咖啡化学成分及其生物活性研究进展
沈晓静, 字成庭, 辉绍良, 杨俊滔, 王青, 曹梦婷, 范江平
咖啡化学成分及其生物活性研究进展
沈晓静a,b, 字成庭b*, 辉绍良b, 杨俊滔b, 王青b, 曹梦婷b, 范江平a*
(云南农业大学, a. 食品科学技术学院; b. 理学院,昆明 650201)
咖啡为茜草科(Rubiaceae)咖啡属()植物,位居世界三大饮品之首,具有降低血糖、保护肝脏和神经保护等作用。咖啡化学成分类别较多,包括生物碱、酚酸类、黄酮类、萜类等。咖啡中的化学成分是发挥其生物学功能和形成特色风味的基础,对其化学成分来源和生物活性进行综述,为进一步发展咖啡产业提供依据和理论支撑。
咖啡;化学成分;生物活性;研究进展
咖啡为茜草科(Rubiaceae)咖啡属()植物,原产于非洲中北部,主要分布在南美、中美洲、非洲和亚洲等少数国家。咖啡属有4组66种,通常所指的咖啡为真咖啡组()的大粒种()、中粒种()、小粒种()和埃塞尔萨种(),其中小粒咖啡气味香醇,品质较好[1]。1892年由法国传教士将咖啡引入云南省宾川县栽培,并不断扩大,目前中国的咖啡主要在云南和海南种植,且99%以上分布在云南[2],以德宏、普洱、保山、西双版纳、临沧为主,云南小粒种咖啡经国际咖啡组织品尝专家评鉴为世界高质量咖啡。此外,根据《中华本草》记载,咖啡微苦,涩,平;具有醒神、利尿、健胃的功效,主治精神倦怠、食欲不振,常作为醒神、利尿和健胃药使用。根据研究报道,咖啡中所含的化学成分主要包括生物碱、酚酸类、黄酮类、萜类、甾醇脂类和挥发性成分等,具有胰岛素增敏、改善糖代谢、抗糖尿病和肝保护作用等多种药理活性。本文对咖啡中的化学成分来源及其生物活性进行综述,旨在为进一步开发咖啡产业提供理论支撑。
1 咖啡的主要成分
1.1 生物碱
咖啡碱(1,3,7-三甲基黄嘌呤, caffeine)为咖啡果实中的主要生物碱成分,是咖啡苦味的来源, 广泛存在于茶叶、可可和咖啡中,是应用较为广泛的精神类药物之一。研究表明,咖啡碱具有缓解老年记忆力下降诱发的健忘症,同时还可以降低患阿尔茨海默病(AD)[3]、帕金森病(PD)[4]等神经退行性疾病的风险。Arendash等[5]的研究表明, 适量摄入咖啡碱可以抑制大鼠记忆力减弱; Zeitlin等[6]证实咖啡碱可能通过促进大脑纹状体和皮层细胞的存活和对细胞凋亡通路的抑制对AD起保护作用;Nakaso等[7]通过人骨髓神经母细胞瘤细胞株(SH-SY5Y) PD模型证实咖啡碱可以降低半胱氨酸的天冬氨酸蛋白水解酶(caspase)-3活性, 减少核碎片和凋亡凝聚的数量。咖啡碱还可以减少1-甲基-4-苯基-1,2,3, 6-四氢吡啶(MPTP)引起的血脑屏障(BBB)渗漏,抑制BBB功能紊乱[8]。常规剂量的咖啡碱有助于改善轻偏瘫性脑卒中。Sun等[9]报道咖啡碱通过其抗氧化性和抗炎性来实现对脑卒中的保护作用。咖啡因还与糖尿病的控制有关[10]。
葫芦巴碱(trigonelline)是一种吡啶衍生物,在咖啡烘焙过程中促进芳香化合物的形成,如N-甲基吡啶(NMP)就是咖啡烘焙过程中葫芦巴碱的热降解产物[11]。近年来关于葫芦巴碱的研究越来越多,生物价值越来越凸显。Liu等[12]报道葫芦巴碱可通过降低血糖,增加胰岛素细胞的表达,调节炎症反应;通过下调细胞凋亡蛋白酶3的表达,抑制细胞部分凋亡,提高抗氧化酶活性,从而对1型糖尿病小鼠起保护作用。人参皂苷Rb1和葫芦巴碱可通过调节mir-3550的表达,作用于Wnt/-catenin信号通路预防糖尿病肾损伤的发展[13]。葫芦巴碱对结肠炎的心脏组织具有潜在的治疗作用[14]。葫芦巴碱具有神经保护作用,是治疗神经退行性疾病的良好药物[15]。Fahanik-Babaei等[16]报道葫芦巴碱具有提高认知和缓解神经丢失的作用;可通过恢复肝脏自噬作用对高胆固醇和高脂饮食引起的肝脏脂质积累和脂肪毒性起到防止作用[17];可抑制胆碱的肠道微生物代谢及其相关心血管风险[18]。
