色酮吡唑啉酮骨架拼接二氢查尔酮类化合物的合成
蒲 健,汪军鑫,韦芳芳,刘雄利,谭 丹*
(1.贵州益佰制药股份有限公司,贵州 贵阳 550025;2.贵州大学 贵州省药食同源植物资源开发工程技术研究中心,贵州 贵阳 550025)
几十年来,由于具有多种生物活性,色酮类化合物一直受到人们的关注[1-3]。因此,开发有效的方法来获取结构多样的色酮衍生物无疑在有机合成中具有吸引力。另一方面,吡唑啉酮[4-6]和查尔酮衍生物[7-9]也是一类重要的具有多种生物活性的生物活性分子。因此,开发高效的含吡唑啉酮和查尔酮骨架的色酮类衍生物的方法也具有重要意义。
最近,色酮-吡唑啉酮合成子[10]为本课题组开发的新的高效的合成子,用于生物重要的吡唑啉酮色酮衍生物的多样性导向合成[11-14]。鉴于色酮、吡唑啉酮和二氢查尔酮的相关性,预期将3个结构基元结合到一个分子中可以得到新的具有潜在的生物学特性化合物[15-16]。本文以色酮-吡唑啉酮合成子1为原料,在三级胺DABCO催化下,第一次与查尔酮发生Michael加成反应,获得了10个新型色酮吡唑啉酮骨架拼接二氢查尔酮类化合物3a~3j,产率79%~90%,dr值6/1~10/1,其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征,化合物3b的相对构型通过单晶进一步进行了确定。该类化合物含有连续两个立体中心,包括一个季碳中心,可以为生物活性筛选提供物质基础。
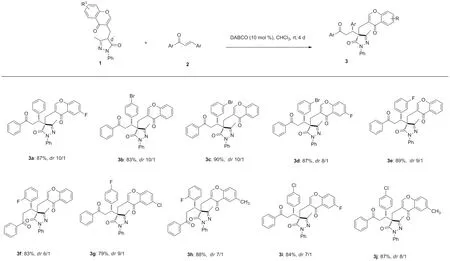
Scheme 1
1 实验部分
1.1 仪器与试剂
WRS-1B型数字熔点仪;Bruker-400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);MicroTMQ-TOF型高分辨质谱仪。
所用试剂均为分析纯。
1.2 3a~3j的合成(以3a为例)
依次加入吡唑啉酮-色酮合成子1a(0.10 mmol),查尔酮2a(0.12 mmol),DABCO(10 mol%,0.01 mmol) 和2.0 mL氯仿,搅拌下反应4 d(TLC检测)。经硅胶柱层析[洗脱剂:V(石油醚)/V(乙酸乙酯)=4/1]纯化得化合物3a。
用类似的方法合成3b~3j。
3a:白色固体,产率87%,m.p.202.3~203.1 ℃;dr10/1;1H NMRδ:2.29~2.34(m,4H),2.93~2.98(m,1H),3.34(d,J=13.6 Hz,1H),3.65~3.72(m,1H),3.95~3.98(m,1H),7.07~7.10(m,1H),7.13~7.16(m,1H),7.21~7.32(m,8H),7.41~7.47(m,3H),7.57~7.59(m,2H),7.66~7.74(m,4H);13C NMRδ:14.1,27.0,38.3,43.6,61.8,109.7(d,JCF=24.3 Hz),116.6,118.3,119.2,119.3,120.9(d,JCF=25.1 Hz),123.5,124.5,126.7,127.0,127.2,127.6,127.8,129.0,132.3,135.6,136.2,136.6,151.3,154.3,158.6(d,JCF=247.2 Hz),161.9,173.2,175.0,195.7;HR-MS(ESI-TOF)m/z:Calcd for C35H27N2O4FNa{[M+Na]+}581.1847,found 581.1853。
3b:白色固体,产率83%,m.p.182.8~183.6 ℃;dr10/1;1H NMRδ:2.25(d,J=14.0 Hz,1H),2.32(s,3H),2.87~2.91(m,1H),3.32(d,J=13.6 Hz,1H),3.58~3.65(m,1H),3.90~3.94(m,1H),7.09~7.12(m,1H),7.18~7.39(m,10H),7.42~7.46(m,1H),7.49~7.54(m,1H),7.58~7.61(m,2H),7.65(s,1H),7.70~7.73(m,2H),8.06~8.08(m,1H);13C NMRδ:14.0,27.1,38.2,42.9,61.5,117.0,117.1,118.3,120.8,122.3,124.2,124.6,124.9,127.0,127.7,127.9,130.3,130.8,132.5,132.7,135.4,135.7,136.2,154.1,155.1,161.8,173.1,175.6,195.4;HR-MS(ESI-TOF)m/z:Calcd for C35H27N2O4BrNa{[M+Na]+}641.1046,found 641.1051。
