SIRT1参与硫化氢改善同型半胱氨酸诱导的海马氧化应激损伤
魏海军 王玥 胡亚 赵美 伍爱荣 赵忠桂 张庆丽

[摘要] 目的 探討沉默信号调节因子1(SIRT1)是否参与硫化氢(H2S)改善同型半胱氨酸 (Hcy)诱导的海马氧化应激损伤。 方法 随机将雄性SD大鼠分成9组,即正常(CON)组、Hcy(0.2 μmol)(H1)组、Hcy(0.6 μmol)(H2)组、Hcy(2.0 μmol)(H3)组、Hcy(0.6 mol)+NaHS(30 μmol/kg)(H2+N1)组、Hcy(0.6 μmol)+NaHS(100 μmol/kg)(H2+N2)组、正常+NaHS(100 μmol/kg)(C+N2)及Hcy(0.6 μmol)+NaHS(100 μmol/kg)+ SIRT1的抑制剂Sirtinol(10 nmol)(H2+N2+S)组、正常+Sirtinol(10 nmol)(C+S)组,每组10只。采用侧脑室微量注射Hcy建立Hcy神经毒性模型,并在Hcy注射前2 d以腹腔注射NaHS及侧脑室注射Sirtinol 共同预处理 SD大鼠2 d,然后与 Hcy共处理7 d;各种试剂盒检测大鼠海马总抗氧化能力(T-AOC)、超氧化物歧化酶(SOD)和丙二醛(MDA)含量。 结果 与CON 组比较,H1、H2组大鼠海马T-AOC、SOD的含量下降(P<0.01)及MDA表达水平升高(P<0.001);与H2组比较,H2+N2组大鼠海马T-AOC、SOD的含量明显上升(P<0.01)及MDA表达水平明显降低(P<0.01)。此外,与H2+N2组比较,H2+N2+S组大鼠海马T-AOC、SOD的含量明显下降(P<0.01)及MDA表达水平明显上升(P<0.01)。 结论 SIRT1 参与H2S减轻Hcy导致的海马氧化应激损伤。
[关键词] SIRT1;硫化氢;同型半胱氨酸;海马氧化应激损伤
[中图分类号] R745.1 [文献标识码] A [文章编号] 1673-9701(2021)36-0033-04
SIRT1 participation in hydrogen sulfide to improve homocysteine-induced hippocampal oxidative stress damage
WEI Haijun WANG Yue HU Ya ZHAO Mei WU Airong ZHAO Zhonggui ZHANG Qingli
Medical School, Hunan Polytechnic of Environment and Biology, Hengyang 421005, China
[Abstract] Objective To explore whether Silent Signal Regulator 1 (SIRT1) participates in hydrogen sulfide (H2S) to improve homocysteine (Hcy)-induced hippocampal oxidative stress damage. Methods Male SD rats were randomly divided into 9 groups, as the normal (CON) group, the Hcy (0.2 μmol) (H1) group, the Hcy (0.6 μmol) (H2) group, the Hcy (2.0 μmol) (H3) group, the Hcy (0.6 mol)+NaHS (30 μmol/kg) (H2+N1) group, the Hcy (0.6 μmol)+NaHS (100 μmol/kg) (H2+N2) group, the normal+NaHS (100 μmol/kg) (C+N2) group, the Hcy (0.6 μmol)+NaHS (100 μmol/kg)+SIRT1 inhibitor Sirtinol (10 nmol) (H2+N2+S) group, and the normal+Sirtinol (10 nmol) (C+S) group, with 10 rats in each group. Microinjection of Hcy into the lateral ventricle was used to establish the neurotoxicity model of Hcy. Two days before Hcy injection, NaHS was injected into the intraperitoneal cavity and Sirtinol was injected into the lateral ventricle to pretreat SD rats for 2 days. Then, the rats were co-treated with Hcy for 7 days. Various kits were used to detect contents of hippocampal total antioxidant capacity (T-AOC), superoxide dismutase (SOD) and malondialdehyde (MDA) in the rats. Results Compared with the CON group, the contents of hippocampal T-AOC and SOD of the H1 and H2 groups were decreased (P<0.01), and the MDA expression levels were increased (P<0.001). Compared with the H2 group, the contents of hippocampal T-AOC and SOD of the H2+N2 group were increased significantly (P<0.01), and the MDA expression level was decreased significantly (P<0.01). Compared with the H2+N2 group, the contents of hippocampal T-AOC and SOD of the H2+N2+S group was significantly decreased (P<0.01), and the MDA expression level was significantly increased (P<0.01). Conclusion SIRT1 participates in H2S to reduce Hcy-induced hippocampal oxidative stress damage.