此外,咖啡中还含有可可碱(theobromine)、茶碱(theophyline)和烟酸(nicotinic acid)。Joseph等[19]报道大果咖啡叶中还有1,3,7,9-四甲基尿酸(theacrine)、大果咖啡碱(liberine)、甲基大果咖啡碱(methylli- betine)(表1)。
1.2 酚酸及其衍生物
目前已从咖啡中分离得到对-羟基苯甲酸(- hydroxybenzoic acid)、香草酸(vanillic acid)、对-香豆酸(-coumaric acid)、阿魏酸(ferulic acid)、绿原酸(chlorogenic acid)、咖啡酸(caffeic acid)、咖啡酰奎宁酸(caffeoylquinic acid)、二咖啡酰奎宁酸(dicaffe- oylquinic acid)、3--阿魏酰奎宁酸(3--feruloyl- quinic acid)、3--阿魏酰-4--咖啡酰奎宁酸(3-- feruloyl-4--caffeoylquinic acid)、3--咖啡酰-4--阿魏酰奎宁酸(3--caffeoyl-4--feruloylquinic acid)等酚酸类及咖啡酸衍生物(表2)。
绿原酸(chlorogenic acid, CGA)是主要的酚酸类化合物,具有降血脂、抗氧化、抗菌等生物学功能。Nishi等[22]报道绿原酸可以显著降低胆固醇、甘油三酯、低密度脂蛋白,增加高密度脂蛋白;Xu等[23]证实绿原酸的活性和DNA保护作用。Shi[24]报道绿原酸能逆转录皮质酮(CORT)诱导的PC12细胞自噬和凋亡,同时还能调节PC12细胞的AKT/mTOR通路;Su等[25]报道绿原酸能抑制铜绿假单胞菌P1细胞的胞内代谢发挥抗菌作用。绿原酸能有效降低Cd在空肠的吸收和积累,保护肠道屏障[26]。人体口服葡萄糖2 h的血糖试验证实绿原酸和葫芦巴碱有降低早期葡萄糖和胰岛素反应的作用[27]。同时, 在咖啡烘焙过程中CGA受热降解导致苦味酚类化合物和酚类芳香化合物的形成,CGA还可通过掺入蛋白黑素的骨架参与咖啡颜色的形成,是造成咖啡色素沉着和涩味的主要原因。
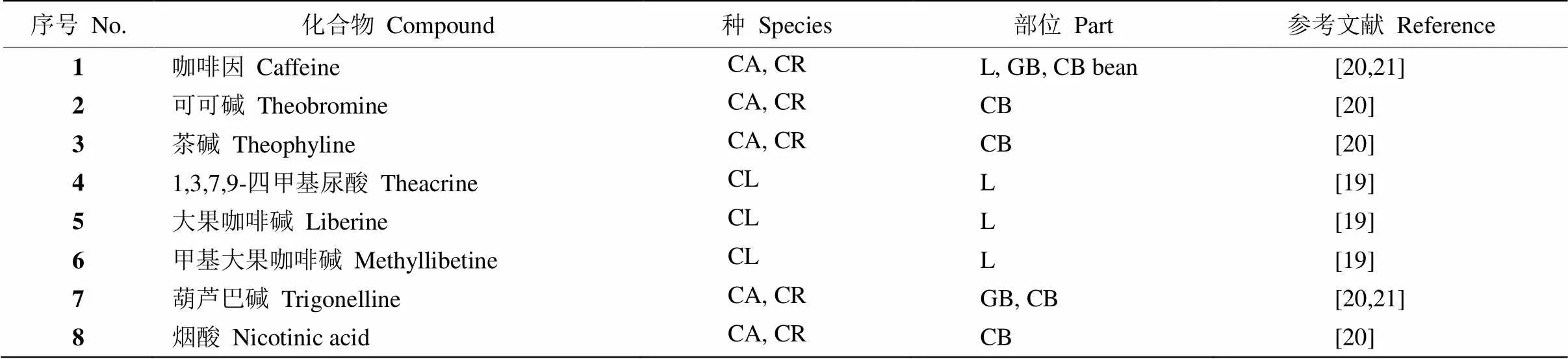
表1 咖啡中的生物碱类化合物
CA: 小粒咖啡; CR: 中粒咖啡; CL: 大粒咖啡; L: 叶; GB: 生豆; CB: 熟豆。下表同。
CA:; CR:; CL:; L: Leaf; GB: Green bean; CB: Roasted bean. The same is following Tables.
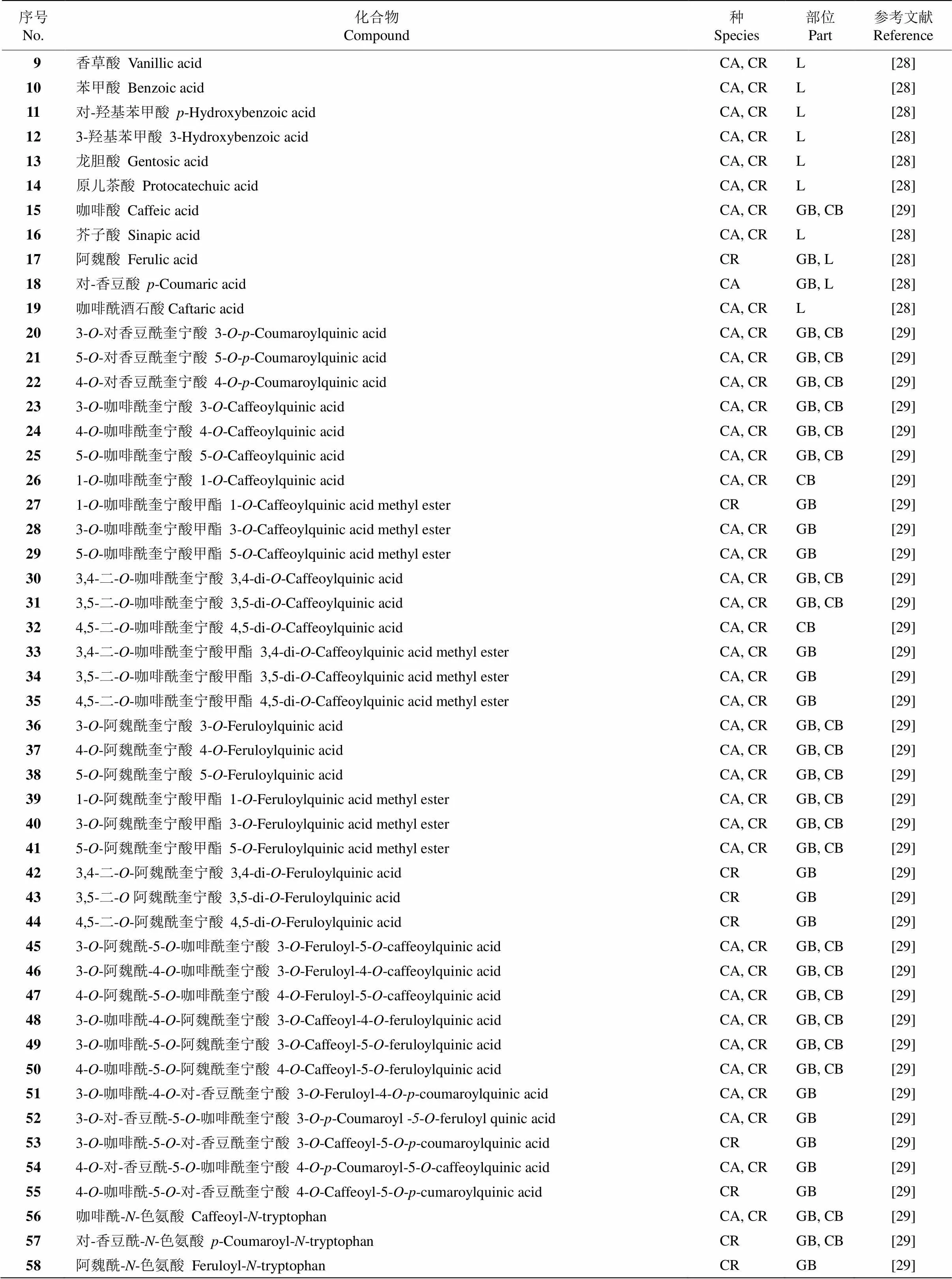
表2 咖啡中的酚酸类化合物
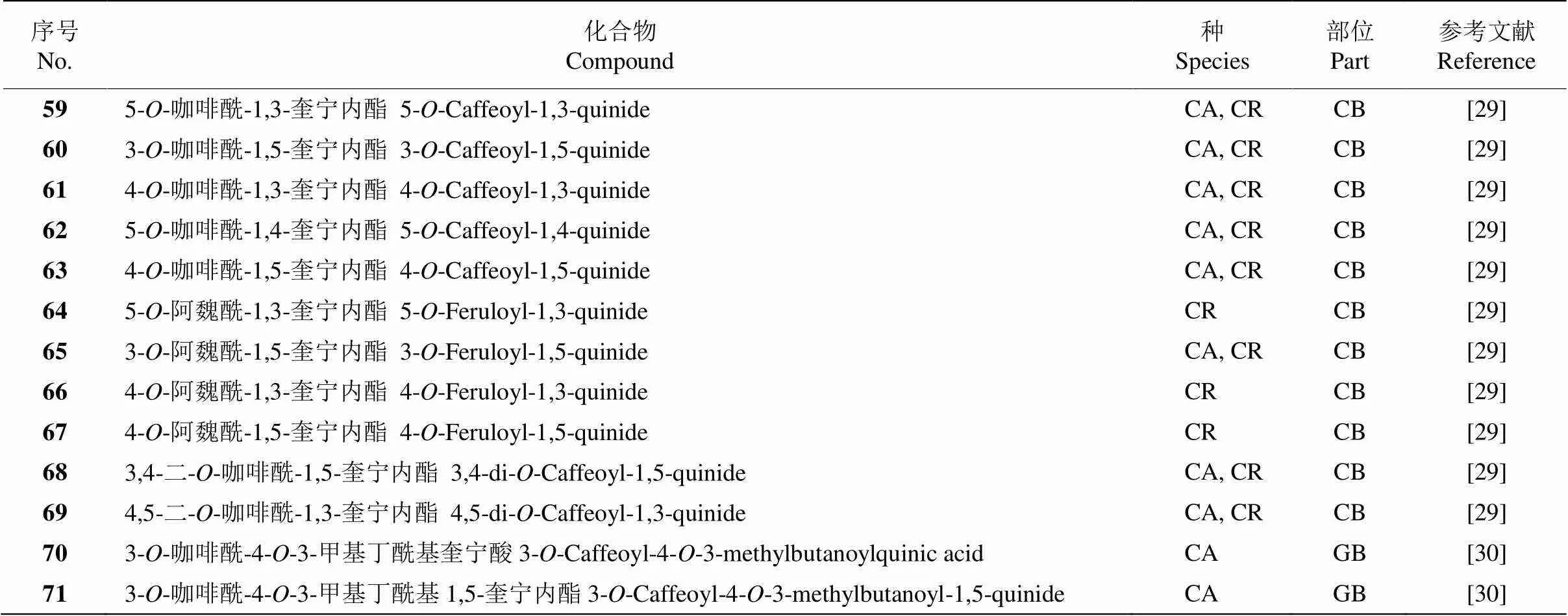
续表(Continued)
1.3 黄酮类
黄酮类化合物是广泛存在于自然界植物中的一类活性成分,具有抗氧化、抗癌、抗炎抗菌等多种活性[31–33]。小粒种咖啡中含有儿茶素、表儿茶素、槲皮素等黄酮类化合物(表3)。
1.4 萜类
咖啡中含有大量的萜类化合物,以对-贝壳杉烷型和咖啡醇二萜为主,其中咖啡豆醇、咖啡醇、16--甲基咖啡醇的含量最高。16--甲基咖啡醇一直作为区别小粒咖啡和中粒咖啡的标志物,Gunning[38]利用600 MHz NMR和LC-MS首次在小粒咖啡烘焙豆中检测到16--甲基咖啡醇和16--甲基咖啡豆醇。