Wittig/Michael串联反应高效合成含有磷叶立德的吲哚并喹唑啉酮衍生物
张 翔,李青竹,黄晴菲,何鑫磊,马恩典,王政博,冯 鑫,李俊龙1,*,王启卫
(1.中国科学院 成都有机化学研究所,四川 成都 610041;2.成都大学 四川抗菌素工业研究所 抗生素研究与再评价四川省重点实验室,四川 成都 610041;3.中国科学院大学,北京 100491)
吲哚骨架广泛存在于天然产物和具有生物活性和药用价值的分子结构中,其在农药、生物医药等领域中均扮演了非常重要的角色[1]。其中,吲哚并喹唑啉酮作为一类具有特殊并环骨架的喹唑啉类生物碱,具有良好的药理活性,如抗菌[2-3]、抗真菌[4]、抗癌[5-7]以及抗利什曼原虫[8]等。但对于此类骨架的官能团修饰和结构多样性拓展尚未有较为高效的合成策略,限制了其更为深入的构效评价及药学相关研究的开展。基于此,本课题组设想在吲哚并喹唑啉骨架上引入一个易于修饰的高活性基团,这对于丰富骨架的结构拓展性、构建具有多重活性的分子有重要意义。
自Wittig反应发现以来[9],磷叶立德被广泛的应用于碳碳键的构建。除了传统的与羰基化合物进行烯基化反应外,磷叶立德也可以作为一种富电子的亲核试剂进攻各种类型的缺电子物种[10]。此类反应途径不仅能够有效提高Wittig试剂的结构复杂性,还可以作为新型的磷叶立德进一步通过串联反应构建多官能团化合物[11-17]。鉴于磷叶立德良好的化学转化潜力,本课题组将磷叶立德作为修饰起点基团引入吲哚并喹唑啉酮骨架,为后续制备具有此类骨架的化合物提供一种潜在的合成试剂。
本文以色胺酮(1a~1o)及酯基磷叶立德(2)为原料,经Wittig/Michael串联反应,高效合成了15个含有吲哚并喹唑啉骨架的磷叶立德衍生物(3a~3o,Scheme 1),分离收率86%~99%,其结构经1H NMR,13C NMR,31P NMR,19F NMR和HR-MS(ESI-TOF)确认,其中代表化合物3a的结构由X-ray单晶衍射确证。
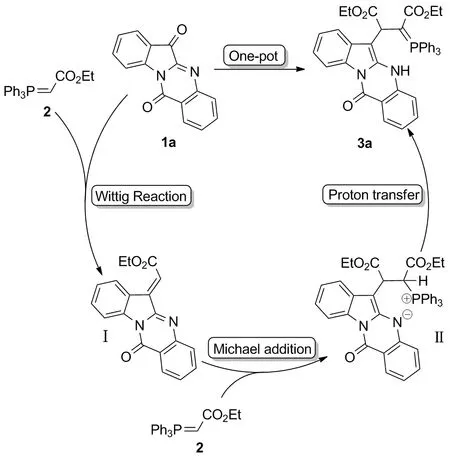
Scheme 2
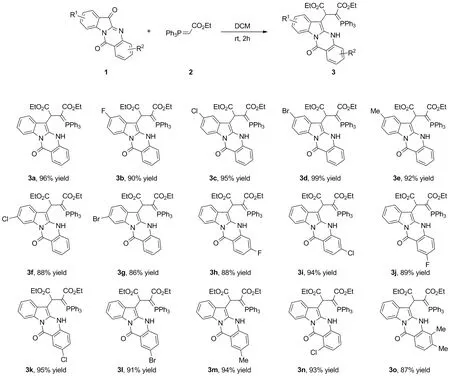
Scheme 1
1 实验部分
1.1 仪器与试剂
WRX-X-4A型熔点仪;JEOL-600 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);Waters SYNAPT G2 型高分辨质谱仪。
化合物1a~1o和2按文献[18-19]方法合成,其余所用试剂均为分析纯。
1.2 合成
(1)1a~1o的合成(以1a为例)[18]
在50 mL圆底烧瓶中依次加入靛红735.5 mg(5.0 mmol),靛红酸酐815.5 mg(5.0 mmol),DABCO 448.8 mg(4.0 mmol)和乙腈20.0 mL,回流反应12 h(TLC监测靛红消耗完毕)。蒸除溶剂,残余物经硅胶柱层析[洗脱剂:A=V(石油醚)/V(乙酸乙酯)=3/1]纯化得1a。用类似的方法合成1b~1o。
(2)3a~3o的合成(以3a为例)
在10 mL反应管中依次加入色胺酮1a24.8 mg(0.1 mmol),三苯基膦乙酸乙酯283.6 mg(0.24 mmol)和二氯甲烷1.0 mL,于室温下反应2 h(TLC监测1a消耗完毕)。反应液经硅胶柱层析(洗脱剂:A=2/1)纯化得3a。
2-(12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3a):黄色固体64.0 mg,收率96%,m.p.160~164 ℃;1H NMR(CDCl3,600 MHz)δ:12.05(s,1H),8.71(d,J=8.4 Hz,1H),8.35(d,J=7.8 Hz,1H),7.64~7.52(m,10H),7.44~7.37(m,6H),7.23(d,J=8.4 Hz,1H),7.09(t,J=8.4 Hz,1H),7.06(t,J=7.2 Hz,1H),6.93(t,J=6.6 Hz,1H),5.91(d,J=7.8 Hz,1H),4.28~4.18(m,3H),3.88~3.73(m,2H),1.28(t,J=7.2 Hz,3H),0.48(t,J=7.2 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.7(d,J=10.1 Hz,C=O),171.7(d,J=11.4 Hz,C=O),160.2(C=O),140.9(Ar),135.1(Ar),134.0(Ar),133.7(d,J=8.6 Hz,Ar),132.0(d,J=2.9 Hz,Ar),130.0(Ar),128.8(d,J=11.4 Hz,Ar),128.5(Ar),128.1(Ar),126.6(d,J=92.0 Hz,Ar),123.1(Ar),119.4(Ar),119.1(Ar),115.6(Ar),115.2(Ar),114.6(Ar),111.9(Ar),94.8(Ar),60.9(CH2),58.5(CH2),46.1(d,J=119.3 Hz,C=P),39.1(d,J=13.1 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:25.00;HR-MS(ESI-TOF)m/z:Calcd for C41H35N2O5P{[M+Na]+}689.2176,found 689.2175。