[Key words] SIRT1; Hydrogen sulfide; Homocysteine; Hippocampal oxidative stress damage
同型半胱氨酸(Homocysteine,Hcy)可引起神经细胞的炎症反应[1]。此外,研究发现Hcy可导致小鼠神经变性[2]。而硫化氢(Hydrogen sulfide,H2S)是一种内源性气体分子,具有神经保护作用[3]。Kang等[4]报道,H2S对Hcy导致的神经细胞衰老具有抑制作用。而在学习和记忆的形成中,沉默信号调节因子1(Silent information regulator,SIRT1)是必需的。Tang等[5]发现,H2S可减轻Hcy引发的大鼠学习记忆损伤,其机制与上调SIRT1表达有关。本研究将从氧化应激的新视角,观察SIRT1 是否与H2S减轻Hcy导致的海马氧化应激损伤有关,现报道如下。
1 资料与方法
1.1一般资料
1.1.1试剂和仪器 NaHS、Hcy及各种检测试剂盒(Sigma公司)。微量注射泵、侧脑室注射用系列套管及大鼠脑立体定位仪(美国 Stoelting 公司)。电子分析天平(日本岛津公司)。
1.1.2 实验动物 购买雄性 SD 大鼠(长沙斯莱克景达实验动物有限公司,260~300 g),将其饲养1周,给予自由饮水及饮食。实验的操作均符合中华人民共和国国家科学技术委员会颁布的《实验动物管理条例》中的规定,并经本单位医学实验动物伦理委员会批准。
1.1.3 药品的配制 ①Hcy注射液的制备:用天平量取 0.33 645 g 固态Hcy,将其放入5 mL生理盐水中,使其充分溶解成为0.5 μmol/μL的母液;②NaHS注射液的制备:用天平量取 100 mg 固态硫氢化钠,将其放入1 mL双蒸水中,使其充分溶解成为100 mg/mL的母液。将二者都保存在 -20℃ 冰箱,备用。
1.2 方法
1.2.1 动物分组 随机将雄性SD大鼠分成9组,即正常(CON)组、Hcy(0.2 μmol)(H1)组、Hcy(0.6 μmol)(H2)组、Hcy(2.0 μmol)(H3)组、Hcy(0.6 mol)+NaHS(30 μmol/kg)(H2+N1)组、Hcy(0.6 μmol)+NaHS(100 μmol/kg)(H2+N2)組、正常+NaHS(100 μmol/kg)(C+N2)及Hcy(0.6 μmol)+NaHS(100 μmol/kg)+SIRT1的抑制剂Sirtinol(10 nmol)(H2+N2+S)组、正常+Sirtinol(10 nmol)(C+S)组,每组10只。
1.2.2 给药方法 H1、H2、H3组连续7 d侧脑室给药Hcy。确定H2组为损伤浓度后,在大鼠侧脑室给药Hcy前2 d,对其进行腹腔注射NaHS(30 μmol/kg或100 μmol/kg),连续 9 d。确定NaHS保护组浓度为100 μmol/kg后,再用Sirtinol(10 nmol/d,icv)及NaHS (100 μmol/kg/d,ip)共同预处理 SD大鼠2 d,然后与 Hcy (0.6 μmol /d,icv)共处理7 d。
1.2.3试剂盒测定大鼠海马中T-AOC、SOD和 MDA表达水平 取9倍体积的 0.9%氯化钠溶液放入各组大鼠的海马组织中,以制备海马组织匀浆,将其离心后取上清,并采取BCA 蛋白定量试剂盒测定蛋白浓度后,再按相应试剂盒说明书上的步骤进行检测。
1.3观察指标及评价标准
观察指标:各种试剂盒测定大鼠海马中T-AOC、SOD和 MDA表达水平。
评价标准:各组比较T-AOC、SOD和 MDA的含量高低,T-AOC、SOD含量降低和MDA含量越高代表氧化应激损伤程度越严重。
1.4 统计学方法
采用SPSS 20.0统计学软件对数据进行分析,计量资料采用均数±标准差(x±s)显示。组间比较及随机成组设计分别用LSD-t检验和单因素方差分析。P<0.05为差异有统计学意义。
2 结果
2.1 Hcy可减少大鼠海马组织中T-AOC、SOD的含量及增加MDA水平
与CON 组比较,H1、H2组大鼠海马T-AOC、SOD的含量下降(P<0.01)及MDA表达水平升高(P<0.001),提示 Hcy可引发大鼠海马氧化应激损伤。见表1。
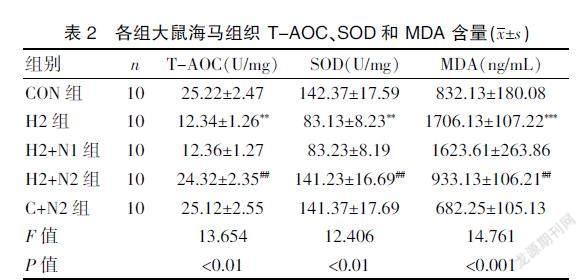
