Serum vitamin D and vitamin-D-binding protein levels in children with chronic hepatitis B
Cai-Zhi Huang, Jie Zhang, Lin Zhang, Cui-Hua Yu, Yi Mo, Li-Ya Mo
Abstract
Key Words: Chronic hepatitis B; Children; Vitamin D; Vitamin-D-binding protein; Hepatitis B virus
INTRODUCTION
Vitamin D is a fat-soluble secosteroid and is essential for humans. Vitamin D is mainly produced in the skin by means of sunlight exposure or retrieved from dietary components. In order to become biologically active, vitamin D needs to be combined with vitamin-D-binding protein (VDBP), which is the major transport protein of vitamin D and its metabolites, and it sequentially converts to its intermediate metabolite calcidiol {25-hydroxy vitamin D [25(OH)D]} and the final active form calcitriol (1,25-dihydroxy vitamin D) by hydroxylation in the liver and kidneys. Twenty-five (OH)D is the main metabolite of vitamin D and is relatively stable in serum. Precise quantification of calcitriol is problematic because of its short half-life and serum concentrations are 1000 times lower compared to those of 25(OH)D. In contrast, 25(OH)D has a half-life of approximately 3 wk, making it a suitable and largely reliable indicator to investigate the individual vitamin D status. Vitamin D promotes absorption of calcium and regulates skeletal function. It is also a powerful modulator of the immune response and is closely associated with many diseases such as immune disease[1], inflammatory bowel disease[2], cancer[3], and metabolic disorders[4].
Hepatitis B virus (HBV) is a serious threat to human health and life. HBV infection is one of the major contributors to the development of chronic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma. About 1 million people die from HBV infection every year[5,6]. One-third of the population around the world are infected by HBV, and it was estimated by the World Health Organization in 2015 that 257 million people were living with chronic hepatitis B infection. Newborn infants and children with acute HBV infection are prone to developing chronic hepatitis B (CHB), and the rate of chronicity in newborn infants, children aged < 6 years, and adults is 80%-90%, 25%-30%, and 1%-5%[7], respectively.
Immune and inflammatory responses participate in chronic liver injuries and fibrogenesis of CHBviaactivating hepatic stellate cells. Due to the heterogeneity of CHB, patients may have different levels of viral replication and severity of hepatic disease. Pathologically, patients infected with HBV are characterized by impairment of T-cell function. The elimination of HBV in CHB patients largely depends on the cellular immune response mediated by T helper (Th)1 cells. Combination of the active form of vitamin D and vitamin D receptor (VDR) may inhibit the proliferation of Th1 cells and the release of cytokines and concurrently activate Th2 cells, thus regulating the immune response. However, HBV-transfected cells downregulate VDR expression, thereby preventing vitamin D from inhibiting transcription and translation of HBVin vitro, and HBV might use this mechanism to avoid the immune system[8]. Therefore, it is believed that vitamin D is closely linked to hepatitis B. In recent years, it has been shown that vitamin D deficiency is related to CHB in adults, and vitamin D level might affect the antiviral effect and disease progression and prognosis[9,10]. Nevertheless, the association of HBV replication and vitamin D level is not consistent[11,12], and there have been few studies about the relationship among vitamin D, VDBP, and CHB in children. Thus, we carried out this study to investigate the association of vitamin D and VDBP with HBV replication and hepatic fibrosis in children with CHB.
MATERIALS AND METHODS
Study population
This research was approved by the Ethics Committee of Hunan Children’s Hospital. The study protocol conformed to the provisions of the Declaration of Helsinki. The diagnostic criteria and definitions for CHB were in accordance with the American Association for the Study of Liver Diseases 2018 Hepatitis B Guidance[13]. Hepatic fibrosis was classified according to the Scheuer Scoring System: Grade 0, no fibrosis; grade 1, enlarged and fibrotic portal tracts; grade 2, periportal or portal-portal septal fibrosis but intact architecture; grade 3, fibrosis with architectural distortion but no obvious cirrhosis; and grade 4, probable or definite cirrhosis.
