Syntheses and structural characterizations of two lanthanide complexes containing bisphosphate ligands
HU Cong, LU Yanlei, WANG Zhiqiang, LI Yingyu, JIN Qionghua
(Department of Chemistry, Capital Normal University, Beijing 100048, China)
Abstract: Two new lanthanide(III)complexes[Nd2L3(NO3)6]n(1)and[CeL(Phen)(NO3)3]n(2)(L = tetraisopropyl 1,2-ethylenediphosphonate, Phen = 1,10-phenanthroline)were synthesized in a mixed solvent of CH3CN and CH3CH2OH, and characterized by single-crystal X-ray diffraction, elemental analysis, IR spectroscopy, 31P NMR spectroscopy and thermal analyses.Complex 1 was obtained by mixing Nd(NO3)3·6H2O and L in the ratio of 2∶3.The O atoms provided by tetraisopropyl 1,2-ethylenediphosphonate and nitrate ion coordinated with Nd3+ ion to form a nine coordination configuration.Complex 1 was formed into a two-dimensional network structure through bridged bisphosphonate ligands.Unlike Complex 1, complex 2 was obtained by mixing Ce(NO3)3·6H2O, L and Phen in the ratio of 1∶1∶1.Complex 2 was connected by L to form a one-dimensional infinite chain, and then formed a three-dimensional stacking structure with the help of a large number of weak forces.
Keywords: lanthanide complexes; tetraisopropyl 1,2-ethylenediphosphonate; 1,10-phenanthroline
0 Introduction
Lanthanide coordination chemistry, a branch of coordination chemistry, has been gradually developed in the second half of the 20th century[1].Unlike transition metals, lanthanide metals belong to the f-block elements and have strong chemical activity.Lanthanide metals usually have soft texture and good ductility.The homologous outer electronic structure makes their properties very similar.Because of their excellent physical and chemical properties, lanthanide metals are used in the fields of luminescent materials[2], medicine[3], catalysis[4]and so on.They are indispensable resources for the production of various advanced materials, that is why they are called “industrial gold”.Lanthanide elements show the unique properties of lanthanide contraction.From La to Lu, the radius of ions decreases with the increase of atomic number, but the radius between adjacent elements does not change greatly, which makes the separation of lanthanide elements very difficult[5].Due to the unique electronic configuration of lanthanide elements, lanthanide complexes have the characteristics of long fluorescence lifetime and a large number of independent 4f electrons, and gradually occupy a place in the fields of light[6], electricity[7]and magnetism[8].

In this paper, two novel lanthanide complexes, namely[Nd2L3(NO3)6]n(1)and[CeL(Phen)(NO3)3]n(2), have been synthesized and characterized by X-ray diffraction, elemental analysis, IR spectroscopy,31P NMR spectroscopy and thermal analyses.
1 Experiments
1.1 Materials and measurements
The reagents and solvents used in the experiment were purchased commercially without further purification.The crystal data was collected by Bruker SMART CCD X-ray single crystal diffractometer and analyzed by SHELEXL program; Elemental analysis(C, H, N)was carried out on an Elementar Vario MICRO CUBE(Germany)element analyzer; FT-IR spectra(KBr pellets)were measured on a Bruker EQUINOX 55 fourier transform infrared spectrometer; NMR experiments were carried out on a VNMRS-600 spectrometer using CDCl3as solvent.
1.2 Synthesis of complexes
Synthesisofcomplex[Nd2L3(NO3)6]n(1).A mixture of Nd(NO3)3·6H2O(0.087 6 g, 0.2 mmol)and L ligand(0.107 5 g, 0.3 mmol)were dissolved in the mixed solvent of 4 mL CH3CN and 6 mL CH3CH2OH.The mixture was stirred at room temperature for 6 hours and filtered into a 20 mL sample bottle.The sample bottle was placed in a wide-mouth bottle containing a certain amount of ether, sealed for about 2 weeks, and a small amount of purple crystals were obtained.Yield(calculated by Nd): 16.3%.Element analysis Calcd.for C42H94N6Nd2O36P6(%): C, 29.10; N, 4.85; H, 5.47; Found(%):C, 29.13; N, 4.87; H, 5.43.IR data(cm-1, KBr pellets): 3 618(w), 3 602(w), 1 592(w), 1 483(m), 1 424(m), 1 384(s), 1 302(m), 1 221(m), 1 103(w), 1 030(m), 842(m), 815(w), 766(w), 725(m), 638(w), 417(w).31P NMR(243 MHz, CDCl3):δ27.44(s, phosphorus from L).
Synthesisofcomplex[CeL(Phen)(NO3)3]n(2).A mixture of Ce(NO3)3·6H2O(0.086 8 g, 0.2 mmol)and L ligand(0.071 7 g, 0.2 mmol)were dissolved in the mixed solvent of 4 mL CH3CN and 6 mL CH3CH2OH, and Phen ligand was added after stirring the mixture at room temperature for 1 hour.Then the mixture was stirred for 6 hours and filtered.The solvent was evaporated at room temperature until colorless transparent crystals appeared 3 weeks later.Yield(calculated by Ce): 25.4%.Element analysis Calcd.for C26H40CeN5O15P2(%): C, 36.16; N, 8.11; H, 4.67; Found(%): C, 36.13; N, 8.15; H, 4.65.IR data(cm-1, KBr pellets): 3 436(m), 2 980(w), 1 621(w), 1 472(s), 1 381(s), 1 323(s), 1 209(m), 1 183(m), 1 141(w), 1 113(w), 1 002(s), 899(w), 846(w), 790(w), 737(w), 630(w), 510(w), 414(w).31P NMR(243 MHz, CDCl3):δ27.43(s, phosphorus from L).
1.3 X-ray crystallography
Single crystals of the title complexes were mounted on a Bruker Smart 1000 CCD diffractometer equipped with a graphite-monochromated CuKα(λ= 0.154 184 nm)radiation at 298 K.Semi-empirical absorption corrections were applied using SADABS program[16].All the structures were solved by direct methods using SHELXS program of the SHELXTL-97 package and refined with SHELXL-97[17].The final refinements were performed by full matrix least-squares methods with thermal parameters for non-hydrogen atoms.The pores and weak forces in the molecular space stacking structure were calculated by using the Platon program.
The crystallographic data and structure refinement data of complexes1and2have been listed in Table 1 in detail.CCDC No.2069756 and 2069755 contained the supplementary crystallographic data for complexes1and2, respectively.These data were obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.

