAPK and AP-1. HaCaT keratinocytes were irradiated with 10 J/cm2 of UVA and treated with/without LEE (50 µg/mL). After incubation for 24 h, phosphorylated and total protein levels of p38, ERK, and JNK (A), and active and total protein levels of c-Fos and c-Jun proteins (B) were evaluated by Western blotting assay. Retinoic acid was used as the positive control. β-actin was used as the loading control.
3.7. Effects of LEE on UVB-induced expressions of MMPs and typeⅠprocollagen
The UVB (20 mJ/cm)-induced changes in the expression of MMP-1, MMP-9, and typeⅠprocollagen and their attenuation by LEE were examined by RT-PCR and Western blotting assay. UVB exposure increased the mRNA and protein expressions of MMP-1 and MMP-9, and exerted an opposite effect on typeⅠprocollagen in HaCaT keratinocytes. Following treatment with LEE, MMP-1 and MMP-9 levels were dose-dependently downregulated at mRNA and protein expression levels. In parallel, typeⅠprocollagen levels were also increased after LEE treatment (Figure 6A and 6B).

Figure 6. Effect of LEE on the expression of MMP-1, MMP-9, and typeⅠprocollagen in UVB-irradiated HaCaT keratinocytes. HaCaT keratinocytes were irradiated with 20 mJ/cm2 of UVB and treated with/without LEE. After incubation for 24 h, the mRNA and protein expressions were determined by RT-PCR(A) and Western blotting assay (B). Retinoic acid was used as the positive control. β-actin was used as the loading control. a-dMeans with different letters are significantly different at P<0.05 level.
3.8. Effect of LEE on UVB-induced MAPK and AP-1 pathway activation
To determine the effect of LEE on the UVB-mediated stimulation of MAPKs and AP-1, their phosphorylation levels were investigated by Western blotting assay. Following UVB exposure (20 mJ/cm),HaCaT keratinocytes displayed elevated phosphorylation levels of MAPKs and AP-1. Treatment with LEE (50 µg/mL) significantly inhibited the phosphorylation levels of p38, whereas it failed to attenuate the levels of JNK and ERK phosphorylation (Figure 7A).Suppression of the phosphorylated p38 by LEE treatment was also accompanied by a decrease in UVB-induced elevation of phospho-c-Fos levels (Figure 7B).
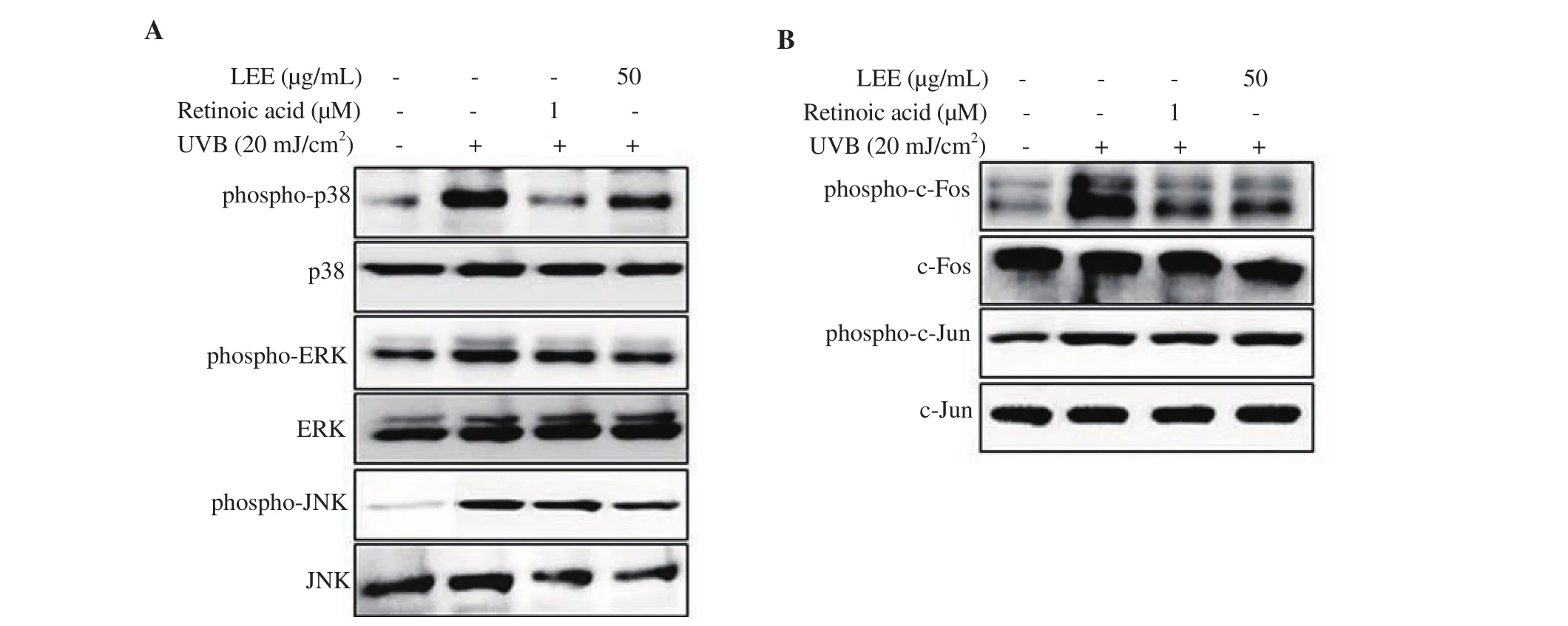
Figure 7. Effect of LEE on the phosphorylation of MAPKs and AP-1. HaCaT keratinocytes were exposed to 20 mJ/cm2 of UVB and treated with/without LEE(50 µg/mL). After incubation for 24 h, phosphorylated and total protein levels of p38, ERK, and JNK (A), and active and total protein levels of c-Fos and c-Jun proteins (B) were evaluated by Western blotting assay. Retinoic acid was used as the positive control. β-actin was used as the loading control.
