Active surveillance in low risk papillary thyroid carcinoma
Fabian Pitoia, Anabella Smulever
Fabian Pitoia, Anabella Smulever, Division of Endocrinology, Hospital de Clínicas, University of Buenos Aires, Buenos Aires 1120, Argentina
Abstract In recent decades, while the incidence of thyroid cancer has increased exponentially around the world, mortality has remained stable. The vast majority of this increase is attributable to the identification of intrathyroidal papillary microcarcinomas, which exhibit slow growth rates with indolent courses. A diagnosis of thyroid cancer based upon the presence of these small tumors could be considered as an overdiagnosis, as the majority of these tumors would not likely result in death if left untreated. Although surgical resection was the classical standard therapy for papillary microcarcinomas, active surveillance (AS)has emerged over the last three decades as an alternative approach that is aimed to recognize a minority group of patients who will clinically progress and would likely benefit from rescue surgery. Despite the encouraging results of AS, its implementation in clinical practice is strongly influenced by psychosocial factors.The aim of this review is to describe the epidemiology, clinical evolution,prognostic factors, and mortality of papillary thyroid microcarcinomas. We also summarize the AS strategy according to published evidence, characterize the criteria for selecting patients for AS according to risk factors and environmental characteristics, as well as analyze the current limitations for AS implementation.
Key words: Active surveillance; Low risk; Papillary thyroid carcinoma; Observation;Papillary thyroid microcarcinoma; Thyroid cancer
INTRODUCTION
The incidence of thyroid cancer has increased over the last few decades, which is likely due to overdiagnosis, especially considering that thyroid cancer mortality remained stable at a rate of approximately 0.5 cases per 100000 persons[1]. Most diagnosed thyroid tumors are papillary microcarcinomas (PMCs) that are characterized by a slow growth rate and indolent course. The majority of these tumors would not likely result in death if left untreated[2,3]. Indeed, the prevalence of occult papillary thyroid microcarcinomas identified in autopsy studies were reported to be as high as 35%[4]. Although thyroidectomy was the standard PMC for years, active surveillance (AS) has emerged as an alternative approach that involves regular observation aimed to recognize a minority of patients who will clinically progress and would likely benefit from rescue surgery[3]. The delay in surgical treatment for properly selected patients with low-risk papillary thyroid carcinomas (PTCs) is not associated with a higher risk of persistent or recurrent structural disease compared to immediate intervention[3,5]. Data from the first researchers that carried out AS trials in PMCs from Japan, and later from diverse countries around the world, have demonstrated that this approach is a safe alternative for managing low risk papillary PTCs[3,6]. However, the implementation of AS in clinical practice is strongly influenced by psychosocial factors, particularly in less economically developed countries in which, as we previously showed, only around one quarter of patients appear to accept this modality[5,7,8].
The aim of this review is to describe the epidemiology, clinical evolution,prognostic factors, and mortality of papillary thyroid microcarcinomas. Here, we also summarize the AS strategy according to published evidence, characterize the criteria for selecting patients for AS according to risk factors and environmental characteristics, as well as analyze the current limitations for AS implementation.
SEARCH RESULTS
Seventy-five studies were included in this review. The search flow diagram is shown in Figure 1.
EPIDEMIOLOGY OF PAPILLARY THYROID MICROCARCINOMAS
Papillary thyroid carcinomas account for about 90% of malignant thyroid neoplasms[9]. Statistical data from the National Cancer Institute in the United States warned that its incidence has tripled in recent decades, increasing from 4.9 cases per 100000 persons in 1975 to 15.3 cases per 100000 persons in 2013[1,10]. The same source informed that there were 54000 new cases of thyroid carcinoma detected in 2018, and it is estimated that 1.2% of the population will be diagnosed with thyroid cancer at some point during their lifetime[10]. This rising trend has been broadly observed around the world. For example, in South Korea, the reported incidence of PTC in 2011 was 15 times higher than in 1993[11]. Additionally, Vaccarellaet al[12]reported a similar phenomenon in many countries from Europe and Oceania. While this upward trend is overwhelming, global statistics agree that these findings are attributed to an increase in the detection of small intrathyroidal papillary carcinomas, especially those smaller than 1 cm, which are also called microcarcinomas[1,12]. Furthermore, mortality related to thyroid cancer has remained stable over the last four decades, ranging from 0.4 to 0.5 deaths per 100000 persons[1,10].