We selected 257 children with CHB who were admitted to Hunan Children’s Hospital in summer and autumn between 2018 and 2019, along with 170 randomly selected healthy controls. Patients were excluded from the present analysis if they met the following exclusion criteria: (1) Patients with infectious liver disease caused by pathogens other than HBV and with other liver diseases such as autoimmune liver disease; (2) Patients with malignant tumors; (3) Patients who have received immunomodulatory therapy or taken vitamin D preparations in the past 4 wk; (4) Patients with comorbidities that may affect the serum levels of vitamin D and VDBP, including malnutrition, renal disease, and diabetes; and (5) Repeated patients during the study period. Lastly, 53 of the patients were excluded, and a total of 204 children were enrolled in the analysis.
The enrolled patients included: 193 hepatitis B surface antigen (HBsAg) positive and 11 HBsAg negative; 164 hepatitis B e antigen (HBeAg) positive and 40 HBeAg negative; 164 with detectable HBV deoxyribonucleic acid (DNA) and 40 with undetectable HBV DNA; 131 with HBV genotype B, 23 with HBV genotype C, and 50 without genotyping due to refusal of guardians; and 124 with histopathological determination of grade of hepatic fibrosis (27 grade 0, 74 grade 1, 12 grade 2, 9 grade 3, and 2 grade 4), and 80 without histopathological examination because of refusal of guardians. The flow chart of the study is shown in Figure 1.
Data collection
All blood tests were provided by the Department of Laboratory Medicine of Hunan Children’s Hospital. Serum 25(OH)D level was determined by chemiluminescent immunoassay (ADVIA Centaur XP; Siemens, Erlangen, Germany). Serum VDBP level was detected by ELISA (reagent kit from China). After collection, the human serum samples for 25(OH)D examination were stored at 4 °C and tested within 24 h, and the samples for VDBP examination were stored at -70 °C and tested within 1 wk. The definition of vitamin D levels in Hunan Children’s Hospital included deficiency (< 37.50 nmoL/L), insufficiency (37.5-50.0 nmoL/L), and sufficiency (50.0-250.0 nmoL/L). Other clinical parameters including liver function tests, serum HBV DNA level, and serological markers of HBV, such as HBsAg and HBeAg, were collected for analysis of the association with 25(OH)D and VDBP levels.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 software (Armonk, NY, United States). Normal distribution measurement data were expressed as mean ± standard deviation and analyzed by thettest to determine the difference between two groups. Non-normal distribution measurement data were expressed as median (interquartile range) and analyzed by Mann-WhitneyUtest or Kruskal-WallisHtest. Numerical data were expressed as rate or percentage and analyzed byχ2test. The Spearman’s rank correlation test was used to determine the correlation between the two groups. Binary multivariate logistic regression analysis was applied to analyze further the statistically significant factors determined by univariate analysis. Statistical analysis was based on the actual number of completed cases if values were missing.P< 0.05 by two-tailed tests was considered statistically significant for tests of all variables.
RESULTS
Analysis of serum levels of 25(OH)D and VDBP in patients with CHB and healthy controls
Serum levels of 25(OH)D, VDBP, calcium, and phosphorus and liver function test results in patients with CHB and healthy controls are shown in Table 1. Patients with CHB had higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBil); longer prothrombin time (PT); and lower serum levels of 25(OH)D, VDBP, calcium, and phosphorus. No difference in albumin (ALB) level was found between patients with CHB and healthy controls. There were 25 cases (12.25%) of vitamin D deficiency, 44 (21.57%) of vitamin D insufficiency, and 135 (66.18%) of vitamin D sufficiency in children with CHB. The cases of vitamin D deficiency, insufficiency, and sufficiency in healthy controls were 3 (1.76%), 12 (7.06%), and 155 (91.18%), respectively. The distribution frequency of vitamin D in CHB patients and healthy controls is shown in Figure 2.