Table 1 Crystallographic data for complexes 1 and 2
2 Results and discussion
2.1 Synthesis of the complexes
Complex[Nd2L3(NO3)6]n(1)was synthesized by using tetraisopropyl 1,2-ethylenediphosphonate and lanthanide nitrate as raw materials, reacting in the mixed solution of ethanol and acetonitrile, filtering and gas-phase diffusion with ether.Later, on this basis, nitrogen-containing ligand 1,10-phenanthroline was introduced, and[CeL(Phen)(NO3)3]n(2)was synthesized under the same experimental conditions but without ether diffusion.However, when the reaction solvent or reaction conditions were changed, the crystals could not be obtained.Many experiments using heavy lanthanides did not get crystals, but oil like compounds, which are similar to other diphosphonate complexes[18-19].

Scheme 1 Structures of the ligands used in the present work

Scheme 2 Routine of synthesis for complexes 1 and 2
2.2 Crystal structure


Fig.1 Structural unit of complex 1(All H atoms have been omitted)

Fig.2 Coordination environments of complex 1

Table 2 Selected bond distances and bond angles for complex 1
In complex1, each L ligand is a bridging ligand, and each Nd3+ion is connected to three adjacent Nd3+ions through bisphosphonate ligands to form a two-dimensional network structure, as shown in Fig.3.When using the Platon program to analyze the weak force of complex1, it is found that there are five kinds of intramolecular hydrogen bonds in the two-dimensional network structure.Among them, C6—H6…O16, C13—H13…O4, C30—H30A…O12 are the hydrogen bonds formed inside the bisphosphonate ligands, and the others are formed by the bisphosphonate ligands and O atoms in the coordinated nitrate ions.The combined influence of these five hydrogen bonds and the coordination environments cause the coordinated bisphosphonate ligands to be distorted to a certain extent.The intramolecular hydrogen bonds have been marked in Fig.4.The detailed data of weak interactions are shown in Table 3.

Fig.3 Two-dimensional network structure of complex 1(All H atoms have been omitted)

Fig.4 Intramolecular hydrogen bonds in the structural unit of complex 1(Most H atoms and some C atoms have been omitted)
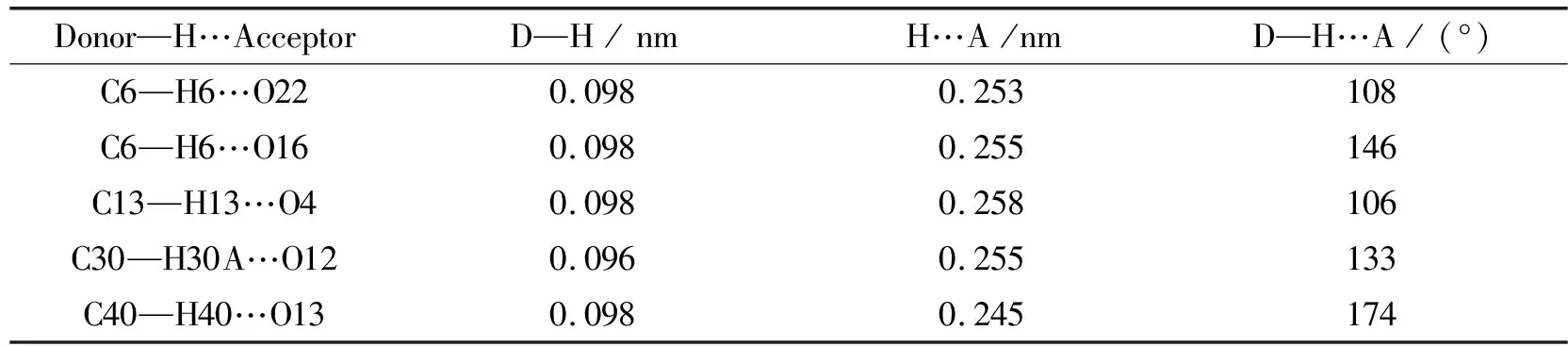
Table 3 Intramolecular hydrogen bonds in the supramolecular structure of complex 1