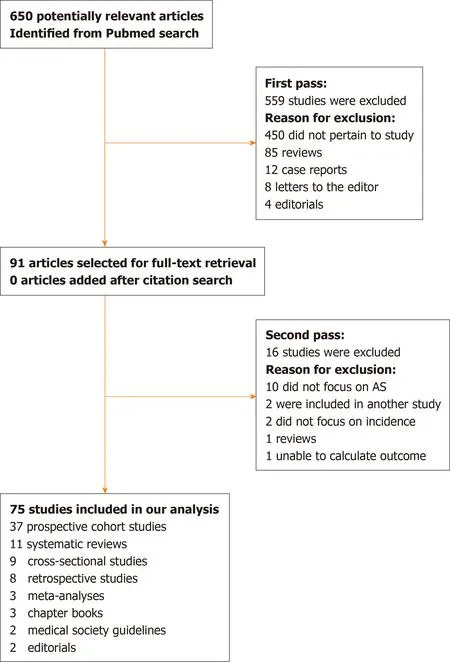
Figure 1 Search strategy for this review. AS: Active surveillance.
INCIDENTAL DETECTION OF MICROCARCINOMAS
Papillary microcarcinoma is the most common type of well-differentiated thyroid cancer[2]. A significant proportion of PMCs are incidentally diagnosed from imaging undertaken for a variety of reasons[13]. Thus, frequencies of thyroid nodules were incidentally reported in up to 67% of neck ultrasounds[14], 25% of computed topographies (CT)[15], and 1-2% of ¹F-fluorodeoxyglucose (¹F-FDG) PET-CT scans[16].Moreover, thyroid cancer diagnoses were observed in 2%, 11%, and 35% of the previously described image studies, respectively[14-16]. Similarly, the incidental finding of PMCs in the anatomopathological study of removed thyroid glands due to benign pathology has been reported to be close to 10%[1]. The higher prevalence of these findings may reflect the wider use of diagnostic imaging technologies, more accurate pathological analysis of the entire thyroid gland, greater access to health care, and improved patient socioeconomic conditions, particularly in developed countries[2,17].
On the other side, several historical autopsy studies showed a high rate of occult orlatentthyroid carcinomas, with a reported prevalence ranging from 4.2-35.6%[4,18,19]. In a recent Korean meta-analysis based on 989 autopsies from 15 studies, it was described that about 80% of occult PMCs did not exceed 1 mm in diameter, with multifocality present in up to half of the cases[19]. Lastly, the prevalence of these asymptomatic tumors that were identified in autopsies was estimated to be between 100 and 1000 times higher than clinical carcinomas[20].
PMC OUTCOMES
As aforementioned, the outcome of PMC patients is typically excellent. The related mortality is very low, reaching to only 0.3% in clinical series, which includes patients with clinical lymph nodes and distant metastases as the initial form of presentation[21].Accordingly, one foci of PMC implies a risk of recurrence between 0.5-1% at 10-12 years, and increases up to 5% when multiple foci or clinical lymph node metastases are evident at the time of diagnosis[13,22]. Wadaet al[23]studied the frequency and pattern of lymph node metastases in 259 patients with PMCs who had undergone total thyroidectomy with lymph node dissection. After prophylactic lymph node dissection, 64% of patients exhibited lymph node metastases in the central neck compartment and 44% in the lateral compartment. A similar risk of recurrence was found between this group and 166 patients with PMCs who underwent total thyroidectomy alone, accounting for 0.4% and 0.6%, respectively[23]. This finding shows the indolence of nodal micrometastases in patients with low risk PMCs[23].Finally, distant metastases in PMCs occur exceptionally, with an estimated frequency less than 1%[24]. Currently, no studies around the world have been published that demonstrate the presence of distant metastases in patients with low-risk PMCs under AS.
From these data, it can be concluded that most low-risk papillary thyroid microcarcinomas have incidental findings, indolent courses, and are not lifethreatening[10,13,22,23].
PAPILLARY THYROID CARCINOMA
AS is a strategic management tool for patients with slow-growing tumors, which can be used to avoid excessive treatments[25]. This approach emerged as an alternative to definitive treatments, including surgery and the risk of its adverse events, until the stage of disease justifies such interventions[25].
AS appeared to be a valid alternative over the past few decades based on the high number of side effects generated by surgical treatment in patients with localized lowrisk prostate cancer[26]. In these patients, the benefits of observation outweighed the risk of health-related quality of life reduction, owing to negative effects on urinary,bowel, and erectile functions[26].
Unlike passive observations, AS involves a close and dynamic follow-up. This approach implies a wide knowledge from both the medical team and the patients, and that clinical progression, if any, would only occur in a minority of cases[25]. For this reason, the term “deferred surgery” as an equivalent expression to AS takes into account these few patients who will be offered, if necessary, the appropriate surgical intervention at the right moment[5,7]. Based on this premise, some researchers from the Kuma Hospital in Kobe, Japan carried out the first clinical trial in 1993 of AS in PMCs.In addition, Itoet al[3]years later published the initial results. After an exhausting analysis of the natural history and biological behavior of PMCs, this study hypothesized that although a small minority of PMCs progress, there have no influence on final disease prognosis if surgery is postponed until progression occurs[3].Moreover, if we consider surgical treatment for all PMC patients, it could result in more harm than benefits[3,27]. Based on the evidence provided by these researchers, AS has progressively become popular, with several studies demonstrating its worldwide applicability .