Differences in serum levels of 25(OH)D and VDBP in CHB children with different conditions
Serum levels of 25(OH)D and VDBP in different groups of children with CHB are shown in Table 2. In children with CHB, no significant difference was found in serum 25(OH)D levels between HBeAg-positive and -negative (P= 0.091), between HBsAgpositive and -negative (P= 0.076), between patients with HBV genotype B and genotype C (P= 0.753), or between patients with detectable and undetectable HBV DNA (P= 0.441). Serum level of 25(OH)D was significantly different among groups with various grades of hepatic fibrosis (P< 0.001). The levels of serum 25(OH)D decreased successively in patients with grade 0, 1, 2, and 3 of hepatic fibrosis, and the difference between any two groups was significant (P< 0.05).
Serum levels of VDBP in children with HBeAg, HBsAg, HBV genotype C, and detectable HBV DNA were significantly lower than those in children without HBeAg and HBsAg and with HBV genotype B and undetectable HBV DNA. There was a significant difference in serum VDBP levels among patients with various grades of hepatic fibrosis (P= 0.032). Patients with grade 2 hepatic fibrosis had higher serum VDBP levels than those with grade 0 or 3 hepatic fibrosis (P< 0.05), and no significant difference was found between any other two groups. The data for hepatic fibrosisgrade 4 were not involved in statistical analysis because there were only 2 cases.

Table 1 Serum levels of 25-hydroxy vitamin D and vitamin-D-binding protein in patients with chronic hepatitis B and healthy controls
Correlation between serum levels of 25(OH)D and VDBP and other clinical parameters in patients with CHB
Spearman’s rank correlation test revealed that serum levels of 25(OH)D were negatively correlated with age and serum TBil level in children with CHB (r= -0.396, -0.280,P< 0.001), but serum 25(OH)D level was not correlated with sex or serum levels of ALT, AST, ALB, PT, calcium, phosphorus, HBV DNA, and VDBP. Serum VDBP level was negatively correlated with serum HBV DNA (log10IU/mL) (r= -0.272,P< 0.001) in children with CHB (Figure 3). No significant correlation was found between VDBP levels and age, sex, or levels of ALT, AST, TBil, ALB, PT, calcium, and phosphorus.
Univariate and multivariate analyses of factors associated with hepatic fibrosis
Univariate analysis revealed that CHB patients with hepatic fibrosis had higher levels of TBil, VDBP, and HBV DNA, lower levels of 25(OH)D, and lower rate of HBeAgpositive to -negative than CHB patients without hepatic fibrosis. Multivariate logistic regression analysis showed that low level of serum 25(OH)D [odds ratio = 0.951, 95% confidence interval: 0.918-0.985] and high level of HBV DNA (odds ratio = 1.445, 95% confidence interval: 1.163-1.794) were both independently correlated with hepatic fibrosis in children with CHB (Table 3).
鱼类。经测产,投放鲤鱼1.1万尾,产量5500公斤;投放鲫鱼4万余尾,产量4800公斤;投放草鱼1920公斤,产量2784公斤,投放鲢鱼2000尾,产量200公斤;总产量为13284公斤,亩产110.7公斤,按当地批发价格23元/公斤计,亩产值2546元。
DISCUSSION
In the present study, the serum levels of 25(OH)D in children with CHB was investigated, along with its correlation with other factors. Children with CHB were found to have lower serum 25(OH)D levels than healthy controls; CHB patients with hepatic fibrosis had lower levels of 25(OH)D than patients without hepatic fibrosis; and low level of serum 25(OH)D was independently correlated with hepatic fibrosis. The results demonstrate the value of maintaining normal levels of 25(OH)D for the prevention and delay of hepatic fibrosis in children with CHB.
Compared with healthy controls, patients with CHB had higher rates of vitamin D deficiency and insufficiency. This may be due to the decrease of vitamin D hydroxylation in the liver in the context of impaired liver function and the increase of vitamin D consumption on account of immune response mediated by vitamin D in patients with CHB.