Fig.5 Structural unit of complex 2(All H atoms have been omitted)
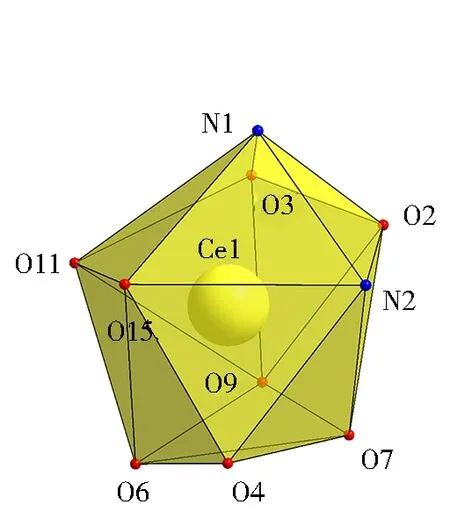
Fig.6 Coordination environment of complex 2
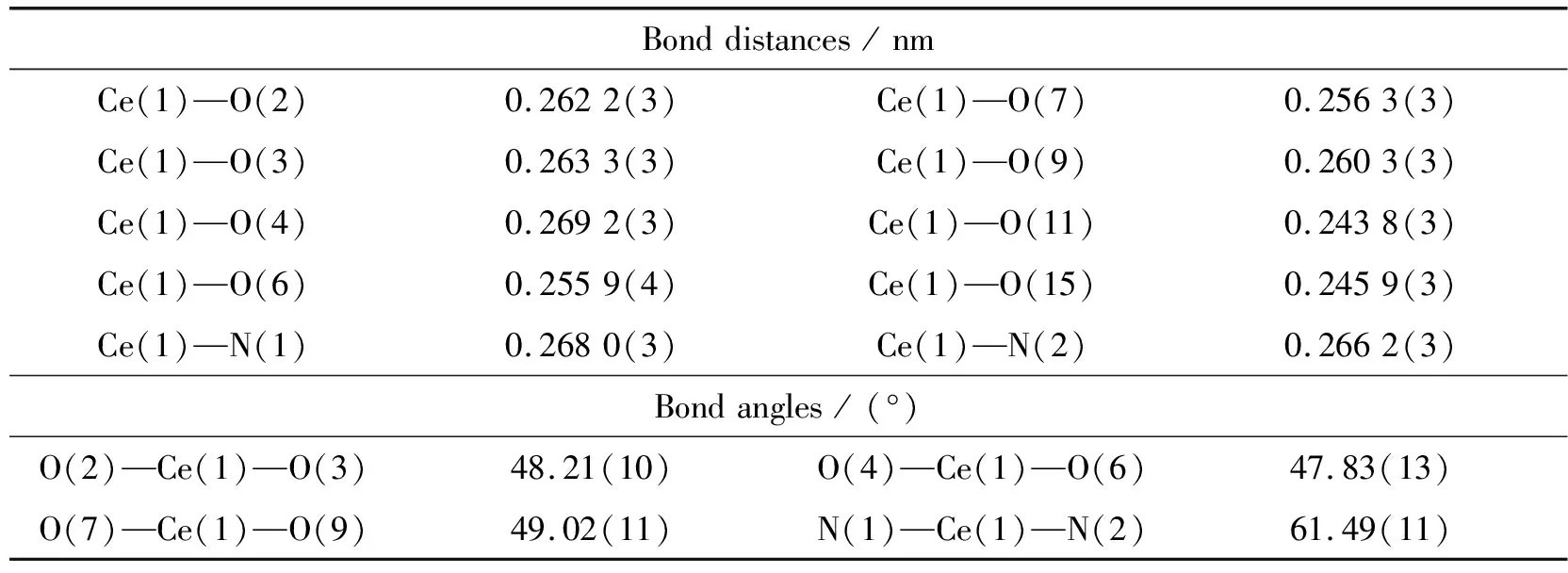
Table 4 Selected bond distances and bond angles for complex 2
According to the analysis of Platon software, complex2has a three-dimensional stacking structure, and the chains bridged by the bisphosphonate ligands are connected by two kinds of intermolecular hydrogen bonds to form a three-dimensional structure.The O atoms forming these two hydrogen bonds come from nitrate ions and the H atoms are all located on the benzene ring of Phen, as shown in Fig.7.There is an intramolecular C—H…π interaction in the stacking structure, which is formed by the interaction between the H atoms from the bisphosphonate ligand and the benzene ring of Phen.In addition, there are three kinds of intramolecular hydrogen bonds, C1—H1…O4(0.258 nm), C20—H20A…O3(0.252 nm), C25—H25…O15(0.255 nm).The existence of a large number of weak interactions makes the crystal structure more stable and distorts the structure of bisphosphonate ligand at the same time.The weak interactions in the complex are listed in Table 5.