ADVERSE EVENTS AND MEDICAL COSTS OF USUAL TREATMENTS IN LOW-RISK PMCs
The most frequent adverse effects of total thyroidectomy include hypothyroidism,hypoparathyroidism, and vocal cord paralysis due to recurrent laryngeal nerve injury.Less frequently, post-surgical hematomas and surgical wound infections may be found, among other complications[28]. These adverse events, transient or permanent,exert a negative impact on patient quality-of-life[27]. In a multicenter study that included 14934 patients undergoing thyroidectomy, the overall complication rate was 17.4%, 7% of which were permanent[28](Table 1). The frequency of postoperative adverse events tended to decrease when surgery was performed by high volume surgeons[29,30]. However, even in surgeries performed by high volume surgeons, the risk of permanent hypoparathyroidism and laryngeal recurrent nerve paralysis may reach 1-3% after total thyroidectomy with prophylactic lymph node dissection, and less than 1% after total thyroidectomy without lymph node dissection or lobectomy[29].Although the risk of permanent hypocalcemia was remarkably lower in patients who underwent lobectomy, one out of five patients required hormonal replacement with levothyroxine[31]. In addition, a considerable proportion of patients claimed to have quality-of-life alterations such as neurocognitive and mood disorders[32].

Table 1 Postoperative adverse events of thyroid surgery in a cohort of 14934 patients[28]
On the other hand, Odaet al[27]compared the outcomes and adverse events of 2153 patients with low-risk PMCs who underwent immediate surgery (45%)vsAS (55%).The oncological outcomes were excellent in both groups. However, the incidence of adverse events such as vocal cord paralysis and temporary or permanent hypoparathyroidism, hypothyroidism, and surgical scars was significantly higher in the immediate surgery group, even though they were all performed by high-volume endocrine surgeons[27](Table 2 ).
Considering the impact of surgeryvsAS on quality-of-life (QoL), a recent published study compared QoL parameters in 43 patients with PMCs under ASvs148 patients who underwent immediate lobectomy. Patients from the immediate surgery group experienced more QoL alterations. These subjects expressed more neuromuscular,throat, and surgical scar-related problems, as well as decreased emotional wellbeing[33].
Regarding the medical cost associated with treating thyroid cancer as a matter of relevance in the health system, the estimated value in 2013 for the care of patients diagnosed with thyroid cancer in the United States after 1985 was USD 1.6 billion, and it is projected to increase to USD $3.55 billion by 2030[34]. In accordance, researchers in Japan and Hong Kong compared the total costs of two groups of patients with PMCs who underwent immediate surgeryvsAS for 10 years[35,36]. Similarly, both found that the total cost of immediate surgery over 10 years was 4.7 to 6.5 times higher than AS.Furthermore, the total cost of immediate surgery, which included the estimated price of an eventual deferred surgery during AS, was still 4.1 times more expensive than AS during the same period of time[35,36]. In Argentina, the same costs of surgery were calculated to be between 3 to 4 times more expensive than AS (unpublished data).
IMPLEMENTATION OF AS: THE CORRECT MOMENT FOR DIAGNOSIS
The worldwide implementation of AS is not uniform. There is a lack of consensus about the minimum diameter of a suspicious thyroid nodule required to undergo a fine needle aspiration biopsy (FNAB) as a requisite to apply for an AS protocol[13,30,37,38].In Japan, for example, high risk ultrasound thyroid nodules greater than or equal to 5 mm are cytologically confirmed by ultrasound-guided FNAB, following the guidelines of the Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons (JSTS/JAES)[37]. This practice is carried out because patients may visit other doctors who may recommend unnecessary surgery[37]. According to these guidelines, cytological diagnosis would provide two additional benefits: Optimize patient adherence to AS programs, and enhance the reliability of research studies[30].Conversely, the 2015 American Thyroid Association (ATA) guidelines[13]recommend AS without FNAB for sub-centimeter thyroid nodules with highly suspicious ultrasonographic features for thyroid cancer. This recommendation is an effort tofurther minimize both the overdiagnosis of patients with microcarcinomas as well as the consequences of overtreatment[13,17,38]. An exception to performing FNAB on a subcentimeter nodule is evidence for aggressiveness features, such as extrathyroidal extension, multiple foci, high risk location for potential local complications, and/or lymph node metastases[13]. Thyroid nodules that are incidentally detected by ¹F-FDG PET, are smaller than 1 cm in diameter, and have ultrasound criteria of malignancy could also benefit from FNAB in the rare cases in which the diagnosis of intrathyroidal metastases affects treatment of the primary cancer[16,39]. A recent Argentinian publication about the experience of AS in Latin America[8]included a