2.2 H2S 可拮抗Hcy降低大鼠海马组织中T-AOC、SOD的含量及升高MDA含量的作用
与H2组比较,H2+N2组大鼠海马T-AOC、SOD的含量明显上升(P<0.01)及MDA表达水平明显降低(P<0.01),说明H2S可减轻Hcy引发的大鼠海马氧化应激损伤。见表2。
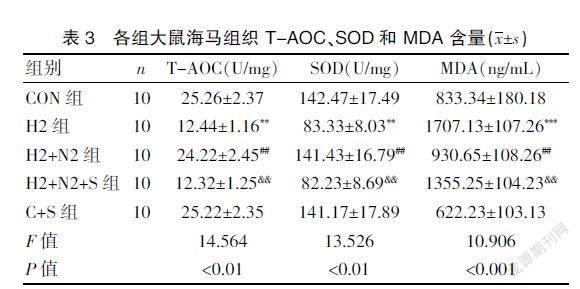
2.3 Sirtinol 可逆转 H2S 对 Hcy 诱导大鼠海马组织中T-AOC、SOD的含量下降及 MDA 水平增加的抑制作用
与H2+N2组比较,H2+N2+S组大鼠海马T-AOC、SOD的含量明显下降(P<0.01)及MDA表达水平明显上升(P<0.01),说明Sirtinol 取消了H2S 可减轻Hcy 引发海马氧化应激损伤的作用,提示SIRT1 参与H2S减轻Hcy引发的海马氧化应激损伤。见表3。
3 讨论
研究证实 Hcy 可损伤PC12细胞[6]及引起动物大脑的线粒体功能障碍[7]。Kumar[8]等报道H2S 可通过增加内源性硫化氢水平来减轻Hcy引起的神经化学变化。此外,研究发现H2S可改善 Hcy 的神经细胞毒性[9]及减弱 Hcy 诱导的SD 大鼠学习记忆能力损害[10]。但是H2S的这种作用,需进一步阐明其机制,氧化应激是引起神经系统方面疾病的一个重要因素。T-AOC含量越高代表组织总抗氧化能力越好,此外,SOD是机体内重要的抗氧化酶,其活性越高代表组织的抗氧化应激能力越强,而MDA是一种氧化应激中脂质过氧化的重要中间产物,可反映海马组织氧化损伤的程度[11]。本研究发现,与CON 组比较,H1、H2组大鼠海马T-AOC、SOD的含量下降及MDA含量增加,而与H2组相比,H2+N2组大鼠海马T-AOC、SOD的含量明显上升而MDA含量明显下降,表明硫化氢可减轻Hcy导致的氧化应激。SIRT1在神经保护方面起着重要作用,研究发现棕榈酸依赖的NAD+耗竭会引起SIRT1的功能障碍,并进一步可能刺激Aβ的产生[12]。Tang等[13]发现五味子酚可改善东莨菪碱所致的AD小鼠认知功能障碍,其机制是与激活SIRT1-PCG1α信号及抑制tau蛋白的磷酸化有关。SIRT1还可调节海马氧化应激损伤[14],本研究发现与H2+N2组比较,H2+N2+S组大鼠海马T-AOC、SOD的含量下降及MDA表达水平明显上升。这说明Sirtinol 取消了H2S 可减轻Hcy引发海马氧化应激损伤的作用,也提示SIRT1 参与H2S减轻Hcy引发的海马氧化应激损伤。此外,顾依静等[15]研究发现,在大鼠H9c2心肌细胞中,用过氧化氢诱导氧化应激模型时,H2S可提高SIRT1的活性及减少MDA的含量。贾强等[11]发现H2S可通过抑制海马组织氧化应激损伤来减轻糖尿病大鼠的学习记忆障碍。此外,Tabassum等[16]报道H2S可通过影响线粒体氧化应激发挥抗氧化功能,Kang等[4]提出H2S可减轻Hcy导致的神经细胞衰老,其机制与SIRT1表达有关。这提示了SIRT1在H2S抗氧化应激损伤中的作用不可忽视,值得进一步深入研究,以明确SIRT1的具体作用机理。
此外,脑源性神经营养因子(Brain-derived neu-rotrophic factor,BDNF)是一种神经保护因子,研究表明H2S可减轻甲醛(Formaldehyde,FA)诱导的大鼠认知功能障碍,其机制是与调节BDNF表达有关[17],同时,Liu等[18]发现BDNF-TrkB通路介导了H2S在糖尿病大鼠中的抗抑郁作用,其机制是通过促进海马自噬,此外,Fahimeh等[19]报道在出生后早期的雄性幼鼠中给予酒精,会引起幼鼠的空间记忆障碍,但是H2S可通过增加BDNF水平来减轻幼鼠的空间记忆障碍。以上提示BDNF也可能在H2S改善Hcy诱导的海马氧化应激损伤中发挥作用。
综上所述,SIRT1参与了H2S改善Hcy导致的海马氧化应激损伤还需更加深入地探讨,本研究为H2S拮抗Hcy神经毒性机制的研究提供了新思路,也为探讨出更多防治与Hcy相关的神经退行性疾病提供了新靶点。
[参考文献]
[1] Kumar M,Sandhir R. Hydrogen sulfide suppresses homocysteine-induced glial activation and inflammatory response[J]. Nitric Oxide,2019,90:15-28.