3c:白色固体,产率90%,m.p.140.5~141.3 ℃;dr10/1;1H NMRδ:2.26(d,J=13.6 Hz,1H),2.32(s,3H),2.89~2.94(m,1H),3.33(d,J=14.0 Hz,1H),3.58~3.65(m,1H),3.90~3.94(m,1H),7.08~7.12(m,2H),7.19~7.33(m,7H),7.43~7.46(m,2H),7.49~7.54(m,1H),7.60(d,J=7.6 Hz,2H),7.67(d,J=7.6 Hz,2H),7.72~7.74(m,2H),8.06~8.08(m,1H);13C NMRδ:14.0,27.1,38.4,43.1,61.5,117.0,117.1,118.3,121.3,122.4,124.2,124.6,124.9,127.0,127.7,127.9,128.7,129.9,132.1,132.5,132.7,135.4,136.2,139.2,154.1,155.1,161.7,173.1,175.6,195.3;HR-MS(ESI-TOF)m/z:Calcd for C35H27N2O4BrNa{[M+Na]+}641.1046,found 641.1047。
3d:白色固体,产率87%,m.p.189.9~190.5 ℃;dr8/1;1H NMRδ:2.28(d,J=14.0 Hz,1H),2.32(s,3H),2.88~2.93(m,1H),3.30(d,J=14.0 Hz,1H),3.58~3.65(m,1H),3.90~3.93(m,1H),7.08~7.12(m,2H),7.19(s,1H),7.23~7.34(m,7H),7.42~7.46(m,2H),7.59~7.61(m,2H),7.67~7.74(m,4H);13C NMRδ:13.9,27.1,38.3,43.0,61.5,109.7(d,JCF=24.3 Hz),116.4,118.3,119.2(d,JCF=8.1 Hz),121.0(d,JCF=26.0 Hz),121.3,123.4,123.5,124.6,127.0,127.7,127.9,128.7,129.9,132.1,132.5,135.3,136.1,139.1,151.3,154.4,158.2(d,JCF=236.3 Hz),161.7,173.0,174.9,195.3;HR-MS(ESI-TOF)m/z:Calcd for C35H26N2O4FBrNa{[M+Na]+}659.0952,found 659.0957。
3e:白色固体,产率89%,m.p.98.4~99.2 ℃;dr9/1;1H NMRδ:2.30(d,J=14.0 Hz,1H),2.34(s,3H),2.90~2.95(m,1H),3.35(d,J=14.0 Hz,1H),3.60~3.67(m,1H),3.95~3.98(m,1H),6.83~6.87(m,1H),7.08~7.12(m,1H),7.18~7.21(m,2H),7.24~7.33(m,7H),7.43~7.46(m,1H),7.50~7.54(m,1H),7.58(s,1H),7.60(s,1H),7.67(s,1H),7.72~7.74(m,2H),8.06~8.09(m,1H);13C NMRδ:14.0,27.0,38.4,43.2,61.6,113.7(d,JCF=21.4 Hz),115.9(d,JCF=21.3 Hz),117.0,117.1,118.3,122.4,124.2,124.5,124.9,127.0,127.7,127.9,128.6,129.9,132.5,132.7,135.4,136.2,154.1,155.1,161.4(d,JCF=257.0 Hz),161.7,173.1,175.6,195.4;HR-MS(ESI-TOF)m/z:Calcd for C35H27N2O4FNa{[M+Na]+}581.1847,found 581.1848。