subgroup of patients in which PTC diagnosis was established after ultrasonographic surveillance of a single thyroid nodule that grew after a previous ultrasonographic follow-up, or a second FNAB changed the original benign cytological diagnosis. We referred to this type of surveillance as “undercover”, and in some cases also as“retrospective”. The outcomes of these cases were similar to those with prospective AS[8](Figure 2). Currently, the Kuma Hospital recommends AS as the first line option in the management of patients with low-risk PMC[30]. Patients who accept this approach are evaluated with thyroid and neck ultrasound every 6 mo during the first year, and then annually thereafter. If primary tumor grows 3 mm or more in the greatest dimension from the baseline, there is evidence that extrathyroidal extension,nodal metastases, progression might occur, and thus surgery is recommended. If the tumor only exhibits enlargement without other aggressive features, some patients may continue AS until the tumor reaches 13 mm[40]. If the diagnosis of lymph node metastasis is confirmed by FNAB and/or thyroglobulin measurements with needle wash-out, total thyroidectomy with lymph node dissection is indicated[6]. In our study[8], 75% of patients who exhibited tumor enlargement refused surgery. These patients continued with AS, and none of them showed evidence of a new increase in tumor diameter, cervical, or distant metastases after a median follow-up period of 42 mo (range 36-84 mo)[8].

Table 2 Unfavorable events following active surveillance and immediate surgery (adapted from Oda et al[27], 2018)
ATA guidelines do not support AS as the first line treatment in patients with PMC,but they consider AS to be an alternative to immediate surgery, mainly in patients with high surgical risk or poor life expectancy due to comorbid conditions (e.g., severe cardiopulmonary disease, other malignant pathologies, advanced age,etc).Furthermore, there may be concurrent medical or surgical issues that take priority over thyroid surgery[13].
The most appropriate moment to decide rescue surgery still remains unclear. In this regard, Tuttleet al[17]demonstrated that an increase in tumor volume greater than 50%precedes enlargement by 3 mm or more in diameter, which might enable an early diagnosis of progression. However, Miyauchiet al[41]emphasized that if this parameter is taken into account, the enlargement of a tumor, for example, from 6 mm× 6 mm × 6 mm to 7 mm × 7 mm × 7 mm, represents a 59% increase in volume. This criterion, therefore, could be too sensitive to diagnose progression, particularly when also considering the limitation of ultrasonography to show only one mm of change.However, none of the patients who underwent AS that were included in clinical trials worldwide developed distant metastases or died from thyroid carcinoma, regardless of the adopted progression criteria[6,17].
Finally, there is a consensus that the proper selection of patients as candidates for this less aggressive approach should be carefully considered, taking into account the set of values and preferences of the patients and their medical team[42].

Figure 2 Decision flow after diagnosis of suspicious ultrasonographic thyroid nodule leading to active surveillance or surgery[8]. FNAB: Fine needle aspirative biopsy; PTC: Papillary thyroid carcinoma.
AS AROUND THE WORLD
Ever since promising results from Japanese clinical trials were reported, which was the pioneering study, AS gradually became popular worldwide. Thus, additional data were obtained from diverse populations with particular cultures, dietary habits,sociological, and psychological characteristics that increased knowledge of the natural history of PMCs. As previously mentioned, some researchers in 1993 proposed AS as an alternative in patients with low-risk PMCs at Kuma Hospital, but it was only after 10 years of prospective follow-up that Itoet al[3]published the initial results, which showed that 70% of 162 patients with PMC under AS did not exhibit progression[3]. In 2010, these authors reported, from a population of 340 eligible patients under AS, that the incidence of tumor growth greater than or equal to 3 mm and diagnosis of lymph node metastases was 15.9% and 3.4%, respectively[43]. Two years later, Dr. Sugitaniet al[44]from the Cancer Institute Hospital in Tokyo showed that from a cohort of 300 patients with asymptomatic PMC, 22 (7%) had an increase of 3 mm in larger diameter and three patients (1%) developed apparent lymph node metastases. Over the years,the prospective enrollment of patients in Japanese AS protocols was exponential. Dr.Itoet al[6]in 2014 finally showed that, out of 1235 patients with PMC over 10 years of follow-up, that there was an 8% rate of tumor growth and 3.8% rate of metastatic lymph node presence. In addition, this was the first study that demonstrated the inverse relationship between disease progression and patient age at diagnosis.Furthermore, none of the included subjects underwent rescue surgeries due to disease progression or died from thyroid cancer-related causes[6,44]. The first prospective AS