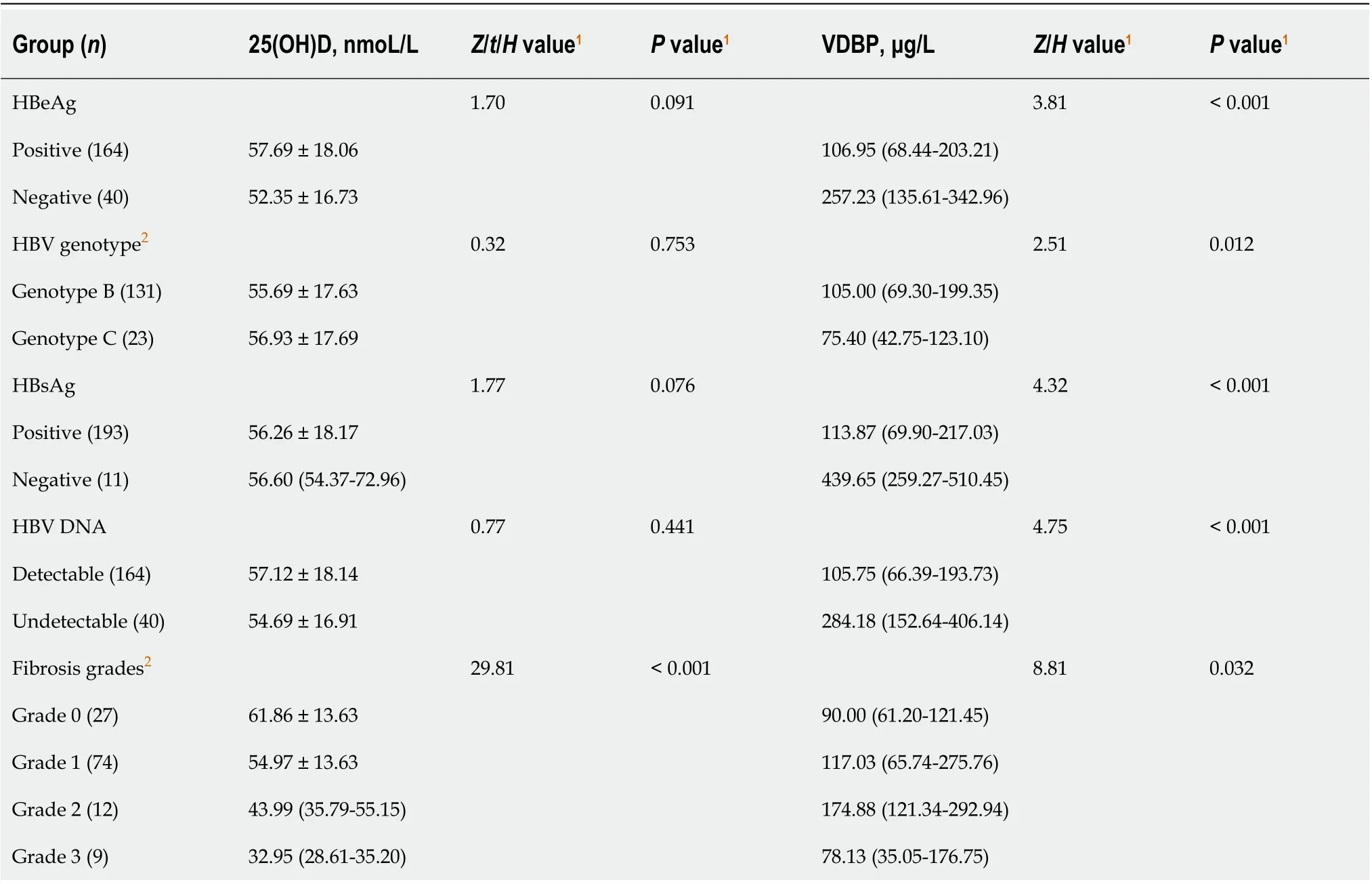
Table 2 Serum levels of 25-hydroxy vitamin D and vitamin-D-binding protein in different groups of children with chronic hepatitis B
Some investigations have indicated that serum 25(OH)D level is negatively correlated with HBV load, and low 25(OH)D level is associated with HBV genotype B, HBsAg, and HBeAg[11,14]. Other studies have shown inconsistent results[8,10,12]. In the present study, 25(OH)D was not correlated with HBV DNA in patients with CHB, and serum levels of 25(OH)D did not differ significantly between patients with or without HBeAg, patients with HBV genotype B or C, patients with or without HBsAg, and patients with or without detectable HBV DNA. These results indicate that serum 25(OH)D levels are not associated with HBV genotype and virus replication. The discrepancy between various studies may have been caused by the different disease course and stage and study population.