3f:白色固体,产率83%,m.p.136.2~137.1 ℃;dr6/1;1H NMRδ:2.28(d,J=13.6 Hz,1H),2.34(s,3H),2.87~2.92(m,1H),3.48(d,J= 13.6 Hz,1H),3.64~3.71(m,1H),4.40~4.43(m,1H),6.98~7.12(m,3H),7.13~7.21(m,2H),7.25~7.33(m,5H),7.41~7.45(m,1H),7.48~7.52(m,1H),7.54~7.58(m,1H),7.62~7.65(m,3H),7.71~7.73(m,2H),8.05~8.08(m,1H);13C NMRδ:13.7,26.1,28.7,37.6,61.2,114.4(d,JCF=23.1 Hz),117.0,118.2,122.4,123.0,123.7,124.2,124.4,124.9,127.0,127.6,127.9,128.2,128.3,129.8(d,JCF=19.7 Hz),132.6,135.4,136.3,154.1,155.1,160.5(d,JCF=244.2 Hz),162.4,173.0,175.6,195.3;HR-MS(ESI-TOF)m/z:Calcd for C35H27N2O4FNa{[M+Na]+}581.1847,found 581.1850。
3g:白色固体,产率79%,m.p.210.3~211.2 ℃;dr9/1;1H NMRδ:2.26(d,J=13.6 Hz,1H),2.33(s,3H),2.88~2.93(m,1H),3.31(d,J=14.0 Hz,1H),3.59~3.66(m,1H),3.93~3.96(m,1H),6.89~6.94(m,2H),7.09~7.12(m,1H),7.18~7.20(m,2H),7.25~7.33(m,4H),7.43~7.47(m,3H),7.59(d,J=7.6 Hz,2H),7.65(s,1H),7.71~7.73(m,2H),8.03(d,J=2.8 Hz,1H);13C NMRδ:14.0,27.1,38.4,42.9,61.7,114.1(d,JCF=19.5 Hz),117.2,118.2,118.9,123.2,124.5(d,JCF=24.1 Hz),127.0,127.6,127.9,130.2,130.6,132.3,132.5,132.9,135.4,136.2,153.4,154.3,161.2(d,JCF=246.1 Hz),161.8,173.1,174.6,195.6;HR-MS(ESI-TOF)m/z:Calcd for C35H26N2O4ClFNa{[M+Na]+}615.1457,found 615.1453。
3h:白色固体,产率88%,m.p.78.4~79.2 ℃;dr7/1;1H NMRδ:2.11(s,3H),2.33(s,3H),2.87(d,J=14.0 Hz,1H),3.48(d,J=13.6 Hz,1H),3.92(d.J=6.8 Hz,2H),4.45~4.49(m,1H),6.75~6.78(m,1H),6.87~6.92(m,1H),6.98~7.04(m,1H),7.06~7.15(m,3H),7.23~7.27(m,2H),7.31~7.38(m,3H),7.45~7.51(m,3H),7.62~7.67(m,1H),7.77(s,1H),7.90~7.92(m,2H);13C NMRδ:13.1,18.2,19.9,26.5,36.7,61.4,114.7(d,JCF=24.3 Hz),116.8,116.9,118.7,122.1,123.3,124.3,124.6,127.1,127.6,127.8,128.1,129.9,132.3,133.9,134.3,135.7,136.2,153.4,153.7,159.5(d,JCF=237.1 Hz),162.0,173.8,175.5,196.4;HR-MS(ESI-TOF)m/z:Calcd for C36H29N2O4FNa{[M+Na]+}595.2004,found 595.2001。