study conducted in West countries was published in 2017 by Dr. Tuttleet al[17]from the Memorial Sloan Kettering Cancer Center Hospital in the United States. This group extended the inclusion criteria to tumors up to 1.5 cm in maximum diameter, and also analyzed the kinetics of tumor volume. From 284 subjects under AS, an increase in diameter ≥ 3 mm was detected in 3.8% of patients, with a cumulative incidence of 2.5% at 2 years and 12.1% at 5 years. In contrast, 12.7% showed ≥ 50% increase in tumor volume, which preceded an 8.2 mo increase detected by diameter measurements[17]. Considering the behavior of the 59 included tumors between 1 and 1.5 cm compared to the 230 remaining PMCs, similar stability rates between groups and/or very slow disease progression rates were found[17]. Tumor volume doubling time was 2.2 years, which demonstrated an exponential growth pattern[17]. In consonance with tumor kinetics analysis, two studies in 2018 proposed that an increase in volume is more likely to be detected than a small increase in diameter[45,46].Miyauchiet al[45]retrospectively studied 169 patients with PMCs followed by AS for 10 years, and were classified into four categories according to tumor volume doubling rates. Therefore, 3% and 22% of patients exhibited fast and slow growth, respectively.Fifty seven percent remained stable, and seventeen percent showed a decrease in size[45]. Concurrently, Ohet al[46]showed from 370 PMCs patients who underwent AS that 86 (23.2%) experienced a volume increase, whereas 13 patients (3.5%) experienced an > 3 mm increase in maximum diameter during a median 32.5 mo follow-up period.The cumulative incidence of tumor volume increase over time was 6.9% at 2 years,17.3% at 3 years, 28.2% at 4 years, 36.2% at 5 years, and 47.5% at 6 years[46]. Findings from these studies revealed that tumor volume doubling time allows for a detection period of rapid growth before the development of clinical presentation, which may be used as an early indicator of progression[45,46].
Finally, a recent prospective study that included 392 patients under AS described the results of 61 patients with low-risk PTCs in the T1b state who refused surgery[47].The range of tumor diameter was 11 to 16 mm with a median of 8 years follow-up period. In this study, 7% of patients had an increase in diameter, and nodal metastases were disgnosed in 3%. On the other hand, the 5-year progression rate was 5%, with no significant differences compared with patients with PMCs. Long-term rescue surgery was not associated with adverse outcomes between groups[47].
Table 3 summarizes the main studies of AS around the world.
AS IN LATIN AMERICA
In Latin America, adverse socioeconomic and cultural circumstances, in addition to inhomogeneous access to expert medical teams and high quality neck ultrasound,may potentially affect the implementation of AS. Nevertheless, some studies show that this approach can be carried out in centers with high expertise in the management of patients with thyroid cancer[8,48,49]. The first Argentine report was carried out from 2014 to 2018, and included 137 patients who attended the Hospital de Clínicas[8]. This study described the frequency of AS acceptance in patients with lowrisk PTCs, which included the clinical characteristics and outcomes of patients who chose this approach. The included patients did not show any evidence of: (1) US extrathyroidal extension; (2) Tumors adjacent to the recurrent laryngeal nerve or trachea; and/or (3) US regional lymph node metastasis or clinical distant metastasis.PTC progression was defined as the presence of: (1) A tumor larger than ≥ 3 mm; (2)Novel appearance of lymph node metastasis; and (3) Serum thyroglobulin doubling time of less than 1 year. For patients with these features, surgery was recommended.Surprisingly, only 34 (25%) of 136 patients eligible for AS accepted this approach, and around 10% of those who accepted abandoned it due to anxiety. The frequency of patients with tumor enlargement was 17% after a median of 4.6 years follow-up without any evidence of nodal or distant metastases, in accordance with other publications. After a median AS time of 4 years, 10 patients underwent surgical treatment due to: Anxiety (n =3), tumor growth (n =1), Tg doubling time < 1 year (n=1) and, finally, in the context of retrospective surveillance, tumors that measured ~15 mm (n= 5). All patients showed no sign of disease after a median follow-up period of 3.8 years after surgery[8]. In Colombia, Sanabriaet al[48]published similar data with regard to tumor stability, with a 12 mo follow-up. From 57 PMCs, 16 (28%)experienced an increase of 2 mm, and two (3.5%) grew more than 3 mm, while 9% of patients underwent surgery[48]. Finally, regarding tumor progression, a recent Brazilian study showed analogous results to the aforementioned Latin American research study[49]. In conclusion, outcomes are similar to those reported by other series around the world, and although AS is not an option that most patients will consider as a first line treatment, this approach is likely to be carried out in our area[8,47,48].