Fig.7 Three-dimensional network structure of complex 2(Some atoms have been omitted)

Table 5 Weak interactions in complex 2
2.3 IR spectra

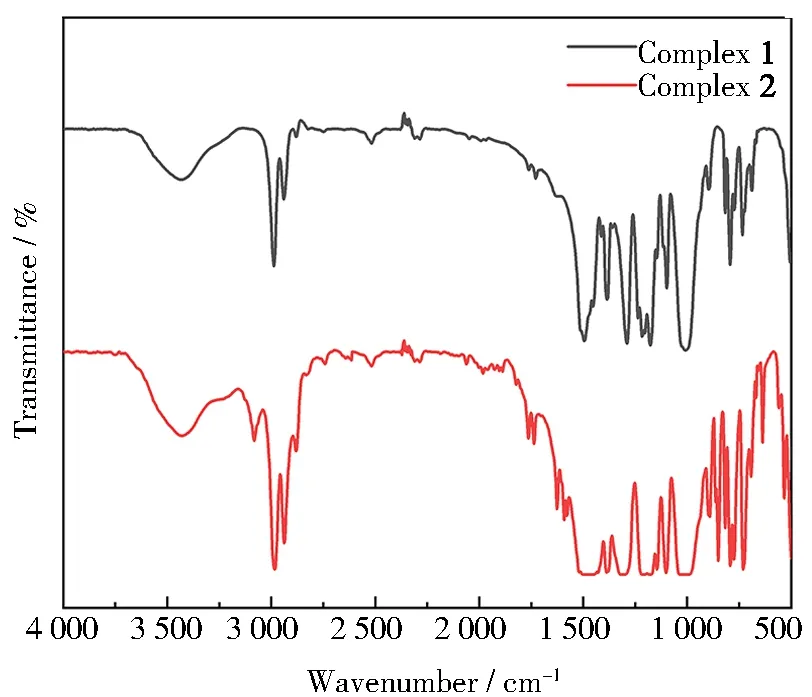
Fig.8 IR spectra of complexes 1 and 2
2.4 UV-Vis spectra
The UV absorption spectrum of complex2is shown in Fig.9.The complex exhibits primary absorption peaks in the range of 200~300 nm.The strongest absorption peak of Phen ligand is at 229 nm[25].The strongest absorption peak of Phen in complex2produces a corresponding blue shift from 229 nm to 225 nm, which suggests the electron distribution of the conjugated system in Phen changes when it is coordinated with Ce3+.The absorption spectrum of the complex shows the intense absorption peaks at 225 nm and 264 nm, which demonstrates that the coordination between Ce3+and Phen ligand forms a more extensive conjugated system[25].

Fig.9 UV-Vis spectrum of complex 2
2.5 TGA curves
In order to check the thermal stability of the two complexes, thermal gravimetric analysis(TGA)is shown in Fig.10.It is clear from the TG curves that two complexes undergo a single-step decomposition and start to decompose at 190 and 204 °C respectively, which proves that there are no solvent molecules in the crystal.

Fig.10 TG curves of two complexes
3 Conclusions
Two novel lanthanide complexes, namely[Nd2L3(NO3)6]n(1)and[CeL(Phen)(NO3)3]n(2), have been synthesized and characterized by single-crystal X-ray diffraction, elemental analysis,31P NMR spectroscopy, and thermal gravimetric analysis.These two complexes have not been obtained in other solvents or reaction conditions, which proves that the solvent system and reaction environments have a great influence on the formation of the complexes, which will be beneficial to the design and synthesis of the complexes in the future.Through IR spectra, the changes of characteristic absorption peaks can be found, and it can be concluded that the coordination reaction may affect the ligand skeleton vibration.The research on the reaction conditions of bisphosphonate ligands and the design of new bisphosphonate ligands will continue.