4. Discussion
A variety of studies demonstrated that UV irradiation induces MMP expression, ROS generation, and inflammatory cytokine secretion[1,5]. Excessive ROS leads to the upregulation of lipid peroxidation, collagen degradation, and DNA damage, causing skin aging[18]. Thus, inhibition of ROS generation is important for preventing skin aging. There are a number of natural antioxidants such as vitamins, polyphenols, flavonoids, anthocyanins, and coumarins, all of which are able to effectively scavenge ROS[19].In this study, L. edodes which is an ingredient for daily diet and traditional folk medicine was extracted with ethanol to obtain LEE.The effect of LEE on the regulation of UVA and UVB-induced ROS generation and MMP expression was evaluated. L. edodes contains various bioactive compounds such as polysaccharides(lentinan), erythadenine, mannan-peptide, and lenthionine. These compounds have several bioactivities including but not limited to antitumor, antibacterial, antiviral, antifungal, hepatoprotective, and antioxidant effects[20]. This report also confirmed that LEE contained polyphenols and beta-glucans. However, in the current study, total phenolic content (18.03 mg/g) of LEE was significantly higher than the contents (2.02 mg/g) reported by an earlier study[21]. The difference between total phenolic contents was considered to be due to the different extraction methods. In a report by Han et al.[21], L.edodes was extracted at room temperature for 72 h whereas LEE was extracted at 80 ℃ for 4 h where both were extracted using the same solvent, ethanol. The extraction of LEE is similar to polyphenol extraction method which was carried out at temperatures above 90 ℃[22]. Thus, it was expected that LEE contained a large amount of polyphenols. Another report showed that ethanol extracts from L. edodes containing polyphenols and flavonoids had antioxidant activities against DPPH radicals and ABTS[10]. In addition to DPPH and ABTS, these components of LEE have antioxidant effects such as inhibition of intracellular ROS production, lipid peroxidation,and DNA oxidation[18,23]. In this study, LEE significantly inhibited UVA- and UVB-induced ROS generation in HaCaT keratinocytes.Hence, ROS scavenging effects of LEE were assumed to be due to active antioxidant components of LEE such as polyphenols, betaglucans, and probably vitamins and so on which are commonly found in similar species[21]. Based on the antioxidative effect of LEE, the effect of L. edodes on UV-exposure-linked changes in MMP and typeⅠprocollagen expressions was investigated.