MODELS OF PROPER PATIENT SELECTION
Proper selection of a candidate patient for AS is the key to reaching successful outcomes with this less aggressive approach. In an effort to establish a patient’s selection of clinical frame, Nickelet al[73]proposed a stratification model for decisionmaking. Based on the features of three complementary domains (patient, medical team, and tumor/imaging characteristics), this author proposed that a classification of patients as appropriate or inappropriate for AS was mandatory[42](Figure 3). Thus,they suggested that patients that were classified as ideal or appropriate should be offered the two management options of AS or immediate surgery, and the final decision would take patient preferences into account. On the other hand, for patients classified as inappropriate, AS would not be an option[7,42]. Certainly, this classification could vary between institutions and countries based on multiple factors. These factors included patient preferences based on their values, beliefs, and socio-cultural environment, the philosophies and experience of the treating medical team, the availability of high quality ultrasound, and access to the health system[42].
Ideal and appropriate patients
Typically, the ideal patient is over 60 years of age, with a single thyroid nodule with well-defined tumor margins by ultrasound, as well as well-defined borders surrounded by normal thyroid parenchyma. In addition, the patient is willing to accept an observation protocol by an experienced multidisciplinary team that uses high quality neck ultrasound[42]. Although the ideal tumor size is ≤ 1 cm in themaximum dimension, patients with tumors between 1-1.5 cm are currently considered as appropriate for AS[42]. As previously mentioned, the favorable results observed in tumors that were classified as T1b in terms of stability may show that a tumor size of 1 cm is an arbitrary cut off[17,40,47]. Moreover, PMCs with minimal extrathyroidal extension on the anterior or lateral surface are not contraindicated for AS[40]. Finally,the ideal candidate for observation is a compliant patient with a “minimalist”mentality, who also believes that a surgical intervention may be necessary in the future[7,42].
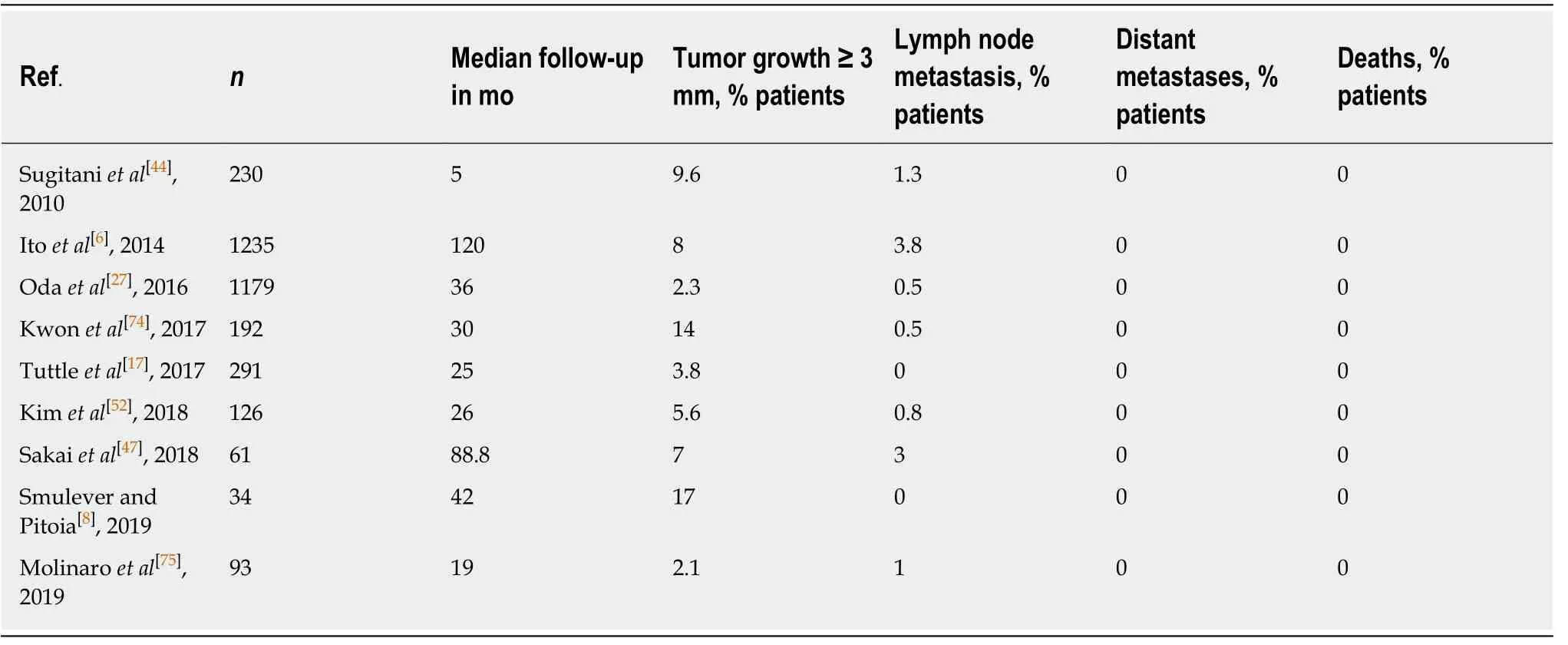
Table 3 Highlighted active surveillance trials
Compared to ideal patients, appropriate patients have a higher risk of disease progression[7]. The latter are usually between the ages of 18 to 59 years at the time of diagnosis, and show presence of benign ultrasound findings (thyroiditis, benign nodules, or reactive lymph nodes)[7]. At present, there is no evidence to suggest that chronic lymphocytic thyroiditis influences the biological behavior of PMC, since both diseases have been shown to have considerably increased detection over the last few decades. Powered prospective studies will be needed to clarify this issue[50]. Finally, a tumor location of 2 mm or less from the thyroid capsule constitutes another characteristic of appropriate patients[7]. In any case, although a 10% progression rate is expected in this cohort, the treatment offered in this instance by an experienced team is very effective and will be associated with excellent clinical outcomes[42].