用类似的方法合成3b~3o。
2-(8-氟-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3b):黄色固体61.7 mg,收率90%,m.p.174~177 ℃;1H NMR(CDCl3,600 MHz)δ:12.20(s,1H),8.62(dd,J=9.0 Hz,4.8 Hz,1H),8.34(d,J=7.2 Hz,1H),7.63~7.53(m,10H),7.45~7.38(m,6H),7.24(d,J=7.8 Hz,1H),7.11(t,J=7.8 Hz,1H),6.73(td,J=9.0 Hz,2.4 Hz,1H),5.45(dd,J=9.6 Hz,1.8 Hz,1H),4.30~4.18(m,2H),4.11(d,J=18.6 Hz,1H),3.85~3.75(m,2H),1.30(t,J=6.6 Hz,3H),0.47(t,J=6.6 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.4(d,J=11.4 Hz,C=O),171.7(d,J=11.6 Hz,C=O),159.9(C=O),159.8(d,J=237.0 Hz,Ar),140.7(Ar),136.5(Ar),134.1(Ar),133.7(d,J=8.6 Hz,Ar),132.2(Ar),131.3(d,J=10.1 Hz,Ar),128.8(d,J=13.1 Hz,Ar),128.0(Ar),126.4(d,J=92.0 Hz,Ar),125.1(Ar),119.8(Ar),116.5(d,J=8.6 Hz,Ar),115.3(Ar),111.9(Ar),106.1(d,J=24.5 Hz,Ar),100.4(d,J=25.8 Hz,Ar),95.0(Ar),61.0(CH2),58.5(CH2),46.2(d,J=120.6 Hz,C=P),39.3(d,J=13.1 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.92;19F NMR(CDCl3,564 MHz)δ:-119.61~-119.72(m,1F);HR-MS(ESI-TOF)m/z:Calcd for C41H34N2O5FP{[M+Na]+}707.2082,found 707.2074。
2-(8-氯-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3c):黄色固体66.6 mg,收率95%,m.p.185~188 ℃;1H NMR(CDCl3,600 MHz)δ:12.25(s,1H),8.60(d,J=8.4 Hz,1H),8.33(d,J=8.4 Hz,1H),7.64~7.55(m,10H),7.46~7.39(m,6H),7.24(d,J=8.4 Hz,1H),7.11(t,J=6.6 Hz,1H),6.98(dd,J=9.0 Hz,2.4 Hz,1H),5.76(d,J=1.8 Hz,1H),4.30~4.18(m,2H),4.11(d,J=18.6 Hz,1H),3.85~3.75(m,2H),1.31(t,J=6.6 Hz,3H),0.48(t,J=7.2 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.4(d,J=10.1 Hz,C=O),171.7(d,J=12.9 Hz,C=O),160.0(C=O),140.8(Ar),136.3(Ar),134.2(Ar),133.7(d,J=8.7 Hz,Ar),132.3(d,J=2.9 Hz,Ar),131.3(Ar),129.0(Ar),128.9(d,J=13.1 Hz,Ar),128.0(Ar),127.0(Ar),126.3(d,J=92.0 Hz,Ar),119.8(Ar),118.9(Ar),116.6(Ar),115.4(Ar),114.1(Ar),111.9(Ar),94.5(Ar),61.1(CH2),58.6(CH2),46.3(d,J=120.6 Hz,C=P),39.1(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.92;HR-MS(ESI-TOF)m/z:Calcd for C41H34N2O535ClP{[M+H]+}701.1967,found 701.1977;C41H3437ClN2O5P{[M+H]+}703.1937,found 703.1940。