近年来,邱明华等[39–42]从小粒咖啡熟豆中分离鉴定了4个新对-贝壳杉型二萜苷(mascarosidesI~II, pani- culoside VI和cofaryloside I)、1个对-滨海孪生花烷型二萜苷类(villanovane I)和7个对-贝壳杉型二萜苷;从生豆中分离到5个对HL60、A-549、SMMC-7721、MCF-7和SW480肿瘤细胞株无抑制作用的对-贝壳杉型二萜(mascaroside III~V和20-nor-cofaryloside I~II)、8个新咖啡二萜内酯化合物(caffarolides A~H), 其中caffarolides C, D和F有一定的体外激活血小板聚集活性,3´10–4g/mL的诱导率分别为(11.4± 5.5)%、(15.8±5.6)%和(7.8±3.3)%; 从日晒豆中分离出4个对-贝壳杉型二萜(caffruenol A, caffruenol B, caffruolide A和caffruolide B),且对脂多糖诱导的264.7巨噬细胞NO的产生具有抑制作用。咖啡中也含有三萜,Wang等[43]首次从云南小粒咖啡干燥果实中分离出4个新达玛烷型三萜(caffruonesA~D)。这大大丰富了咖啡中萜类化合物的类型, 并对云南小粒咖啡的深入研究提供了大量的参考依据。
目前对咖啡中萜类活性成分的研究主要集中于咖啡醇和咖啡豆醇。Hiroaki等[44]报道咖啡豆醇乙酸酯和咖啡醇对人前列腺癌细胞具有抑制作用,并呈剂量依赖关系。Suck等[45]报道咖啡豆醇通过诱导caspase 3依赖途径抑制乳腺癌细胞增殖和诱导细胞死亡。Lima等[46]研究了咖啡醇对NB4、K562、HL60和KG1白血病细胞系的作用,结果表明咖啡醇对HL60和KG1细胞的细胞毒性最高,能使HL60细胞的增殖降低100%。Ferdrik等[47]研究表明,当咖啡醇浓度为10–8和10–6mol/L时,分别能使胰岛素分泌增加12%和16%,若长期暴露则能增加34%和68%,且在10–8mol/L时,人体骨骼肌细胞对葡萄糖的吸收能显著增加8%。Seo等[48]报道咖啡豆醇能降低脂多糖诱导的白细胞介素1、1、6和肿瘤坏死因子的产生,可抑制脂多糖引起的肝脏炎症。
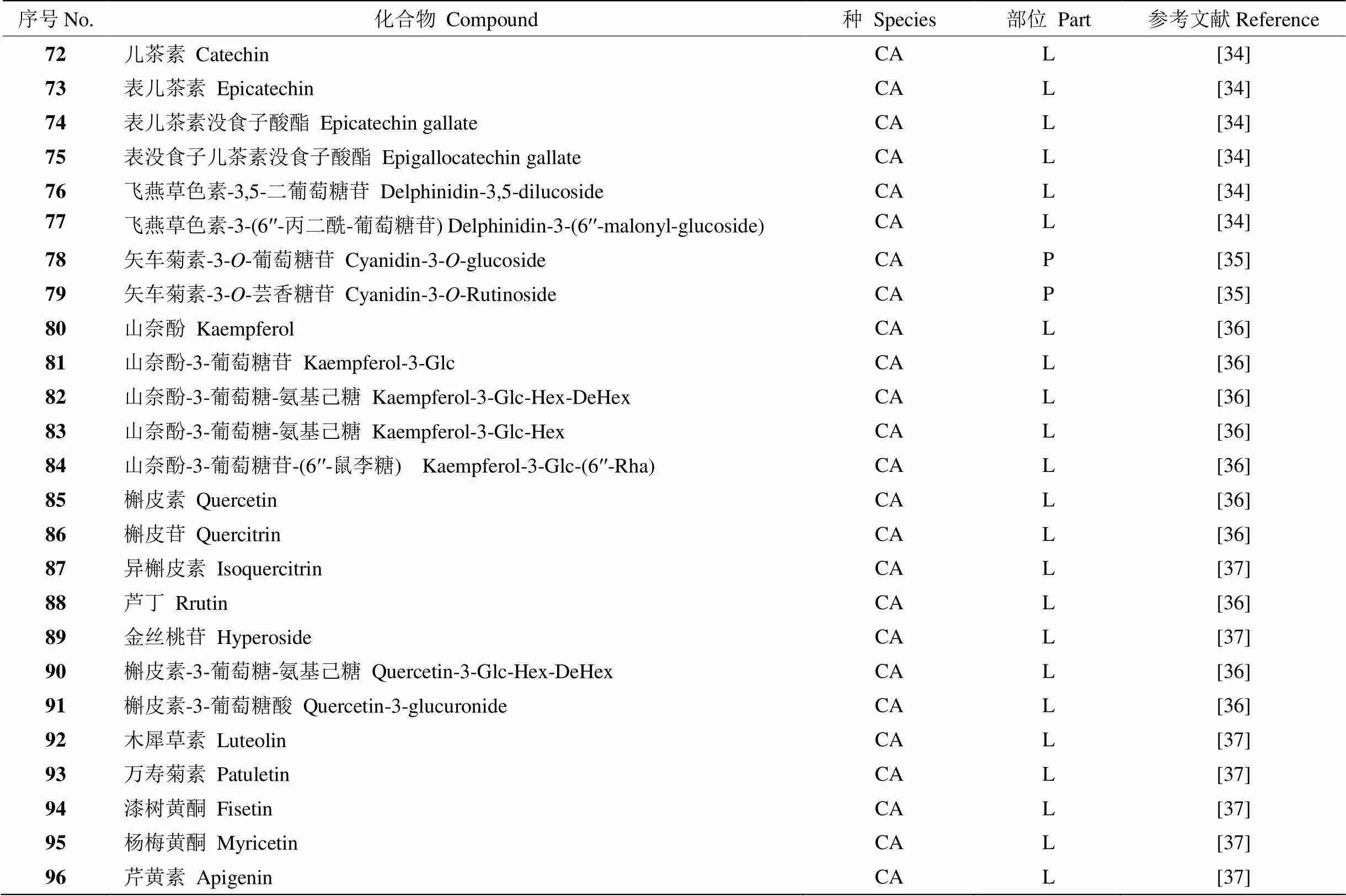
表3 咖啡中的黄酮类化合物
P: 果皮。
P: Peel.

表4 咖啡中的萜类化合物

续表(Continued)
SB: 日晒豆。
SB: Sun bean.
1.5 风味物质
20世纪60年代人们开始对咖啡豆风味物质进行研究,70年代报道了咖啡生豆中风味前体与烘焙咖啡中香气成分的相关性[53]。细胞壁多糖、脂质、蛋白质、蔗糖、绿原酸、咖啡因和葫芦巴碱是成熟咖啡种子的主要贮藏化合物,这些物质在烤焙过程中主要通过Maillard反应、Strecker降解、焦糖化反应形成咖啡香味。咖啡的特征性风味物质主要有28种[54],(1) 醛酮类物质与焦糖味/甜味有关:异丁醛、2-甲基丁醛、异戊醛、2,3-丁二酮、2,3-戊二酮、4-羟基-2,5-二甲基-3(2H)-呋喃酮、5-乙基-4-羟基-2-甲基-3(2H)-呋喃酮和香兰素;(2) 含硫化合物与硫磺/焙炒气味有关:2-糠基硫醇、2-甲基-3-呋喃硫醇、3-甲硫基丙醛、3-硫基-3-甲基-1-甲酸丁酯、3-甲基-2-丁烯-1-硫醇、甲硫醇和二甲基三硫化合物;(3) 吡嗪类化合物与泥土气味有关:2-乙基-3,5-二甲基吡嗪、2-乙烯基-3,5-二甲基吡嗪、2,3-二乙基- 5-甲基吡嗪、2-乙烯基-3-乙基-5-甲基吡嗪和2-甲氧基-3-异丁基吡嗪;(4) 酚、醛类化合物与烟熏/酚类香味有关:愈创木酚、4-乙基愈创木酚、4-乙烯基愈创木酚、乙醛、丙醛和(F)--大马酮;(5) 呋喃酮类化合物与辛辣味有关:3-羟基-4,5-二甲基-2(5H)-呋喃酮和3-羟基-4-甲基-5-乙基-2(5H)-呋喃酮。陈祎平等[55]从咖啡油的水蒸气蒸馏部分中共鉴定了以醛类、呋喃类、酚类、噻唑类、烯烃类、烷烃类、酯类、酮类、吡咯类、噻吩类、羧酸类、吡嗪类为主的12类挥发性成分。詹家芬等[56]从老挝咖啡中鉴定了包括醇类、酚类、醚类、醛类、酮类、酸类、酯类、烃类及氮氧化合物的77种挥发性成分。Hafsah等[57]利用气相色谱-质谱联用技术(GC-MS)从中粒咖啡花中鉴定了101种挥发性化合物,从咖啡豆中共鉴定了72种挥发性化合物。
1.6 其他成分