[2] Nibendu N,Himanshu KP,Munish K. Cerebroprotective effects of hydrogen sulfide in homocysteine-induced neurovascular permeability:Involvement of oxidative stress,arginase,and matrix metalloproteinase-9[J]. J Cell Physiol,2019,234(3):3007-3019.
[3] Tang YY,Tang XQ. Research progress in the neurobiological effects of hydrogen sulfide[J]. Sheng Li Ke Xue Jin Zhan,2017,48(1):42-51.
[4] Kang X,Li C,Xie X,et al. Hydrogen Sulfide Inhibits Homocysteine-Induced Neuronal Senescence by Up-Regulation of SIRT1 [J]. Int J Med Sci,2020,17(3):310-319.
[5] Tang YY,Wang AP,Wei HJ,et al. Role of silent information regulator 1 in the protective effect of hydrogen sulfide on homocysteine-induced cognitive dysfunction: Involving reduction of hippocampal ER stress[J]. Behav Brain Res,2018,342:35-42.
[6] Wang CY,Zou W,Liang XY,et al. Hydrogen sulfide prevents homocysteine-induced endoplasmic reticulum stress in PC12 cells by upregulating SIRT-1[J]. Mol Med Rep,2017,16(3):3587-3593.
[7] Kumar M,Sandhir R. Hydrogen sulfide attenuates hyperhomocysteinemia-induced mitochondrial dysfunctions in brain[J]. Mitochondrion,2020,50:158-169.
[8] Kumar M,Modi M,Sandhir R. Hydrogen sulfide attenuates homocysteine-induced cognitive deficits and neurochemical alterations by improving endogenous hydrogen sulfide levels[J]. Biofactors,2017,43(3):434-450.
[9] Kumar M,Ray RS,Sandhir R. Hydrogen sulfide attenuates homocysteine-induced neurotoxicity by preventing mitochondrial dysfunctions and oxidative damage:In vitro and in vivo studies[J]. Neurochemistry International,2018, 120:87-98.
[10] Li M,Zhang P,Wei HJ,et al. Hydrogen sulfide ameliorates homocysteine-induced cognitive dysfunction by inhibition of reactive aldehydes involving upregulation of ALDH2[J]. Int J Neuropsychopharmacol,2017,20(4):305-315.
[11] 賈强,李焱,刘小粉.硫化氢对糖尿病大鼠空间学习记忆和海马组织氧化应激的影响[J]. 蚌埠医学院学报,2020,45 (4):447-451.
[12] Manuel FL,Martha PD,Rodrigo GB,et al. Palmitic acid-induced NAD+depletion is associated with the reduced function of SIRT1 and increased expression of BACE1 in hippocampal neurons[J]. Neurochem Res,2019,44:1745-1754.
[13] Tang YW,Shi CJ,Yang HL,et al. Synthesis and evaluation of isoprenylation- resveratrol dimer derivatives against alzheimer's disease[J]. Eur J Med Chem,2019,163:307-319.
[14] 石麗娜. Sirt1调节氧糖剥夺后海马神经元的氧化应激[D].郑州:郑州大学,2020.
[15] 顾依静,武丹,祝德秋.硫化氢通过SIRT1途径对大鼠H9c2心肌细胞的保护作用[J]. 江苏医药,2020,46(11):1085-1089.
[16] Tabassum R,Jeong NY. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases[J]. Int J Med Sci,2019,16(10):1386-1396.
[17] Li X,Zhuang YY,Wu L,et al. Hydrogen sulfide ameliorates cognitive dysfunction in formaldehyde-exposed rats:Involvement in the upregulation of brain-derived neurotrophic factor[J].Neuropsychobiology,2020,79(2):119-130.
[18] Liu HY,Wei HJ,Wu L,et al. BDNF-TrkB pathway mediates antidepressant-like roles of H 2 S in diabetic rats via promoting hippocampal autophagy[J]. Clin Exp Pharmacol Physiol,2020,47(2):302-312.
[19] Fahimeh M,Farzaneh B,Raheleh R,et al. Hydrogen sulfide improves spatial memory impairment via increases of BDNF expression and hippocampal neurogenesis following early postnatal alcohol exposure[J]. Physiol Behav,2020,215:112 784.
(收稿日期:2021-03-02)