2-(8-溴-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3d):黄色固体73.9 mg,收率99%,m.p.185~187 ℃;1H NMR(CDCl3,600 MHz)δ:12.26(s,1H),8.56(d,J=7.8 Hz,1H),8.33(d,J=8.4 Hz,1H),7.63~7.56(m,10H),7.46~7.40(m,6H),7.25(d,J=8.4 Hz,1H),7.15~7.07(m,2H),5.94(d,J=1.8 Hz,1H),4.30~4.18(m,2H),4.10(d,J=18.0 Hz,1H),3.85~3.75(m,2H),1.31(t,J=6.6 Hz,3H),0.48(t,J=7.8 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.3(d,J=10.1 Hz,C=O),171.7(d,J=12.9 Hz,C=O),160.0(C=O),140.8(Ar),136.1(Ar),134.2(Ar),133.6(d,J=10.1 Hz,Ar),133.5(Ar),132.3(d,J=1.8 Hz,Ar),131.7(Ar),128.8(d,J=11.6 Hz,Ar),128.0(Ar),127.4(Ar),126.3(d,J=90.5 Hz,Ar),121.6(Ar),119.8(Ar),116.99(Ar),116.96(Ar),115.4(Ar),111.9(Ar),94.3(Ar),61.1(CH2),58.6(CH2),46.3(d,J=120.8 Hz,C=P),39.1(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.86;HR-MS(ESI-TOF)m/z:Calcd for C41H3479N2O5BrP{[M+H]+}745.1461,found 745.1470;Calcd for C41H3481N2O5BrP{[M+H]+}747.1441,found 747.1449。
2-(8-甲基-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3e):黄色固体62.7 mg,收率92%,m.p.179~182 ℃;1H NMR(CDCl3,600 MHz)δ:12.04(s,1H),8.56(d,J=7.8 Hz,1H),8.33(d,J=8.4 Hz,1H),7.65~7.58(m,6H),7.58~7.53(m,4H),7.45~7.38(m,6H),7.21(d,J=8.4 Hz,1H),7.08(t,J=7.8 Hz,1H),6.87(dd,J=8.4,1.2 Hz,1H),5.68(s,1H),4.23(t,J=7.2 Hz,2H),4.19(d,J=18.6 Hz,1H),3.86~3.74(m,2H),2.23(s,3H),1.29(t,J=7.8 Hz,3H),0.47(t,J=7.2 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.8(d,J=10.1 Hz,C=O),171.7(d,J=12.9 Hz,C=O),160.1(C=O),140.8(Ar),135.2(Ar),133.9(Ar),133.7(d,J=8.7 Hz,Ar),133.5(Ar),131.9(d,J=2.7 Hz,Ar),130.2(Ar),128.7(d,J=11.6 Hz,Ar),128.5(Ar),128.0(Ar),126.7(d,J=93.3 Hz,Ar),120.3(Ar),119.3(Ar),115.3(Ar),115.1(Ar),114.6(Ar),111.9(Ar),94.5(Ar),61.0(CH2),58.5(CH2),46.2(d,J=120.8 Hz,C=P),39.0(d,J=12.9 Hz,CH),21.6(CH3),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.92;HR-MS(ESI-TOF)m/z:Calcd for C42H37N2O5P{[M+H]+}681.2513,found 681.2524。