A previous study revealed that vitamin D can reduce profibrotic factors and collagen expression and that it has immunomodulatory effects on immune cells, including T cells[15]. Vitamin D has been found to have an antifibrotic effects on human primary transforming-growth-factor-β-stimulated hepatic stellate cells[16]. In the present study, serum levels of 25(OH)D in CHB children with hepatic fibrosis were markedly decreased, and the higher the grade of hepatic fibrosis, the lower the level of serum 25(OH)D. Multivariate logistic regression analysis also revealed that low level of 25(OH)D was independently associated with hepatic fibrosis, which suggested that maintaining normal levels of 25(OH)D in children with CHB may have a positive effect on the prevention and delay of hepatic fibrosis.
VDBP, also known as gc-globulin, is a plasma glycoprotein with a molecular weight of 56-58 kDa, composed of 458 amino acids. VDBP is primarily synthesized in the liver and expressed in several organs and tissues, including the liver, kidneys, gonads and fat, and neutrophils. Unlike serum vitamin D following seasonal variation, serum concentration of VDBP is stable during the year[17]. VDBP has numerous physiological roles, including transporting vitamin D and its metabolites, regulating bone development, scavenging actin, and regulating immune and inflammation process[18].
In the present study, serum VDBP levels in CHB children were significantly lowerthan those in healthy controls, and the levels of VDBP were markedly decreased in patients with HBV genotype C, HBsAg, HBeAg, and detectable HBV DNA. VDBP levels were negatively correlated with HBV DNA levels in children with CHB. The results demonstrated that serum levels of VDBP were associated with HBV genotype and negatively correlated with HBV replication. The reasons for the decrease in VDBP may be lower production in impaired hepatic cells and greater consumption of scavenging actin, mediating monocyte and neutrophil chemotaxis to inflammatory foci caused by HBV replication[18].
