Synthesis and Magnetocaloric Effects of FeNi@SiO2 Nanocomposite Particles
ZHANG Ying, ZHOU Meiting, GAO Yiming , XIONG Xi, LIU Shuli, MA Song, ZHANG Zhidong
(1.Key Laboratory of the Ministry of Education, Shenyang Pharmaceutical University, Shenyang 110016, China; 2.Shenyang National Research Center for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China)
【Abstract】 Large-scale synthesis and prominent magnetocaloric effects of magnetic particles in low temperature and normal temperature ranges have theoretical value for the application of tissue rewarming and magnetic hyperthermia. Iron-nickel nanoparticles with an average diameter of 80nm were prepared on a large scale by high-temperature arc discharge method following the process of classification and sedimentation. Iron-nickel silica nanoparticles with an average diameter of 100 nm were obtained by the sol-gel method and the coating thickness was 15-20 nm. The saturation magnetization (Ms) was measured to be 80 emu/g by using a superconducting quantum interferometer. Under the high-temperature standard, the iron-nickel silica nanoparticles mainly show the magnetocaloric effect, and the average heating rate is 3.6 ℃/min, which is enough to consider the need for magnetic hyperthermia. In the simulation condition of tissue rewarming, iron-nickel silica nanoparticles exhibit prominent magnetocaloric efficiency with an average heating rate of 61.8 ℃/min in the presence of Vs55. The iron-nickel silica nanocomposite particles exhibit prominent magnetocaloric effects in different solvents and temperature ranges so that they can be used as a temperature activating agent for tissue rewarming and hyperthermia. The results benefit the applications of hyperthermia and tissue rewarming.
【Key words】 Magnetic nanoparticles; Magnetocaloric effects; Heating rate; Hyperthermia; Tissue rewarming
1 Introduction
Magnetic particles due to their biocompatibility, low toxicity, and high saturation magnetization, have been widely used in biomedical applications such as in photocatalyst, drug delivery, and separation of biological macromolecules[1-2]. In recent years, magnetocaloric effects under the alternating magnetic field[3-4]have advanced a new application in magnetic hyperthermia and resuscitation of frozen tissue. When the magnetic nanoparticles are targeted at a tumor and warmed to 43 ℃, the tumor cells are killed[5]. Several clinic hyperthermia tests with magnetic particles have been carried on in China and Germany, and the effectiveness of hyperthermia has been proved. When the tissue and the organs are preserved at low-temperature, rapid non-destructive rewarming is a key issue to be solved for the resuscitation of frozen tissue[6]. Etheridge[7]conducted a preliminary exploration of magnetic nanoparticle rapid rewarming of frozen solutions which are used to store tissues or bodies for a long time. Tissues in small volumes have obtained resuscitation with the warming of magnetic particles. For the tumors in deep regions and tissues in large volume, it is still in great demand of magnetic particles with prominent magnetocaloric effects. Therefore, large-scale synthesis and magnetocaloric effects of magnetic particles in low temperature and normal temperature regions have theoretical value for the application of tissue rewarming and magnetic hyperthermia.
In this paper, iron-nickel silica nanocomposites were prepared on a large-scale and the magnetocaloric effects of the composites were measured as shown in scheme 1. Firstly, iron-nickel nanoparticles were prepared by the high-temperature arc discharge method[8]. Following the process of classification and sedimentation, iron-nickel particles with uniform morphology of 80 nm and 155 mg were obtained. By the sol-gel method, iron-nickel particles were coated with a 15 nm silica shell. Using the high-frequency induction heating machine, the thermal performance of the magnetic nanoparticles in different temperature ranges (normal temperature and low temperature) and different solvents (low temperature freezing liquid, ethanol, and water) were observed. Compared with the current magnetic nanoparticles, it shows a stronger temperature active characteristic for the resuscitation of frozen tissue and magnetic hyperthermia.
2 Experimental
2.1 Materials
Anhydrous ethanol, Ammonia (25%), Tetraethylorthosilicate (TEOS), 1,2-Propanediol, Dimethyl Sulfoxide (DMSO), Carboxamide, Formamide, Pyridine (PY), Chromium Trioxide(CT), Dichloromethane(DM).