此外,咖啡中还含有蒽酮类化合物芒果苷(mangiferin)和异芒果苷(isomangiferin),香豆素类化合物东崀菪素(scopoletin),以及类胡萝卜素和叶黄素类化合物[28]。小粒咖啡熟豆中还含有五羟色胺类化合物scorodocarpines D~F[58]。咖啡种子中含有-谷甾醇(-sitosterol)、豆甾醇(stigmasterol)、菜油甾醇(campesterol)、胆甾醇(cholesterol)、5-燕麦甾烯醇(5-avenasterol)、7-燕麦甾烯醇(7-avenasterol)、7-豆甾烯醇(7-stigmastenol)等甾醇成分[59];脂类主要有肉豆寇酸、棕榈酸、硬脂酸、油酸、亚油酸、花生酸油等[59]。
2 咖啡的生物活性
咖啡中含有大量的生物活性物质,具有抗氧化、降脂、降血糖、神经保护等多种生物活性。
2.1 抗氧化
Yashin等[60]对咖啡的抗氧化活性进行了全面的综述,包括体外抗氧化性研究和测定方法,指出烤焙咖啡的抗氧化活性和总抗氧化物含量与茶、可可和红酒相当,且咖啡豆中的主要抗氧化剂绿原酸含量在烤焙后显著降低。
2.2 降脂作用
Duangjai等[61]采用3T3-L1脂肪细胞来评估不同颜色(绿色、黄色和红色)的咖啡果对脂肪生成和/或脂肪分解的影响,结果表明不同颜色的生咖啡果在3T3-L1脂肪细胞中均具有抑制脂肪生成的活性,其中红色干咖啡能减少约47%的脂肪积累。此外,除了黄色新鲜咖啡外的所有咖啡提取物主要成分(苹果酸、奎宁酸和绿原酸)都能增加甘油的释放。同时研究还证实了小粒咖啡果肉能通过下调NPC1L调制的LXR活性和胶束配合物的形成抑制肠内胆固醇的吸收而在体外和体内发挥降低胆固醇的作用[62]。
2.3 降血糖
糖尿病(DM)是一种由胰岛素相对或绝对缺乏引起的血糖水平增加的慢性疾病,在糖尿病的治疗中以药物治疗和饮食管理为主。咖啡中的咖啡因、绿原酸、葫芦巴碱和其他主要成分都有降低血糖的作用。Sake等[63]对小粒咖啡及其叶乙醇提取物进行降血糖药效研究,咖啡提取物可以显著降低小鼠的血糖含量。咖啡醇具有潜在的抗糖尿病作用,可以增加葡萄糖刺激的胰岛素分泌,增加人体骨骼肌细胞对葡萄糖的摄取[64]。
2.4 神经保护
流行病学研究表明,习惯性饮用咖啡可能会降低患阿尔茨海默病的风险[65],且男性原发性帕金森病患者的咖啡摄入量与震颤严重程度负相关[66]。在阿尔茨海默病的APP/PS2转基因小鼠模型中,证实咖啡中的绿原酸具有预防认知功能障碍的作用。免疫组织化学分析表明,咖啡多酚对淀粉样蛋白(A)海马的斑块数量有明显减少作用。
2.5 其他作用
云南小粒咖啡果皮的粗提物对受损人脐静脉内皮细胞有一定的保护和恢复作用[67]。咖啡提取物与Vc联合使用可起到抗肿瘤作用[68]。绿咖啡豆的甲醇提取物具有一定的抗炎活性[69]。咖啡具有肝脏保护作用,饮用咖啡能降低肝细胞癌复发的风险并增加原位肝移植后的生存机会[70]。咖啡与非酒精性脂肪肝的患病风险呈负相关[71]。
3 展望
咖啡作为世界三大饮品之首,与我们的日常生活息息相关,对咖啡化学成分的研究是进一步开发和提升咖啡利用的重要环节。当前,我国咖啡产业面临的主要问题是缺乏品牌效应和附加值低。为解决我国咖啡产业面临的这一困境,我国咖啡加工企业积极探索咖啡的精深加工工艺,力求将资源优势转变为经济优势。但这种局面的改变必须建立在对我国咖啡深入研究的基础上。
(1) 咖啡的化学成分丰富,是影响咖啡生物活性和风味的关键,因此对其化学成分的研究将是进一步完善和推进咖啡研究的基础,也是提高咖啡风味的关键。
(2) 通过对咖啡进行全面深入的研究,综合利用将是进一步开发利用咖啡的重要环节,如咖啡花、咖啡叶、咖啡残渣等。咖啡花中含有酚类、咖啡因和葫芦巴碱等活性物质,具有抗氧化能力,且具有转化为生物糖的潜质。其次,咖啡叶中所含咖啡因较少,可作为茶替代品。
(3) 云南小粒咖啡品质较好,其二萜类成分具有独特性,因此探明其化学成分与风味之间的关系,对提高咖啡品质及其重要。