2-(9-氯-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3f):黄色固体61.7 mg,收率88%,m.p.173~178 ℃;1H NMR(CDCl3,600 MHz)δ:12.12(s,1H),8.73(d,J=1.8 Hz,1H),8.32(dd,J=8.4 Hz,1.2 Hz,1H),7.61~7.53(m,10H),7.44~7.38(m,6H),7.22(d,J=7.8 Hz,1H),7.10(t,J=7.8 Hz,1H),6.88(dd,J=8.4 Hz,1.8 Hz,1H),5.75(d,J=7.8 Hz,1H),4.28~4.18(m,2H),4.16(d,J=18.0 Hz,1H),3.85~3.75(m,2H),1.29(t,J=6.6 Hz,3H),0.48(t,J=7.8 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.5(d,J=11.4 Hz,C=O),171.7(d,J=13.1 Hz,C=O),160.1(C=O),140.9(Ar),135.5(Ar),134.3(Ar),133.6(d,J=10.1 Hz,Ar),132.1(d,J=2.9 Hz,Ar),128.8(d,J=11.6 Hz,Ar),128.44(Ar),128.40(Ar),128.1(Ar),126.5(d,J=90.5 Hz,Ar),124.3(Ar),123.3(Ar),119.7(Ar),115.7(Ar),115.3(Ar),115.2(Ar),111.7(Ar),94.7(Ar),61.0(CH2),58.5(CH2),46.0(d,J=120.6 Hz,C=P),39.2(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.92;HR-MS(ESI-TOF)m/z:Calcd for C41H3435ClN2O5P{[M+H]+}701.1967,found 701.1974;C41H3437ClN2O5P{[M+H]+}703.1937,found 703.1944。
2-(9-溴-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3g):黄色固体64.2 mg,收率86%,m.p.181~184 ℃;1H NMR(CDCl3,600 MHz)δ:12.13(s,1H),8.88(d,J=1.2 Hz,1H),8.32(dd,J=8.4 Hz,1.8 Hz,1H),7.62~7.53(m,10H),7.44~7.38(m,6H),7.22(d,J=8.4 Hz,1H),7.11(t,J=8.4 Hz,1H),7.01(dd,J=9.0 Hz,2.4 Hz,1H),5.70(d,J=7.8 Hz,1H),4.29~4.17(m,2H),4.15(d,J=18.6 Hz,1H),3.86~3.73(m,2H),1.28(t,J=7.2 Hz,3H),0.47(t,J=7.2 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.5(d,J=11.4 Hz,C=O),171.8(d,J=11.6 Hz,C=O),160.1(C=O),140.9(Ar),135.5(Ar),134.3(Ar),133.7(d,J=10.1 Hz,Ar),132.1(d,J=2.6 Hz,Ar),129.3(Ar),128.9(d,J=12.9 Hz,Ar),128.4(Ar),128.1(Ar),126.5(d,J=92.0 Hz,Ar),126.0(Ar),119.8(Ar),118.5(Ar),115.6(Ar),115.3(Ar),111.7(Ar),111.7(Ar),94.7(Ar),61.1(CH2),58.6(CH2),46.0(d,J=120.8 Hz,C=P),39.2(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.89;HR-MS(ESI-TOF)m/z:Calcd for C41H3479N2O5BrP{[M+H]+}745.1461,found 745.1466;Calcd for C41H3481N2O5BrP{[M+H]+}747.1441,found 747.1447。