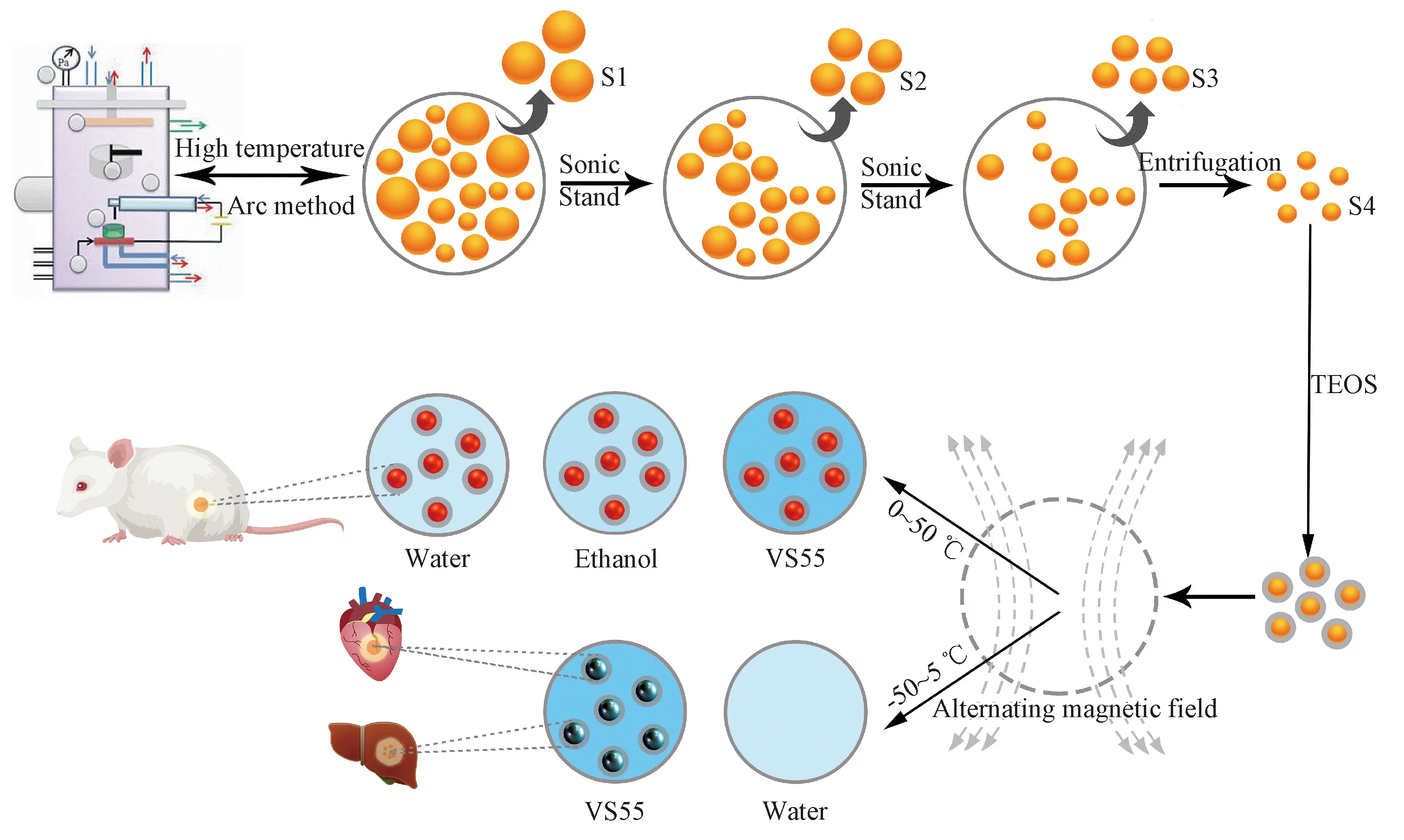
Scheme 1 Preparation and magnetocaloric performance test of iron-nickel silica nanocompostite
2.2 Preparation of cryogenic protectant Vs55
15.82 ml of pyridine was poured into a round bottom flask and placed in an ice-water bath. 2.083 g of CT was taken, ground, and crushed, and the pyridine in that flask was added slowly with a magnetic stirrer. The solution was kept below 10 ℃. The precipitated yellow complex was transferred into a beaker, and 30 ml of DM was added to obtain Collins reagent[9]. The Collins reagent was mixed with 1.8 ml DMSO, 3.0 ml formamide, and 1.4 ml 1,2-propanediol to prepare a 8.4 mol/L solution of Vs55 containing 50 ml of substance (carboxamide 3.1 mol/L; dimethyl sulfoxide 3.1 mol/L; 1,2-propanediol 2.2 mol/L).