Tuttleet al[7]described that only 4% of their AS cohort were classified as ideal candidates and 94% as appropriate. Thus, if AS were only offered to patients denominated as ideal, most low-risk PTCs would be incorrectly classified as not applicable for an observational approach.
Tumor characteristics for the proper selection of AS candidates are illustrated in Figure 3.
Inappropriate patients: Contraindications for AS
There are several contraindications for AS that have been sorted into two main categories that are related to aggressive tumor characteristics and localization. The first category includes the presence of lymph node and/or distant metastases(extremely rare), vocal cord paralysis due to invasion of the recurrent laryngeal nerve by the tumor, and/or cytological evidence of an aggressive variant (e.g., tall cell variant or poorly differentiated thyroid carcinoma)[7,30,42]. The second includes tumors attached to the trachea or located along the path of the recurrent laryngeal nerve(RLN). The rationale of this relative contraindication is not associated with aggressive tumor behavior, but instead with potential tumor invasion into local structures, even if it occurred in minimal proportion[6,30,42]. Itoet al[51]characterized an association between the angles formed by the tumor and tracheal surface with the chance of tracheal invasion. In that study, 12 (24%) of 51 PMCs greater than 7 mm in maximal diameter, which were composed of obtuse angles, showed tracheal invasion that required resection of cartilage and mucosa, while none of the 286 PMCs that formed acute or almost right angles showed significant tracheal invasion[51]. On the other hand, the risk of RLN invasion was associated with the absence of normal tissue interposed between the tumor and thyroid surface in the direction of the nerve. Thus,9% of 98 PMCs greater than 7 mm without normal interposed tissue required a partial or segmental RLN resection, while none of the 776 PMCs with normal borders exhibited microscopic RLN invasion. As expected, none of the PMCs lower than 7 mm showed tracheal or RLN invasion[51](Figure 4).
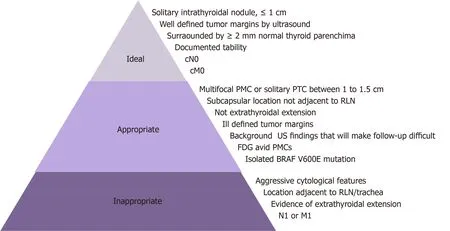
Figure 3 Tumor characteristics for proper selection of candidates for active surveillance[7]. PMC: Papillary thyroid microcarcinoma; RLN: Recurrent laryngeal nerve; US: Ultrasound.
Other authors propose that the tumor volume doubling time (TVDT) variable should be incorporated when classifying patients for AS according to tumor size[7,17].Hence, the TVDT would allow for the differentiation of patients with slow-growing tumors (3 to 5 years) from those with relatively rapid progression (1 to 2 years)[17]. An increase of 3 mm or more in size, or 50% or more of tumor volume, would classify a patient as inappropriate. Nonetheless, this may not require immediate rescue surgery,especially if the nodule is small, limited to the thyroid, grows very slowly, and/or the patient is older or presents comorbidities[7].
Finally, from a psychosocial point-of-view, which will be developed later, the inappropriate patient for AS is generally unable to accept this approach in the context of a "maximalist" mentality[7,42].
RISK FACTORS FOR TUMOR PROGRESSION
The identification of risk factors that allow the prediction of tumor progression in patients during AS is a current research topic of interest[52]. Although the data provided by prospective studies are scarce, the following predictive biological elements were proposed.