[1] HUANG J X, LI G P, YANG S G. Brief introduction of coffee species and fine varieties [J]. Nongcun Shiyong Jishu, 2009(1): 42–43.黄家雄, 李贵平, 杨世贵. 咖啡种类及优良品种简介 [J]. 农村实用技术, 2009(1): 42–43.
[2] LI W R, ZHOU S Z. Development status and prospects of coffee industry in China [J]. Chin J Trop Agric, 2011, 31(10): 105–108. doi: 10.3969/j.issn.1009-2196.2011.10.025. 李维锐, 周仕峥. 我国咖啡产业发展现状及前景 [J]. 热带农业科学, 2011, 31(10): 105–108. doi: 10.3969/j.issn.1009-2196.2011.10.025.
[3] LAURENT C, EDDARKAOUI S, DERISBOURG M, et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology [J]. Neurobiol Aging, 2014, 35(9): 2079–2090. doi: 10.1016/ j.neurobiolaging.2014.03.027.
[4] SINGH S, SINGH K, PATEL S, et al. Nicotine and caffeine-mediated modulation in the expression of toxicant responsive genes and vesi- cular monoamine transporter-2 in 1-methyl 4-phenyl-1,2,3,6-tetra- hydropyridine-induced Parkinson’s disease phenotype in mouse [J]. Brain Res, 2008, 1207: 193–206. doi: 10.1016/j.brainres.2008.02.023.
[5] ZEITLIN R, PATEL S, BURGESS S, et al. Caffeine induces beneficial changes in PKA signaling and JNK and ERK activities in the striatum and cortex of Alzheimer’s transgenic mice [J]. Brain Res, 2011, 1417: 127–136. doi: 10.1016/j.brainres.2011.08.036.
[6] ARENDASH G W, REZAI-ZADEH K, CAO C H, et al. Caffeine: Evidence for protection against, and treatment for, Alzheimer’s disease by direct suppression of disease pathogenesis [J]. Alzheimers Dement, 2007, 3(S3): S166. doi: 10.1016/j.jalz.2007.04.355.
[7] NAKASO K, ITO S, NAKASHIMA K. Caffeine activates the PI3K/ Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells [J]. Neurosci Lett, 2008, 432(2): 146–150. doi: 10.1016/j.neulet.2007.12.034.