2.3 Preparation of iron-nickel nanoparticles by high temperature arc method
The plasma arc furnace was used to prepare nanoparticles. A high-purity graphite negative electrode was prepared and aluminum alloy anodizing was performed in advance for the furnace. Before turning on the power switch, the water cooling system started running and the vacuum package of the reaction chamber was carried out to make the vacuum value 5×10-3Pa or less. 30 ml of anhydrous ethanol was injected into the chamber as a carbon source. A small amount of argon gas and hydrogen gas were also running through. As the ionized plasma, the volume ratio of Ar/H2gas mixture was 5∶1. Adjusting the working voltage and current knobs to 20 V, the arc was formed by changing the distance between the cathode and the iron-nickel alloy target. After successfully arcing, the value of voltage and current was monitored and adjusted in real-time to stabilize it at the set point. Adjust the distance between the cathode and anode as much as possible to ensure that the arc continuously uninterrupted. After sufficient time, the main power switch was turned off, the reaction chamber was cooled, and appropriate argon gas was added to it, letting the passivation hour more than 8 hours. Collect 1.719 g of iron-nickel nanoparticles and disperse them in 500 ml of industrial ethanol for 30 minutes by ultrasonic. Let it stand for 3 minutes, the lower sediment solid in a beaker was collected and dried that was numbered S1. The suspension was suspended with ultrasonic for 20 minutes and stood for 10 minutes. The lower solid in the beaker was collected and dried which was numbered S2. The suspension was suspended with ultrasonic for 20 minutes and stood for 30 minutes. The precipitated solid in the beaker was collected which was numbered S3. The supernatant mixture was suction filtered at a rate of 3000 rpm/min for five minutes, and the solid serial number after suction filtration was S4. Each of the S1, S2, S3, and S4 products was weighed after drying naturally.
2.4 Preparation of iron-nickel silica nanocompostite particles
12 mg of S4 powder and 30 ml of anhydrous ethanol were added respectively in a beaker, soaked in a water bath for 40 minutes, and then mixed continuously with probe ultrasound for 25 minutes. Then the solution was transferred to a three-necked flask and 20 ml of purified water and 50 ml of industrial ethanol were added. Futhermore, 6 ml of 25% ammonia in a constant temperature water bath at 35 ℃, and 2 ml 1∶1 TEOS compound were added and. mixed slowly but strongly in also a constant temperature water bath at 35 ℃ for 6 hours. The whole thing above was centrifuged at 4500 rpm/min for 5 minutes to ensure that the unreacted ester did not precipitate. It was finally washed twice with 15 ml of alcohol and slightly dried at 60 ℃.
2.5 Magnetocaloric performance test of the magnetic nanoparticles
The magnetocaloric efficacy of the nanoparticles was accurately measured. Before application, the nanoparticles were soaked in an ultrasonic bath for 20 minutes, and the concentration of the tested product was 10 mg/ml. Here we set the current to 25.5 A, and 40 A of the magnetic induction heating machine which was used to mesure the carloric data. The properties of the magnetic nanoparticles were characterized by using different disperse phases: water, ethanol and cryogenic liquid Vs55. The cryo-protectant group was placed in liquid nitrogen for 24 hours, and the temperature was lowered to below 0 ℃ and measured.
3 Results and discussion
3.1 Screening results of magnetic nanoparticles
Because of the non-equilibrium characteristics of the low-temperature plasma arc method, the nanoparticles obtained by individuals have certain specifications with a typical range of 50-200 nm. The particles can be uniformly dispersed in anhydrous ethanol, turning out into a black solution. Different sizes of particles have a different interactions with solvent, and the small particles for the strong interaction need more time to sedimentation. In the experiment, 0.263 g (15.3%) of the product was lost. The result is shown in Table 1 and Fig.1. The method of screen based on the interaction between particles and solvents can grade nanoparticles so that the uniform size of the particles can be obtained.