MMPs have a central role in the different processes of aging,especially in skin aging. UV irradiation induces the upregulation of MMP expression and destroys skin connective tissue[3,5]. Among MMPs that digest the ECM proteins, MMP-1 is a member of the collagenase subfamily of MMPs and initially degrades fibrous collagen when activated. Degraded collagens are then cleaved to the smaller fragments by the activities of other MMPs: MMP-2 and MMP-9. Overexpression of MMPs causes the skin to lose the elasticity and form wrinkles, leading to skin aging. Treatment with LEE suppressed the levels of MMP-1 and MMP-9 and increased the expression of typeⅠprocollagen in both UVA- and UVB-irradiated HaCaT keratinocytes. Several studies demonstrated that polyphenols and beta-glucans decreased the expression of MMPs[4,15,24-27]. In addition, antioxidant components are known to have a downregulation effect on UV-induced MMP expression[6,7,17].Current results suggested that LEE could effectively prevent the overexpression of UV-induced MMP-1 and MMP-9 and it could enhance the expression of typeⅠprocollagen.
Studies confirmed that the interaction between MAPK and AP-1 pathways regulates most of the MMP expression. UV-induced activation of AP-1, which is a dimeric protein composed of c-Jun and c-Fos proteins, is preceded by the activation of MAPK pathways. The MMP-1 and MMP-9 genes are then expressed by the transcriptional activity of AP-1. Activation of these cascades is induced by excessive generation of UV-mediated ROS, which in turn increases the levels and activities of MMP-1 and MMP-9.
MAPKs are a family of proline-directed Ser/Thr kinases including p38, JNK, and ERK. Some reports showed that JNK and p38 are related to the expression of c-Jun and ERK is crucial to the activation and dimerization c-Fos[28-30]. Treatment with LEE suppressed the phosphorylation of p38, but the phosphorylation of JNK and ERK were not affected by LEE in UVA-irradiated HaCaT keratinocytes. Suppression of the phosphorylated p38 expectedly resulted in the downregulation of UVA-mediated elevation in c-Fos levels. These results are similar to a previous report[31]where scopoletin downregulated MMP-1 expression by inhibiting only p38 phosphorylation without suppression of JNK and ERK phosphorylation. According to a study by Tanos et al.[29],the UV-induced phosphorylation of c-Fos was dependent on p38 kinases, but not ERK and JNK in AP-1 activation. These results were in accordance with LEE treatment in UVB-irradiated HaCaT keratinocytes suggesting a similar mechanism.
Therefore, these results showed that LEE effectively inhibited the expressions of MMP-1 and MMP-9 and increased the expression of typeⅠprocollagen via suppression of p38 MAPK/c-Fos signaling pathway in UVA and UVB-irradiated HaCaT keratinocytes.However, current study presented the effect of L. edodes in vitro models whereas further studies using in vivo skin models and detailed analysis of compounds are needed to establish L. edodes as a cosmetic agent. Nevertheless, the present study suggests that L.edodes may be developed as a cosmetic material to suppress UV-induced skin aging.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This research was supported by the Ministry of Trade, Industry& Energy, Korea Institute for Advancement of Technology and Busan Institute For Regional Program Evaluation through the Encouragement Program for the Social and Economic Innovation Growth (project number, P0008724).
Authors' contributions
JIL, JHO, BJJ and CSK conceived the idea, designed, and supervised the study. JIL, JHO, SYP, HRK, HJJ and KIJ performed the analysis and investigation, interpreted the results, and visualized the data. JIL and FK wrote and revised the manuscript. BJJ and CSK provided the resources.
 Asian Pacific Journal of Tropical Biomedicine2021年4期
Asian Pacific Journal of Tropical Biomedicine2021年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cannabinoid CB2 receptors and spinal microglia are implicated in tingenonemediated antinociception in mice
- Morin attenuates L-arginine induced acute pancreatitis in rats by downregulating myeloperoxidase and lipid peroxidation
- Natural compounds as potential inhibitors of SARS-CoV-2 main protease: An insilico study
- Reactive oxygen species and senescence modulatory effects of rice bran extract on 4T1 and NIH-3T3 cells co-treatment with doxorubicin