[8] CHEN X S, LAN X, ROCHE I, et al. Caffeine protects against MPTP- induced blood-brain barrier dysfunction in mouse striatum [J]. J Neurochem, 2008, 107(4): 1147–1157. doi: 10.1111/j.1471-4159.2008. 05697.x.
[9] SUN L Y, TIAN X, GOU L S, et al. Beneficial synergistic effects of concurrent treatment with theanine and caffeine against cerebral ischemia-reperfusion injury in rats [J]. Can J Physiol Pharmacol, 2013, 91(7): 562–569. doi: 10.1139/cjpp-2012-0309.
[10] BOJAR D, SCHELLER L, HAMRI G C E, et al. Caffeine-inducible gene switches controlling experimental diabetes [J]. Nat Commun, 2018, 9(1): 2318. doi: 10.1038/s41467-018-04744-1.
[11] RIEDEL A, HOCHKOGLER C M, LANG R, et al. N-methyl-pyridi- nium, a degradation product of trigonelline upon coffee roasting, stimulates respiratory activity and promotes glucose utilization in HepG2 cells [J]. Food Funct, 2014, 5(3): 454–462. doi: 10.1039/c3fo 60320b.
[12] LIU L, DU X H, ZHANG Z, et al. Trigonelline inhibits caspase 3 to protectcells apoptosis in streptozotocin-induced type 1 diabetic mice [J]. Eur J Pharmacol, 2018, 836: 115–121. doi: 10.1016/j.ejphar.2018. 08.025.
[13] SHAO X N, CHEN C, MIAO C S, et al. Expression analysis of microRNAs and their target genes during experimental diabetic renal lesions in rats administered with ginsenoside Rb1 and trigonelline [J]. Die Pharm, 2019, 74(8): 492–498. doi: 10.1691/ph.2019.8903.
[14] OMIDI-ARDALI H, LORIGOOINI Z, SOLTANI A, et al. Inflam- matory responses bridge comorbid cardiac disorder in experimental model of IBD induced by DSS: Protective effect of the trigonelline [J]. Inflammopharmacology, 2019, 27(6): 1265–1273. doi: 10.1007/s1078 7-019-00581-w.
[15] ZHOU J Y, ZHOU S W. Protection of trigonelline on experimental diabetic peripheral neuropathy [J]. Evid Based Compl Alternat Med, 2012, 2012: 164219. doi: 10.1155/2012/164219.
[16] FAHANIK-BABAEI J, BALUCHNEJADMOJARAD T, NIKBAKHT F, et al. Trigonelline protects hippocampus against intracerebral A(1– 40) as a model of Alzheimer's disease in the rat: insights into under- lying mechanisms [J]. Metab Brain Dis, 2019, 34(1): 191–201. doi: 10. 1007/s11011-018-0338-8.
[17] SHARMA L, LONE N A, KNOTT R M, et al. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6J mice, via restoration of hepatic auto- phagy [J]. Food Chem Toxicol, 2018, 121: 283–296. doi: 10.1016/j. fct.2018.09.011.
[18] ANWAR S, BHANDARI U, PANDA B P, et al. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiova- scular risk [J]. J Pharm Biomed Anal, 2018, 159: 100–112. doi: 10. 1016 j.jpba.2018.06.027.
[19] PETERMANN J B, BAUMANN T W. Metabolic Relations between methylxanthines and methyluric acids inL. [J]. Plant Physiol, 1983, 73(4): 961–964. doi: 10.1104/pp.73.4.961.
[20] RODRIGUES N P, BRAGAGNOLO N. Identification and quanti- fication of bioactive compounds in coffee brews by HPLC-DAD-MSn [J]. J Food Compos Anal, 2013, 32: 105–115. doi: 10. 1016/j.jfca.2013. 09.002.
[21] CAPORASO N, WHITWORTH M B, GREBBY S, et al. Non- destructive analysis of sucrose, caffeine and trigonelline on single green coffee beans by hyperspectral imaging [J]. Food Res Int, 2018, 106: 193–203. doi: 10.1016/j.foodres.2017.12.031.
[22] NISHI, AHAD A, KUMAR P. Hypolipidemic effect of chlorogenic acid in a hypercholesterolemic rat model [J]. Int J Pharm Bio Sci, 2013, 4(1): 582–586.
[23] XU J G, HU Q P, LIU Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers [J]. J Agric Food Chem, 2012, 60(46): 11625–11630. doi: 10.1021/jf303771s.
[24] SHI X W, ZHOU N, CHENG J Y, et al. Chlorogenic acid protects PC12 cells against corticosterone-induced neurotoxicity related to inhibition of autophagy and apoptosis [J]. BMC Pharmacol Toxicol, 2019, 20(1): 56. doi: 10.1186/s40360-019-0336-4.
[25] SU M M, LIU F, LUO Z, et al. The antibacterial activity and mecha- nism of chlorogenic acid against foodborne pathogen[J]. Foodborne Pathog Dis, 2019, 16(12): 823–830. doi: 10.1089/fpd.2019.2678.
[26] XUE Y W, HUANG F, TANG R X, et al. Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague-Dawley rats [J]. Food Chem Toxicol, 2019, 133: 110751. doi: 10.1016/j.fct.2019.110751.
[27] van DIJK A E, OLTHOF M R, MEEUSE J C, et al. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance [J]. Diabetes Care, 2009, 32(6): 1023–1025. doi: 10.2337/dc09-0207.
[28] CHEN X M. A review on coffee leaves: Phytochemicals, bioactivities and applications [J]. Crit Rev Food Sci Nutri, 2019, 59(6): 1008–1025. doi: 10.1080/10408398.2018.1546667.
[29] ASAMENEW G, KIM H W, LEE M K, et al. Comprehensive charac- terization of hydroxycinnamoyl derivatives in green and roasted coffee beans: A new group of methyl hydroxycinnamoyl quinate [J]. Food Chem, 2019, X2: 100033. doi: 10.1016/j.fochx.2019.100033.
[30] SITTIPOD S, SCHWARTZ E, PARAVISINI L, et al. Identification of flavor modulating compounds that positively impact coffee quality [J]. Food Chem, 2019, 301: 125250. doi: 10.1016/j.foodchem.2019.125250.
[31] SHI X W, ZHOU N, CHENG J Y, et al. Chlorogenic acid protects PC12 cells against corticosterone-induced neurotoxicity related to inhibition of autophagy and apoptosis [J]. BMC Pharmacol Toxicol, 2019, 20(1): 56. doi: 10.1186/s40360-019-0336-4.
[32] WENZEL U, KUNTZ S, BRENDE M D, et al. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells [J]. Cancer Res, 2000, 60(14): 3823–3831.