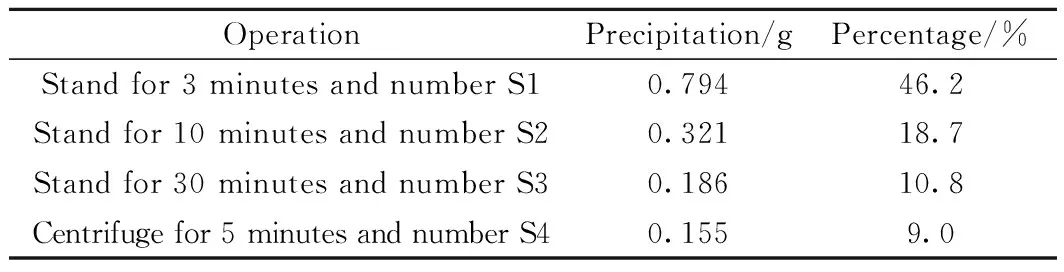
Table 1 Screening results of iron-nickel nanoparticles
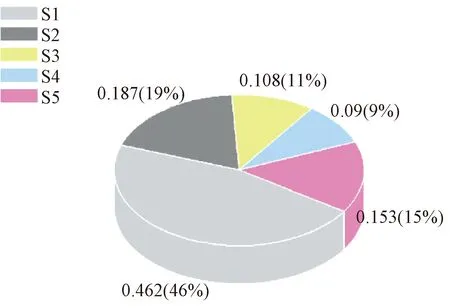
Fig.1 Screening results of magnetic nanoparticles
3.2 X-ray diffraction peak (XRD) analysis of the nanocomposite
XRD was performed on the Rigaku D/Max-2400 transilluminator equipped with Cu Kα radioation. The scanning angle 2θ was 10-85°, and the scanner stride was 0.02°. The recorded XRD pattern was compared with the standard PDF card. The XRD result suggests that the composition of the prepared nanoparticles contains FeNi3, Ni, and Fe2.5C6. Carbon was derived from anhydrous ethanol. At high temperatures, metal atoms or clusters evaporate and ethanol vapors dissociate into C, H, and O atoms, which in turn promoted metal evaporation. Because the total contact area between the shape memory alloy and the graphite crucible is not large, most of the carbon comes from alcohol, not from the crucible, but is concentrated in the charge and discharge area instead of the solution. According to Fig.2(a), the iron-nickel alloy nanoparticles are composed of Fe, Ni, and C. The carbon content is extremely low.
3.3 Magnetic properties
The accurate magnetic measurement results are shown in Fig.2(b). The iron-nickel nanoparticles are superparamagnetic, and the saturation magnetization (Ms) is 80 emu/g. The higher the magnetization, the better the actual effect and high heating efficiency. Compared with the 20 nm magnetic metal oxide nanoparticles whose Ms is generally in the range of 60 - 70 emu/g, the prepared iron-nickel alloy particles mainly exhibit higher Ms.
3.4 Transmission electron microscopy (TEM) charac-terization
TEM photographs are shown in Fig.3. As shown in Fig.3(a), iron-nickel nanoparticles of S4 have the excellent dispersing ability and a spherical shape with an average particle size of 100 nm. The spherical shape is an iron-nickel alloy core with an average particle size of 70 nm and a silica shell with a thickness of 15 nm. The dark brown lined loops are carbon because the application of volatile alcohol will dissociate the carbon package, and the thickness of the shell is not large, as shown in Fig.3(b). The lightly saturated ring is the silica casing. The thickness of the silica casing is between 5 and 20 nm. The thickness of most silica layers is 15 nm. The average particle size is 100 nm.