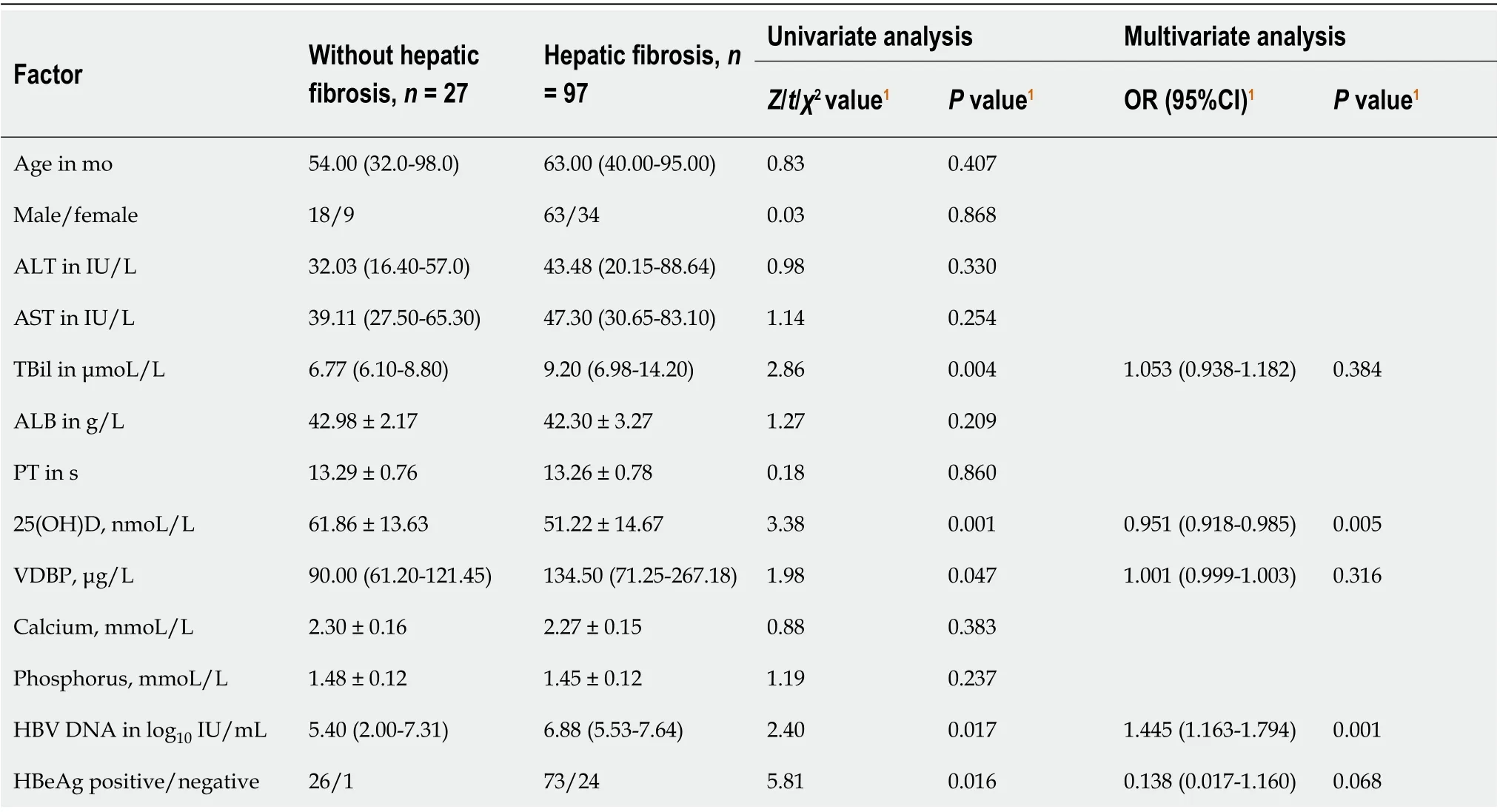
Table 3 Univariate and multivariate analyses of hepatic fibrosis in children with chronic hepatitis B
Children with CHB and hepatic fibrosis had higher VDBP level, and VDBP levels increased in patients with grade 1 and 2 but decreased in patients with grade 3 fibrosis. Although it has been reported that hepatocytes are compensatory in the early stage of fibrosis and that the remaining normal hepatocytes produce more VDBP to replenish the shortage in the circulation caused by massive consumption[19], the detailed mechanism of VDBP variation in different stages of hepatic fibrosis is still unknown. The association between VDBP and hepatic fibrosis and the underlying mechanisms warrant further investigation.
Serum levels of 25(OH)D and VDBP were both decreased in children with CHB, but there was no significant correlation between the two markers. This may be due to the multifunctional property of VDBP. VDBP has many other important physiological functions other than being a transporter and < 5% of the binding sites on VDBP are occupied by 25(OH)D[18]. Most VDBP may be involved in the immune response and inflammatory process caused by HBV infection. The different biological functions of the two markers result in the incompletely consistent variation.
CONCLUSION
Both serum 25(OH)D and VDBP levels are significantly decreased in children with CHB, VDBP may be negatively correlated with HBV replication, and low level of serum 25(OH)D is an independent factor associated with the development of hepatic fibrosis. Therefore, maintaining sufficient 25(OH)D level in children with CHB could be valuable to prevent or delay the process of fibrosis, and the development and application of VDBP analogues might be a consideration for the management of CHB.

Figure 1 Flow chart of the study. 25(OH)D: 25-hydroxy vitamin D; CHB: Chronic hepatitis B; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; VDBP: Vitamin D binding protein.