[33] ZENG C Z, LIU Z X, WU Y X, et al. Studies on flavonoids extraction by ultrasonic technology from glycyrrhiza and their bacteriostatic activity [J]. Lishizhen Med Mat Med Res, 2007, 18(10): 2402–2403. doi: 10.3969/j.issn.1008-0805.2007.10.036.曾超珍, 刘志祥, 吴耀辉, 等. 超声波提取甘草黄酮及其抑菌活性研究 [J]. 时珍国医国药, 2007, 18(10): 2402–2403. doi: 10.3969/j. issn.1008-0805.2007.10.036.
[34] RATANAMARNO S, SURBKAR S. Caffeine and catechins in fresh coffee leaf () and coffee leaf tea [J]. Maejo Int J Sci Technol, 2017, 11(3): 211–218.
[35] ZHANG Y H, FU X P, LIANG W J, et al. Antioxidant activity and compsition of anthocyanins of crude extracts from Yunnan arabica coffee husk [J]. Food Sci Technol, 2016, 41(5): 219–223. doi: 10. 13684/j.cnki.spkj.2016.05.041.张云鹤, 付晓萍, 梁文娟, 等. 云南小粒种咖啡果皮粗提物花青素成分及抗氧化活性研究 [J]. 食品科技, 2016, 41(5): 219–223. doi: 10.13684/j.cnki.spkj.2016.05.041.
[36] SAMUEL C V M, WAGNER L A, TOHGE T, et al. In high-light- acclimated coffee plants the metabolic machinery is adjusted to avoid oxidative stress rather than to benefit from extra light enhancement in photosynthetic yield [J]. PLoS One, 2014, 9(4): e94862. doi: 10.1371/ journal.pone.0094862.
[37] PATAY E B, NÉMETH T, NÉMETH T S, et al. Histological and phytochemical studies ofB. Heyne ex Schult., compared withL. [J]. Farmacia, 2016, 64(1): 125–130.
[38] GUNNING Y, DEFERNEZ M, WATSON A D, et al. 16--methyl- cafestol is present in ground roast arabica coffees: Implications for authenticity testing [J]. Food Chem, 2018, 248: 52–60. doi: 10.1016/j. foodchem.2017.12.034.
[39] WANG X, PENG X R, LU J, et al. Ent-kaurane diterpenoids from the cherries of[J]. Fitoterapia, 2019, 132: 7–11. doi: 10. 1016/j.fitote.2018.08.023.
[40] WANG X, MENG Q Q, PENG X R, et al. Identification of new diterpene esters from green Arabica coffee beans, and their platelet aggregation accelerating activities [J]. Food Chem, 2018, 263: 251–257. doi: 10.1016/j.foodchem.2018.04.081.
[41] SHU Y, LIU J Q, PENG X R, et al. Characterization of diterpenoid glucosides in roasted puer coffee beans [J]. J Agric Food Chem, 2014, 62(12): 2631–2637. doi: doi.org/10.1021/jf500788t.
[42] CHU R, WAN L S, PENG X R, et al. Characterization of new ent- kaurane diterpenoids of Yunnan Arabica coffee beans [J]. Nat Prod Bioprospect, 2014, 6(4): 217–223. doi: 10.1007/s13659-016-0099-1.
[43] WANG X, PENG X R, LU J, et al. New dammarane triterpenoids, caffruones A-D, from the cherries of[J]. Nat Prod Bioprospect, 2018, 8(6): 413–418. doi: 10.1007/s13659-018-0181-y.
[44] IWAMOTO H, IZUMI K, NATSAGDORJ A, et al. Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells [J]. Prostate, 2019, 79(5): 468– 479. doi: 10.1002/pros.23753.
[45] OH S H, HWANG Y P, CHOI J H, et al. Kahweol inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2- overexpressing cancer cells [J]. Food Chem Toxicol, 2018, 121: 326– 335. doi: 10.1016/j.fct.2018.09.008.
[46] LIMA C S, SPINDOLA D G, BECHARA A, et al. Cafestol, a diterpene molecule found in coffee, induces leukemia cell death [J]. Biomed Pharmacother, 2017, 92: 1045–1054. doi: 10.1016/j.biopha.2017.05.109.
[47] MELLBYE F B, JEPPESEN P B, HERMANSEN K, et al. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: Studies[J]. J Nat Prod, 2015, 78(10): 2447–2451. doi: 10.1021/acs.jnatprod.5b00481.
[48] SEO H Y, KIM M K, LEE S H, et al. Kahweol ameliorates the liver inflammation through the inhibition of NF-κB and STAT3 activation in primary kupffer cells and primary hepatocytes [J]. Nutrients, 2018, 10(7): 863. doi: 10.3390/nu10070863.
[49] LANG R, FROMME T, BEUSCH A, et al. 2---d-Glucopyranosyl- carboxyatractyligenin fromL. inhibits adenine nucleotide translocase in isolated mitochondria but is quantitatively degraded during coffee roasting [J]. Phytochemistry, 2013, 93: 124–135. doi: 10. 1016/j.phytochem.2013.03.022.
[50] SPEER K, HRUSCHKA A, KURZROCK T, et al. Diterpenes in coffee [M]// PARLIAMENT T H, HO C T, SCHIEBERLE P. Caffeinated Beverages, Health Benefits, Physiological Effects, and Chemistry. Washington, DC: American Chemical Society, 2000: 241–251.
[51] LANG R, FROMME T, BEUSCH A, et al. Raw coffee based dietary supplements contain carboxyatractyligenin derivatives inhibiting mito- chondrial adenine-nucleotide-translocase [J]. Food Chem Toxicol, 2014, 70: 198–204. doi: 10.1016/j.fct.2014.05.017.
[52] LAM L K T, SPARNINS V L, WATTENBERG L W. Isolation and identification of kahweol palmitate and cafestol palmitate as active constituents of green coffee beans that enhance glutathione S-trans- ferase activity in the mouse [J]. Cancer Res, 1982, 42(4): 1193–1198.
[53] ADANE T G, BYUNG S C. Coffee Flavor [M]// Encyclopedia of Food Chemistry. Amsterdam: Elsevier, 2019: 48–53.
[54] ZENG F K, OU S Y. Coffee Flavor Chemistry [M]. Guangzhou: Jinan University Press, 2014: 1–128. 曾凡逵, 欧仕益. 咖啡风味化学 [M]. 广州: 暨南大学出版社, 2014: 1–128.
[55] CHEN Y P, LIANG Z Y, LIN Y Q, et al. Preliminary analysis of odor composition in coffee oil from coffee grounds [J]. Food Sci, 2004, 25(11): 230–232. doi: 10.3321/j.issn:1002-6630.2004.11.058.陈祎平, 梁振益, 林尤全, 等. 咖啡油香气成分的初步分析 [J]. 食品科学, 2004, 25(11): 230–232. doi: 10.3321/j.issn:1002-6630.2004.11.058.