3i:白色固体,产率84%,m.p.213.8~214.2 ℃;dr7/1;1H NMRδ:2.26(d,J=14.0 Hz,1H),2.32(s,3H),2.86~2.91(m,1H),3.30(d,J=14.0 Hz,1H),3.58~3.65(m,1H),3.92~3.95(m,1H),7.09~7.13(m,1H),7.19~7.34(m,8H),7.42~7.47(m,4H),7.59~7.61(m,2H),7.66(s,1H),7.69~7.73(m,2H);13C NMRδ:14.0,27.1,38.3,42.9,61.5,109.6(d,JCF=25.0 Hz),116.4,118.2,119.3,121.1(d,JCF=24.5 Hz),124.6,127.0,127.4,127.7,127.9,130.4,132.5,132.7,135.1,135.4,136.2,154.3,158.4(d,JCF=253.2 Hz),161.8,172.2,173.1,174.7,195.4;HR-MS(ESI-TOF)m/z:Calcd for C35H26N2O4ClFNa{[M+Na]+}615.1457,found 615.1458。
3j:白色固体,产率87%,m.p.200.4~201.2 ℃;dr8/1;1H NMRδ:2.25(d,J=14.0 Hz,1H),2.33(s,3H),2.34(s,3H),2.87~2.92(m,1H),3.34(d,J=14.0 Hz,1H),3.59~3.66(m,1H),3.91~3.95(m,1H),7.09~7.13(m,2H),7.19(s,1H),7.21(s,1H),7.25~7.33(m,5H),7.43(d,J=8.4 Hz,3H),7.58~7.63(m,3H),7.71~7.73(m,2H),7.85(s,1H);13C NMRδ:14.0,19.9,27.2,38.3,42.9,61.6,116.7,116.9,118.3,122.0,124.2,124.6,127.0,127.4,127.6,127.9,130.4,132.5,132.6,133.9,134.3,135.2,135.4,136.2,153.4,154.0,161.9,173.2,175.7,195.5;HR-MS(ESI-TOF)m/z:Calcd for C36H29N2O4ClNa{[M+Na]+}611.1708,found 611.1714。
2 结果与讨论
2.1 合成
在该Michael加成环化反应中,对反应溶剂和催化剂进行了筛选,发现氯仿作溶剂,DABCO为碱,反应4 d,产率能达到87%,dr值达到10/1。
对底物通过扩展,发现该反应产率普遍较高,在溶剂氯仿中反应4 d,TLC检测基本反应完全,目标化合物3产率为79%~90%,dr值为6/1~10/1。

表1 反应条件的优化Table 1 Optimization of reaction conditions
2.2 化合物3b的单晶制备
在无水乙醇溶剂中对化合物3b进行了单晶培养,分析确证白色晶体3b(CCDC:1941654)的结构。图1 为化合物3b的单晶结构图。由图1分析可知,化合物3b属monoclinic晶系,P21/n空间群,晶胞参数a=10.0962(8) Å,b=25.9866(16) Å,c=11.1225(7) Å,α=90°,β=104.840(7)°,γ=90°。
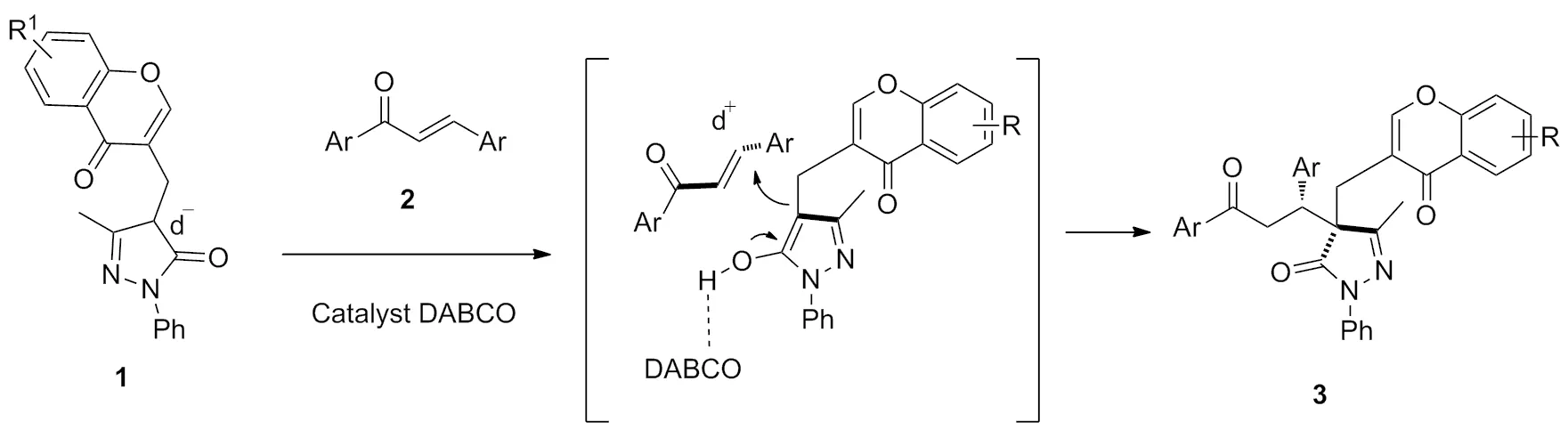
Scheme 2
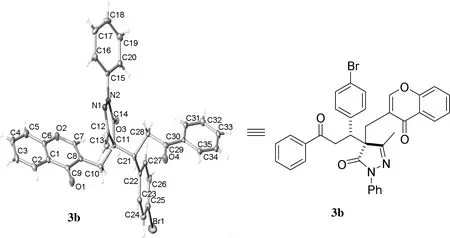
图1化合物3b的单晶结构Figure 1Single crystal structure of compound 3b
2.3 反应机理
该反应可能机理如Schem 2所示:色酮-吡唑啉酮合成子1在DABCO催化活化下,合成子1的3-位进攻发生查尔酮2的β-位,发生Michael加成反应,得目标产物色酮吡唑啉酮骨架拼接二氢查尔酮类化合物3。
合成了10个新型色酮吡唑啉酮骨架拼接二氢查尔酮类化合物3a~3j,产率79%~90%,dr值为6/1~10/1。该类化合物包含色酮吡唑啉酮骨架和二氢查尔酮骨架,且含有连续两个立体中心,包括一个季碳中心,可以为生物活性筛选提供物质基础。