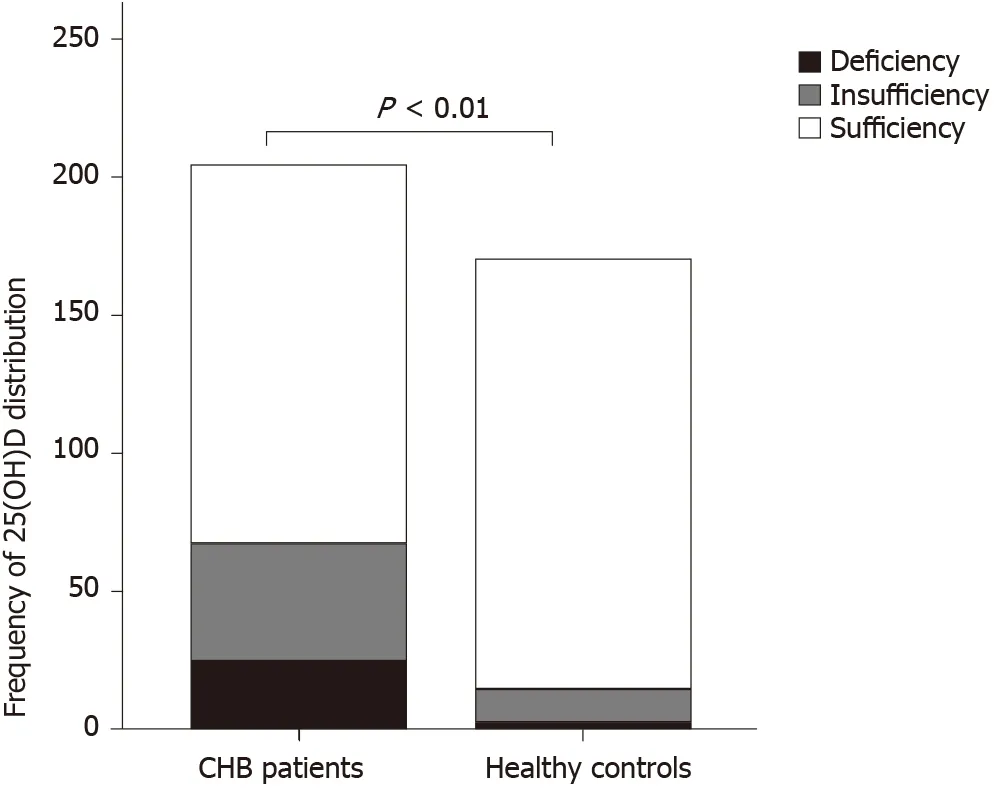
Figure 2 Distribution frequency of serum 25-hydroxy vitamin D in chronic hepatitis B patients and healthy controls. 25(OH)D: 25-hydroxy vitamin D; CHB: Chronic hepatitis B.

Figure 3 Distribution and correlation of serum 25-hydroxy vitamin D and vitamin-D-binding protein levels with other parameters in children with chronic hepatitis B. A: Correlation of serum 25-hydroxy vitamin D [25(OH)D] level with age; B: Correlation of serum 25(OH)D level with serum total bilirubin level; C: Correlation of serum vitamin-D-binding protein level with serum hepatitis B virus deoxyribonucleic acid. 25(OH)D: 25-hydroxy vitamin D; DNA: Deoxyribonucleic acid; HBV: Hepatitis B virus; TBil: Total bilirubin; VDBP: Vitamin-D-binding protein.
ARTICLE HIGHLIGHTS


Research motivation
Few studies have been reported about the serum levels of vitamin D and VDBP and their clinical significance in children with CHB. Some studies have revealed that vitamin D can reduce profibrotic factors and collagen expression and that they have immunomodulatory effects on immune cells, including T cells. Patients infected with HBV is pathologically characterized as the functional impairment of T cells. The elimination of HBV in CHB patients largely depends on the cellular immune response mediated by T helper (Th)1 cells. Combination of the active form of vitamin D and vitamin D receptor may inhibit the proliferation of Th1 cells and the release of cytokines, and concurrently activate Th2 cells, thus regulating immune responses. Vitamin D and its metabolites in the circulation are mainly transported by VDBP, which is a multifunctional plasma glycoprotein that can mediate monocyte and neutrophil chemotaxis to inflammatory foci caused by HBV replication. The present study aimed at exploring the clinical significance of serum 25(OH)D and VDBP levels in children with CHB. The results will help to illustrate whether vitamin D and VDBP levels are associated with HBV replication and hepatic fibrosis.
Research objectives
The main objective of this study was to investigate the serum levels of vitamin D and VDBP in children with CHB and analyze the association of vitamin D and VDBP with HBV replication and hepatic fibrosis.