[56] ZHAN J F, LU S M, QU G F, et al. Analysis on volatile and semi- volatie components of Laos’s coffee [J]. Food Res Dev, 2008, 29(2): 125–129. doi: 10.3969/j.issn.1005-6521.2008.02.041.詹家芬, 陆舍铭, 曲国福, 等. 老挝咖啡的挥发和半挥发性成分提取分析 [J]. 食品研究与开发, 2008, 29(2): 125–129. doi: 10.3969/j. issn.1005-6521.2008.02.041.
[57] HAFSAH H, IRIAWATI I, SYAMSUDIN T S. Dataset of volatile compounds from flowers and secondary metabolites from the skin pulp, green beans, and peaberry green beans of robusta coffee [J]. Data Brief, 2020, 29: 105219. doi: 10.1016/j.dib.2020.105219.
[58] SHU Y. Studies on the chemical constituents and bioactivity of roasted coffee beans ofgrowing in Yunnan [D]. Hefei: Hefei University of Technology, 2014. 疏义. 云南小粒咖啡熟豆化学成分和生物活性研究 [D]. 合肥: 合肥工业大学, 2014.
[59] QIU M H, ZHANG Z R, LI Z R, et al. Review of research on the chemical constituents and bioactivities of coffee [J]. Plant Sci J, 2014, 32(5): 540–550. doi: 10.11913/PSJ.2095-0837.2014.50540.邱明华, 张枝润, 李忠荣, 等. 咖啡化学成分与健康 [J]. 植物科学学报, 2014, 32(5): 540–550. doi: 10.11913/PSJ.2095-0837. 2014.50540.
[60] YASHIN A, YASHIN Y, WANG J Y, et al. Antioxidant and antiradical activity of coffee [J]. Antioxidants, 2013, 2(4): 230–245. doi: 10.3390/ antiox2040230.
[61] DUANGJAI A, NUENGCHAMNONG N, SUPHROM N, et al. Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes [J]. Kobe J Med Sci, 2018, 64(3): E84– E92.
[62] ONTAWONG A, DUANGJAI A, MUANPRASAT C, et al. Lipid- lowering effects ofpulp aqueous extract in caco-2 cells and hypercholesterolemic rats [J]. Phytomedicine, 2019, 52: 187–197. doi: 10.1016/j.phymed.2018.06.021.
[63] MARTINA S J, GOVINDAN P A P, WAHYUNI A S, et al. The difference in effect of Arabica coffee Gayo beans and leaf (Gayo) extract on decreasing blood Sugar levels in healthy mice [J]. Open Access Maced J Med Sci, 2019, 7(20): 3363–3365. doi: 10.3889/oamjms.2019.423.
[64] MELLBYE F B, JEPPESEN P B, SHOKOUH P, et al. Cafestol, a bioactive substance in coffee, has antidiabetic properties in KKAy mice [J]. J Nat Prod, 2017, 80(8): 2353–2359. doi: 10.1021/acs.jnatprod.7b0 0395.
[65] ISHIDA K, YAMAMOTO M, MISAWA K, et al. Coffee polyphenols prevent cognitive dysfunction and suppress amyloidplaques in APP/ PS2 transgenic mouse [J]. Neurosci Res, 2020, 154: 35–44. doi: 10. 1016/j.neures.2019.05.001
[66] CHO B H, CHOI S M, KIM B C. Gender-dependent effect of coffee consumption on tremor severity in de novo Parkinson’s disease [J]. BMC Neurol, 2019, 19(1): 194. doi: 10.1186/s12883-019-1427-y.
[67] FU X P, ZHANG Y H, GU D H, et al. Effect on anti-oxidative injuries of human umbilical vein endothelial cell of crude extracts from Yunnan arabica coffee husk [J]. Food Sci Technol, 2016, 41(12): 183–188. doi: 10.13684/j.cnki.spkj.2016.12.037.付晓萍, 张云鹤, 谷大海, 等. 云南小粒种咖啡果皮粗提取物对人脐静脉内皮细胞抗氧化损伤的研究 [J]. 食品科技, 2016, 41(12): 183–188. doi: 10.13684/j.cnki.spkj.2016.12.037.
[68] El-GARAWANI I M, EL-NABI S H, EL-SHAFEY S, et al.bean extracts and vitamin C: A novel combination unleashes MCF-7 cell death [J]. Curr Pharm Biotechnol, 2020, 21(1): 23–26. doi: 10.2174/1389201020666190822161337.
[69] PERGOLIZZI S, D’ANGELO V, ARAGONA M, et al. Evaluation of antioxidant and anti-inflammatory activity of green coffee beans methanolic extract in rat skin [J]. Nat Prod Res, 2020, 34(11): 1535– 1541. doi: 10.1080/14786419.2018.1523161.
[70] WILTBERGER G, WU Y, LANGE U, et al. Protective effects of coffee consumption following liver transplantation for hepatocellular carci- noma in cirrhosis [J]. Aliment Pharmacol Ther, 2019, 49(6): 779–788. doi: 10.1111/apt.15089.
[71] VITAGLIONE P, MAZZONE G, LEMBO V, et al. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut-liver axis [J]. J Nutri Sci, 2019, 8: e15. doi: 10.1017/jns. 2019.10.
Advances on Chemical Components and Biological Activities of Coffee
SHEN Xiao-jinga,b, ZI Cheng-tingb*, HUI Shao-liangb, YANG Jun-taob, WANG Qingb, CAO Meng-tingb, FAN Jing-pinga*
(a. College of Food Science and Technology; b. College of Science, Yunnan Agricultural University, Kunming 65020, China)
Coffee, one of the three top drinks in the world, belongs to(Rubiaceae), which can reduce blood sugar, protect liver and neuro. The chemical constituents of coffee are rich, including alkaloids, phenolic acid, flavonoid, terpene, etc. The chemical constituents are the basis of biological activities and forming the characteristic flavor of coffee. The main chemical constituents and biological activities of coffee were comprehensivelyreviewed, which would provide relevant basis and theoretical support for the further development of coffee industry.
Coffee; Chemical constituents; Biological activities; Review
10.11926/jtsb.4249
2020–05–13
2020–07–01
国家自然科学基金云南联合基金项目(U1902206); 云南省教育厅科学研究基金项目(2020J0241)资助
This work was supported by the Nation-Yunnan Joint Natural Science Foundation of China (Grant No. U1902206); and the Project for Scientific Research of Yunnan Education Department (Grant No. 2020J0241).
沈晓静(1988~ ),女,实验师,主要从事天然产物研究、食品功能因子开发和利用。E-mail: 690361382@qq.com
E-mail: zichengting@126.com; 1993033@ynau.edu.cn