2-(3-氟-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3h):黄色固体60.3 mg,收率88%,m.p.167~169 ℃;1H NMR(CDCl3,600 MHz)δ:12.28(s,1H),8.66(d,J=8.4 Hz,1H),8.33(dd,J=9.0,6.6 Hz,1H),7.64~7.53(m,9H),7.45~7.38(m,6H),7.06(t,J=6.6 Hz,1H),6.92(t,J=6.6 Hz,1H),6.86(dd,J=10.2 Hz,2.4 Hz,1H),6.78(td,J=9.0 Hz,3.0 Hz,1H),5.88(d,J=7.2 Hz,1H),4.28~4.17(m,3H),3.85~3.75(m,2H),1.29(t,J=7.2 Hz,3H),0.48(t,J=6.6 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.6(d,J=11.6 Hz,C=O),171.8(d,J=11.6 Hz,C=O),166.6(d,J=251.4 Hz,Ar),159.5(C=O),142.6(d,J=12.9 Hz,Ar),134.9(Ar),133.7(d,J=10.1 Hz,Ar),132.1(d,J=3.0 Hz,Ar),131.0(d,J=10.1 Hz,Ar),129.8(Ar),128.8(d,J=11.6 Hz,Ar),126.5(d,J=92.0 Hz,Ar),123.2(Ar),119.5(Ar),115.6(Ar),114.8(Ar),108.8(Ar),108.0(d,J=23.0 Hz,Ar),100.8(d,J=25.8 Hz,Ar),95.5(Ar),61.0(CH2),58.6(CH2),46.4(d,J=120.6 Hz,C=P),39.0(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.97;19F NMR(CDCl3,564 MHz)δ:-104.05(dd,J=17.5 Hz,8.5 Hz,1F);HR-MS(ESI-TOF)m/z:Calcd for C41H34N2O5FP{[M+Na]+}707.2082,found 707.2087。
2-(3-氯-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3i):黄色固体65.9 mg,收率94%,m.p.173~175 ℃;1H NMR(CDCl3,600 MHz)δ:12.30(s,1H),8.67(d,J=8.4 Hz,1H),8.25(d,J=7.8 Hz,1H),7.65~7.53(m,9H),7.47~7.38(m,6H),7.23(d,J=1.8 Hz,1H),7.07(t,J=6.6 Hz,1H),7.01(dd,J=8.4 Hz,1.8 Hz,1H),6.92(t,J=6.6 Hz,1H),5.87(d,J=7.8 Hz,1H),4.31~4.17(m,3H),3.82(q,J=7.8 Hz,2H),1.30(t,J=6.6 Hz,3H),0.49(t,J=7.2 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.5(d,J=11.6 Hz,C=O),171.8(d,J=11.4 Hz,C=O),159.5(C=O),141.5(Ar),140.2(Ar),134.7(Ar),133.7(d,J=10.1 Hz,Ar),133.0(Ar),132.1(d,J=2.9 Hz,Ar),129.8(Ar),129.6(Ar),128.8(d,J=13.1 Hz,Ar),126.5(d,J=92.0 Hz,Ar),123.2(Ar),119.9(Ar),119.5(Ar),115.6(Ar),114.8(Ar),114.6(Ar),110.5(Ar),95.7(Ar),61.0(CH2),58.6(CH2),46.4(d,J=120.6 Hz,C=P),39.0(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:25.09;HR-MS(ESI-TOF)m/z:Calcd for C41H3435N2O5ClP{[M+Na]+}723.1786,found 723.1783;C41H3437N2O5ClP{[M+Na]+}725.1757,found 725.1752。
2-(2-氟-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3j):黄色固体61.0 mg,收率89%,m.p.143~147 ℃;1H NMR(CDCl3,600 MHz)δ:12.13(s,1H),8.68(d,J=8.4 Hz,1H),7.99(dd,J=9.0 Hz,3.6 Hz,1H),7.62~7.53(m,9H),7.44~7.37(m,6H),7.35~7.29(m,1H),7.19(dd,J=9.0,4.2 Hz,1H),7.06(t,J=6.6 Hz,1H),6.93(t,J=8.4 Hz,1H),5.88(d,J=8.4 Hz,1H),4.27~4.16(m,3H),3.84~3.74(m,2H),1.28(t,J=7.2 