Research methods
We enrolled 204 children with CHB who were admitted to Hunan Children’s Hospital in summer and autumn between 2018 and 2019 and 170 healthy controls. Children with CHB included: 164 hepatitis B e antigen (HBeAg) positive and 40 HBeAg negative; 193 hepatitis B surface antigen (HBsAg) positive and 11 HBsAg negative; 164 with detectable HBV DNA and 40 with undetectable HBV DNA; 131 with HBV genotype B and 23 with HBV genotype C; and 97 with hepatic fibrosis and 27 without hepatic fibrosis. Serum levels of 25(OH)D, VDBP, liver function markers, and other clinical parameters were collected for analysis of the association with vitamin D and VDBP. Mann-WhitneyUtest, Kruskal-WallisHtest, orttest was used to analyze serum 25(OH)D and VDBP levels in different groups of subjects. Spearman rank correlation test was utilized to analyze the correlation of 25(OH)D and VDBP with other markers. Finally, statistically significant factors for hepatic fibrosis determined by univariate analysis were further analyzed by binary multivariate logistic regression analysis.
Research results
Children with CHB had lower serum 25(OH)D (56.64 ± 17.89 nmoL/L) and VDBP [122.40 (70.74-262.84 μg/L)] levels than healthy controls had (P< 0.001). Serum 25(OH)D and VDBP levels were significantly different among the stages of hepatic fibrosis (P< 0.05). Patients with HBV genotype C, HBsAg, HBeAg, and detectable HBV DNA had lower VDBP levels than patients with HBV genotype B, without HBsAg and HBeAg, and undetectable HBV DNA (P< 0.05). Serum 25(OH)D level was negatively correlated with age and serum total bilirubin level (r= -0.396 and -0.280, respectively,P< 0.001). Serum VDBP level was negatively associated with HBV DNA (log10IU/mL) (r= -0.272,P< 0.001). Serum 25(OH)D level was not correlated with VDBP level (P> 0.05). Univariate (P< 0.05) and multivariate logistic regression analysis showed that low level of 25(OH)D (odds ratio = 0.951, 95% confidence interval: 0.918-0.985) and high level of HBV DNA (odds ratio = 1.445, 95% confidence interval: 1.163-1.794) were independently associated with hepatic fibrosis (P< 0.01).
Research conclusions
Children with CHB have lower serum levels of 25(OH)D and VDBP. Serum VDBP level is negatively correlated with replication of HBV. Low level of 25(OH)D is independently associated with hepatic fibrosis in CHB children. There is no significant association between serum levels of 25(OH)D and VDBP.
Research perspectives
Previous studies and our data indicate that vitamin D and VDBP are involved in the inflammatory response and immunological regulation in children with CHB. Vitamin D may play an antifibrotic role by reducing stimulation of hepatic stellate cells, profibrotic factors, and collagen expression. VDBP may have a positive effect on alleviating liver injury caused by HBV replication. We speculate that sufficient concentration of vitamin D and VDBP in children with CHB may be beneficial for the progress and prognosis of the disease. The potential value of vitamin D detection in monitoring hepatic fibrosis development and VDBP detection in evaluating the degree of inflammation and liver injury, more detailed mechanism of vitamin D and VDBP on the progression of CHB, and whether VDBP analogs may become a treatment measure for children with CHB warrant further investigation.
ACKNOWLEDGEMENTS
The authors thank Professor Lian Tang from the Department of Liver Disease in Hunan Children’s Hospital, China for her advice and language modification of this manuscript.
 World Journal of Gastroenterology2021年3期
World Journal of Gastroenterology2021年3期
- World Journal of Gastroenterology的其它文章
- Peritoneal dissemination of pancreatic cancer caused by endoscopic ultrasound-guided fine needle aspiration: A case report and literature review
- Comparative study on artificial intelligence systems for detecting early esophageal squamous cell carcinoma between narrow-band and white-light imaging
- Trends in the management of anorectal melanoma: A multi-institutional retrospective study and review of the world literature
- Circular RNA AKT3 governs malignant behaviors of esophageal cancer cells by sponging miR-17-5p
- Screening colonoscopy: The present and the future