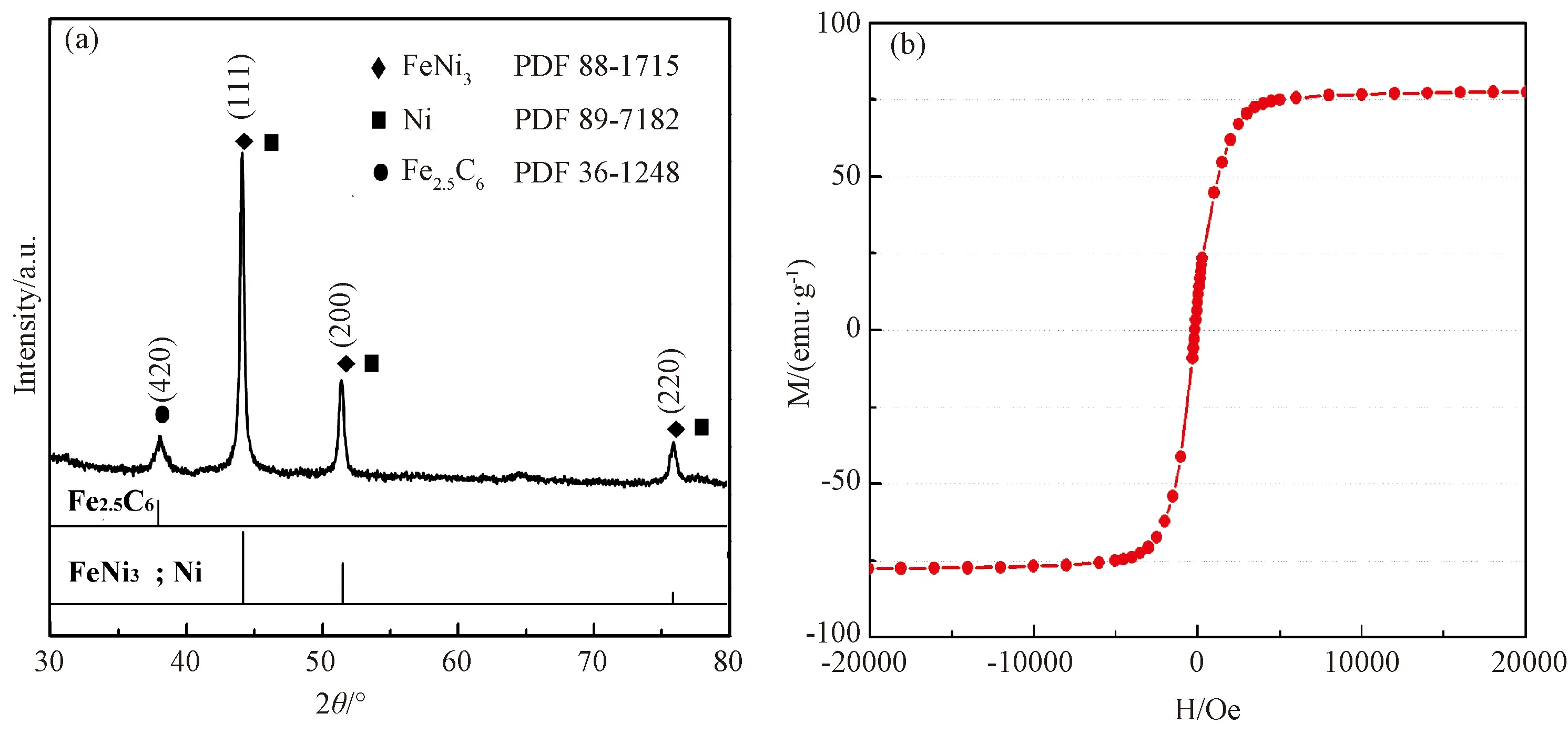
Fig.2 Iron-nickel nanoparticles S4 of XRD spectrum (a) and magnetic-hysteresis loops (b)
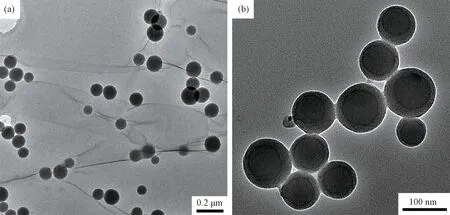
Fig.3 Transmission electron microscope photographs of iron-nickel silica nanocomposite particles S4. (a) at low magnification (b) at high magnification
3.5 Magnetic thermal characterization of magnetic nanoparticles
The key to the high efficiency of magnetic-thermal with magnetic nanoparticles lies in the current intensity and the frequency of the alternating magnetic field and the characteristics of the nanoparticles themselves[10]. Fig.4 shows the magnetic thermal efficiency of iron-nickel nanocomposite particles in the normal temperature range. The nanoparticles exhibit better thermal efficiency at a magnetic field generalized by the current of 40 A than that of 25.5 A. As shown in Fig.4(a), when the solvent was Vs55, the temperature increases from zero to 51.2 ℃ only within 300 seconds and the heating rate is 10.2 ℃/min. At a magnetic field generalized by the current of 25.5 A, there is an obvious decrease in Vs55 with the heating rate of 5.5 ℃/min. As shown in Fig.4(b), when the solvent is pure water, the average temperature of the nanoparticles increases by 3.6 ℃/min (the specific absorption rate 53 W/g can be calculated[11], I25.5 A). As the polarity of the solvent increases, the magnetocaloric efficiency of the magnetic nanoparticles decreases. Compare with the iron particles that have been proved to be highly effective in into hyperthermal (specific absorption rate of 602 W/g can be calculated) within 2 min once in vivo photothermal by Chang[12]etal, longer thermal time is needed for iron-nickel nanocomposite. For the application of magnetic hyperthermia, the specific absorption rate of 53 W/g is a critical value within 20 minutes.