Hz,3H),0.47(t,J=7.2 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.7(d,J=10.1 Hz,C=O),171.8(d,J=11.6 Hz,C=O),159.4(C=O),156.3(d,J=237.0 Hz,Ar),137.6(Ar),135.0(Ar),133.7(d,J=10.1 Hz,Ar),132.1(d,J=2.7 Hz,Ar),130.0(Ar),128.8(d,J=12.9 Hz,Ar),128.5(Ar),126.6(d,J=90.5 Hz,Ar),123.3(Ar),122.6(d,J=25.8 Hz,Ar),119.2(Ar),116.8(d,J=7.2 Hz,Ar),115.7(Ar),114.6(Ar),112.7(d,J=24.5 Hz,Ar),112.2(d,J=7.2 Hz,Ar),94.6(Ar),61.0(CH2),58.5(CH2),46.2(d,J=120.6 Hz,C=P),39.1(d,J=12.9 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.95;19F NMR(CDCl3,564 MHz)δ:-123.24(dd,J=10.7,6.2 Hz,1F);HR-MS(ESI-TOF)m/z:Calcd for C41H34N2O5FP{[M+Na]+}707.2082,found 707.2082。
2-(2-氯-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3k):黄色固体66.6 mg,收率95%,m.p.171~173 ℃;1H NMR(CDCl3,600 MHz)δ:12.24(s,1H),8.67(d,J=8.4 Hz,1H),8.29(d,J=3.0 Hz,1H),7.63~7.53(m,9H),7.48(dd,J=8.4 Hz,1.8 Hz,1H),7.44~7.38(m,6H),7.17(d,J=8.4 Hz,1H),7.06(t,J=6.6 Hz,1H),6.93(t,J=8.4 Hz,1H),5.89(d,J=7.8 Hz,1H),4.28~4.17(m,3H),3.84~3.74(m,2H),1.29(t,J=6.6 Hz,3H),0.47(t,J=7.8 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.6(d,J=10.1 Hz,C=O),171.8(d,J=12.9 Hz,C=O),159.1(C=O),139.4(Ar),134.7(Ar),134.2(Ar),133.7(d,J=8.7 Hz,Ar),132.1(d,J=2.7 Hz,Ar),131.9(Ar),129.9(Ar),128.8(d,J=11.6 Hz,Ar),127.3(Ar),126.5(d,J=91.8 Hz,Ar),124.3(Ar),123.3(Ar),119.4(Ar),116.8(Ar),115.7(Ar),114.7(Ar),112.7(Ar),95.3(Ar),61.0(CH2),58.6(CH2),46.3(d,J=120.8 Hz,C=P),39.1(d,J=13.1 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.97;HR-MS(ESI-TOF)m/z:Calcd for C41H3435ClN2O5P{[M+Na]+}723.1786,found 723.1793;C41H3437N2O5ClP{[M+Na]+}725.1757,found 725.1748。
2-(2-溴-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3l):黄色固体67.9 mg,收率91%,m.p.174~178 ℃;1H NMR(CDCl3,600 MHz)δ:12.26(s,1H),8.67(d,J=7.8 Hz,1H),8.44(d,J=3.0 Hz,1H),7.63~7.53(m,10H),7.44~7.38(m,6H),7.11(d,J=9.0 Hz,1H),7.06(t,J=6.6 Hz,1H),6.93(t,J=8.4 Hz,1H),5.89(d,J=7.8 Hz,1H),4.27~4.16(m,3H),3.85~3.73(m,2H),1.29(t,J=6.6 Hz,3H),0.47(t,J=6.6 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.5(d,J=10.1 Hz,C=O),171.8(d,J=12.9 Hz,C=O),159.0(C=O),139.7(Ar),136.8(Ar),134.6(Ar),133.7(d,J=8.6 