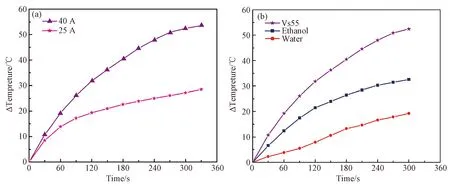
Fig.4 Magnetocaloric efficiency of iron-nickel silica nanocomposite particles S4 (in normal temperature range) at magnetic fields generalized by in different currents (a) and solvents (b).
In the low-temperature range, the temperature rises from -38.6 ℃ to -23 ℃ within 120 s at the average heating rate of 7.8 ℃/min (specific absorption rate of 115 W/g, I40 A) which is shown in Fig.5(b). In the presence of iron-nickel alloy nanoparticles (10 mg/ml), the temperature of Vs55 rises rapidly, and the average heating rate is 61.8 ℃/min, which is about 7.9 times as much as that of water (Fig.5(a)). The heating behavior of Vs55 was extremely different from water. Scientific research[13-14]explained: the faster the cooling rate, the more beneficial it is to prevent the glassy devitrification of Vs55. Conversely, in the whole process of heating, the faster the heating rate, the more beneficial the warming of human organs, thereby preventing physiological damage. The results show that Vs55 has a significant temperature transformation capability in the presence of iron-nickel alloy nanotechnology composite particles. The application of iron-nickel nanoparticles as a temperature activating agent for tissue rewarming is promising.
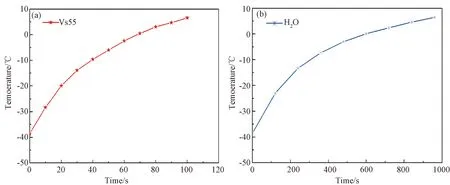
Fig.5 Magnetocaloric efficiency of iron-nickel silica nanocomposite particles S4 (in low temperature range) in Vs55(a) and water (b) at a magnetic field generalized by the current of 40 A
4 Conclusions
1. Iron-nickel nanoparticles were prepared by high-temperature arc method following the process of classification and sedimentation, iron-nickel particles with uniform morphology of 80 nm and 155 mg were obtained. By the sol-gel method, iron-nickel silica nanocomposites coated with 15 nm silica shells were obtained. The saturation magnetization (Ms) was measured to be 80 emu/g.
2. The magnetocaloric effects were measured in a high-frequency induction heating machine. As the current increased from 25.5 A to 40 A, the magnetocaloric efficiency of the magnetic nanoparticles increases. With the increase in solvent polarity, the magnetoelectric efficiency of magnetic nanoparticles is reduced. Under the high-temperature standard of simulation, Fe-Ni silica nanoparticles mainly show the high efficiency of magneto-caloric and the average heating rate is 3.6 ℃/min (specific absorption rate of 53 W/g, I25.5 A) which is a critical value for magnetic hyperthermia. In the simulated condition of tissue rewarming, iron-nickel silica nanoparticles exhibit the prominent magnetocaloric efficiency with an average heating rate of 61.8 ℃/min in the presence of Vs55, which is nearly 7.9 times of the water.