Hz,Ar),132.1(d,J=1.5 Hz,Ar),130.4(Ar),129.9(Ar),128.8(d,J=12.9 Hz,Ar),128.4(Ar),126.4(d,J=92.0 Hz,Ar),123.3(Ar),119.4(Ar),117.0(Ar),115.7(Ar),114.8(Ar),113.2(Ar),111.2(Ar),95.5(Ar),61.0(CH2),58.6(CH2),46.3(d,J=119.3 Hz,C=P),39.1(d,J=14.4 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.97;HR-MS(ESI-TOF)m/z:Calcd for C41H3479N2O5BrP{[M+Na]+}767.1281,found 767.1277;Calcd for C41H3481N2O5BrP{[M+Na]+}769.1260,found 769.1255。
2-(2-甲基-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3m):黄色固体64.0 mg,收率94%,m.p.149~154 ℃;1H NMR(CDCl3,600 MHz)δ:11.89(s,1H),8.70(d,J=8.4 Hz,1H),8.14(s,1H),7.62~7.52(m,9H),7.42~7.36(m,7H),7.14(d,J=9.0 Hz,1H),7.04(t,J=7.2 Hz,1H),6.91(t,J=8.4 Hz,1H),5.89(d,J=7.8 Hz,1H),4.25~4.16(m,3H),3.85~3.73(m,2H),2.43(s,3H),1.27(t,J=7.2 Hz,3H),0.46(t,J=7.8 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.9(d,J=11.6 Hz,C=O),171.7(d,J=13.1 Hz,C=O),160.4(C=O),138.9(Ar),135.5(Ar),135.3(Ar),133.7(d,J=8.6 Hz,Ar),132.0(d,J=2.6 Hz,Ar),130.1(Ar),128.8(d,J=12.9 Hz,Ar),128.5(Ar),128.4(Ar),127.4(Ar),126.7(d,J=90.5 Hz,Ar),123.1(Ar),118.8(Ar),115.6(Ar),115.1(Ar),114.5(Ar),111.7(Ar),94.2(Ar),61.0(CH2),58.5(CH2),46.1(d,J=119.3 Hz,C=P),39.2(d,J=14.3 Hz,CH),20.8(CH3),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.83;HR-MS(ESI-TOF)m/z:Calcd for C42H37N2O5P{[M+H]+}681.2513,found 681.2516。
2-(1-氯-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3n):黄色固体65.2 mg,收率93%,m.p.179~181 ℃;1H NMR(CDCl3,600 MHz)δ:12.29(s,1H),8.73(d,J=8.4 Hz,1H),7.62~7.53(m,9H),7.44~7.35(m,7H),7.12(d,J=7.8 Hz,1H),7.09~7.02(m,2H),6.92(t,J=6.6 Hz,1H),5.87(d,J=7.8 Hz,1H),4.26~4.15(m,3H),3.83~3.73(m,2H),1.27(t,J=7.8 Hz,3H),0.46(t,J=6.6 Hz,3H);13C NMR(CDCl3,150 MHz)δ:173.6(d,J=11.6 Hz,C=O),171.8(d,J=12.9 Hz,C=O),158.3(C=O),143.2(Ar),135.8(Ar),133.9(Ar),133.7(d,J=10.1 Hz,Ar),133.3(Ar),132.1(d,J=1.8 Hz,Ar),129.9(Ar),129.1(Ar),128.8(d,J=12.9 Hz,Ar),126.5(d,J=92.0 Hz,Ar),123.2(Ar),122.5(Ar),119.4(Ar),116.0(Ar),114.6(Ar),114.5(Ar),108.4(Ar),95.0(Ar),61.0(CH2),58.5(CH2),46.5(d,J=120.6 Hz,C=P),39.0(d,J=13.1 Hz,CH),14.4(CH3),14.0(CH3);31P NMR(CDCl3,243 MHz)δ:24.97;HR-MS(ESI-TOF)m/z:Calcd for C41H3435N2O5ClP{[M+Na]+}723.1786,found 723.1777;C41H3437N2O5ClP{[M+Na]+}725.1757,found 725.1749。
2-(3,4-二甲基-12-氧代-5,12-二氢吲哚并[2,1-b]喹唑啉-6-基)-3-(三苯基-λ5-膦叉)琥珀酸二乙酯(3o):黄色固体60.5 mg,收率87%,m.p.168~170 ℃;1H NMR(CDCl3,600 MHz)δ:11.37(s,1H),8.73(d,J=7.8 Hz,1H),8.15(d,J=7.8 Hz,1H),7.68~7.61(m,6H),7.59~7.53(m,3H),7.46~7.39(m,6H),7.06(t,J=7.8 Hz,1H),6.96(d,J=7.8 Hz,1H),6.90(t,J=8.4 Hz,1H),5.82(d,J=7.2 Hz,1H),4.33~4.18(m,3H),3.85~3.70(m,2H),2.51(s,3H),2.43(s,3H),1.27(t,J=7.8 Hz,3H),0.43(t,J=7.8 Hz,3H);13C NMR(CDCl3,150 MHz)δ:174.0(d,J=10.1 Hz,C=O),171.3(d,J=12.9 Hz,C=O),160.5(C=O),142.7(Ar),139.8(Ar),134.9(Ar),133.7(d,J=8.7 Hz,Ar),131.9(d,J=1.5 Hz,Ar),130.2(Ar),128.7(d,J=11.6 Hz,Ar),128.6(Ar),126.9(d,J=90.5 Hz,Ar),124.9(Ar),122.9(Ar),122.0(Ar),121.5(Ar),119.1(Ar),115.6(Ar),114.5(Ar),110.3(Ar),95.0(Ar),60.8(CH2),58.3(CH2),45.4(d,J=122.0 Hz,C=P),39.4(d,J=12.9 Hz,CH),21.2(CH3),14.4(CH3),13.9(CH3),13.3(CH3);31P NMR(CDCl3,243 MHz)δ:24.52;HR-MS(ESI-TOF)m/z:Calcd for C43H39N2O5P{[M+Na]+}717.2489,found 717.2482。
2 结果与讨论
2.1 反应条件优化
以3a的合成反应为模板反应,对反应进行了考察。从表1可见,无论是在非极性溶剂,中等极性溶剂,还是极性溶剂中,色胺酮均能够在相应的时间内消耗完毕,顺利转化得到3a。在极性溶剂乙腈及二甲基亚砜中反应时,能以78%~87%的收率得到目标产物(Entry 6~7)。在醚类溶剂四氢呋喃和1,4-二氧六环中反应时,不仅反应时间大幅延长,且收率严重下降(Entry 4~5)。当以甲苯作为为溶剂时,底物的溶解性不佳,导致反应时间较长(Entry 1)。在溶解性较好的二氯甲烷和氯仿中反应时(Entry 2~3),反应能够高效的进行,收率优异(82%~96%)。因此,将二氯甲烷作为最优溶剂,于室温下进行反应。

表1 3a合成反应条件的优化Table 1 Optimization of the synthesis condition of 3a
2.2 底物扩展
在优化的条件下,对反应的底物普适性进行了研究,结果见Scheme 1。首先以吲哚环上不同取代位点及不同电子效应基团为底物进行反应,均能以良好到优秀的收率分离得到目标化合物3b~3g,最高可达99%(3d)。随后,进一步对喹唑啉环不同取代位点及不同电子效应基团的底物兼容性进行了考查(3i~3o),结果表明反应对喹唑啉环上的取代基同样表现出较好的普适性(87%~95%),对于双取代的色胺酮3o,反应收率也未收到明显影响(87%)。
2.3 产物结构
通过对产物3a(CCDC:1974569)进行X射线单晶衍射实验,我们确证了产物的分子结构(图1)。其余化合物的分子结构通过类推得到。

图1 化合物3a的单晶结构Figure 1The crystal structure of compound 3a
2.4 反应机理
根据实验结果和文献[20]报道,提出了该反应可能的反应机理(Scheme 2)。首先,色胺酮3a与三苯基膦乙酸乙酯2发生Wittig反应,在吲哚并喹唑啉骨架上引入酯烯基,得到中间体Ⅰ;随后,中间体Ⅰ与另一分子的三苯基膦乙酸乙酯发生Michael加成,得两性离子中间体Ⅱ;最后,中间体Ⅱ通过分子内质子转移得到目标化合物3a。
通过色胺酮与酯基Wittig试剂的Wittig/Michael串联反应,高效合成了一系列吲哚并喹唑啉骨架的磷叶立德衍生物。该反应普适性较好、操作简单、条件温和,为后续制备具有此类骨架的化合物提供了一种潜在的合成试剂。