Age at diagnosis
Thanks to an outstanding publication in 2014, Itoet al[6]showed there is an inversely proportional relationship between age at diagnosis and risk of disease progression.Converse to more advanced stages of thyroid cancer, elderly patients were less likely to progress and were the best candidates for AS[6]. The 10-year clinical progression rate in patients under 40 was 8.9% (including a higher probability of developing lymph node metastases), while patients between 40 and 60 years of age and over 60 had progression rates of 3.5% and 1.6%, respectively[6]. Recently, this group estimated a lifetime probability of disease progression during AS for each age decade between the 20s and 70s[45]. Thus, in patients in their 20s, 30s, 40s, 50s, 60s, and 70s the probabilities of progression were 48.6%, 25.3%, 20.9%, 10.3%, 8.2%, and 3.5%,respectively[45]. However, while patients who are in their 20s at diagnosis were more likely to progress, only half would require rescue surgery during their lives and likely none of them would show life-threatening recurrence or die from thyroid carcinoma[45]. Finally, in consonance with these findings, Tuttleet al[17]demonstrated that patients younger than 50 years at diagnosis had a nearly 5-fold higher likelihood of experiencing tumor growth compared with patients 50 years or older (27.3%vs4.6% at 5 years, respectively).
Serum thyrotropin levels
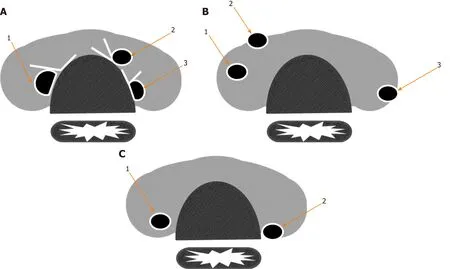
Figure 4 Risk of cervical structure invasion related to tumor location (adapted from Miyauchi et al[40], 2018). Tumors composing obtuse angles with trachea,absence of normal thyroid tissue interposed between tumor and course of recurrent laryngeal nerve (RLN), and tumor location close to posterior thyroid capsule represent high risk features related to cervical structure invasion. References: A: Angles between tumor and tracheal surface: (1) Obtuse; (2) Acute; (3) Almost straight; B: Tumor location: (1) Central; (2) Anterior capsule; (3) Posterior capsule; C: Normal thyroid tissue in direction of RLN: (1) Present; (2) Absent.
The role of thyrotropin (TSH) in the initiation and progression of PTC has already been demonstrated[53]. A Korean study divided patients with PMC under AS into three groups according to highest, middle, and lowest TSH levels[54]. One third of patients who developed more than a 50% increase in tumor volume were classified into the higher TSH levels group, which is in the upper tertile of normal TSH levels(2.3-4.5 mUI/mL). The multivariate analysis was adjusted for age, sex, and other factors, and revealed that higher TSH levels were an independent risk factor for PMC progression[54]. According to statistical analysis, this study estimated that serum TSH levels greater than 2.5 mUI/L could be considered a progression predictive cutoff and a suitable range for the target TSH level during AS[54]. In contrast to these findings, a previous study found no association between TSH levels and an increase in tumor diameter in patients with PMC[55]. These contrasting findings could be explained by the different definitions of tumor progression taken into account at the time of analysis[54,55]. To date, this topic remains unclear, since there are no prospective studies that compared outcomes from subjects under AS with and without TSH suppression.In conclusion, future research is required to evaluate the impact of levothyroxine treatment, especially in higher risk groups such as the younger population.
Pregnancy
It has been well-established that Human Chorionic Gonadotropin (HCG) produced by syncytiotrophoblastic cells of the placenta generates a physiological increase in thyroid hormone levels and glandular trophism, which is secondary to the direct stimulation of the TSH receptor from follicular cell membranes[56]. Hence, pregnancy is a condition that predisposes cells to neoplastic stimulation and therefore implies a risk of tumor progression[56]. Currently, ATA guidelines recommend that PTCs diagnosed in the early stages of pregnancy should be monitored by ultrasound.Surgery should be indicated if there is a 20% detected increase in two-dimensional tumor size and/or 50% or greater increase in tumor volume[13].
However, tumor behavior in patients under AS during pregnancy remains unclear,since data from prospective studies are scarce. In a cases series report from 51 labors,four patients (8%) showed a PMC enlargement of ≥ 3 mm, one patient (2%) showed a decrease of ≥ 3 mm, and the remaining 44 patients (45 events, 90%) showed stable disease. None of the patients had a novel appearance of lymph node metastases during pregnancy. After delivery, the PMC of one of these four patients that experienced progression remained stable, and another showed a decrease in PMC size[56]. This 8% frequency of tumor enlargement during pregnancy is almost the same as that observed by the same group of investigators during more than 10 years followup[6], and likely suggests that pregnancy constitutes a risk factor for tumor growth.
Another case published series on nine pregnant women with PMCs under AS showed that tumor enlargement was detected in four (44.4%), whereas it was only observed in three (11.1%) of the 27 patients in the control group[57]. In the Argentine cohort, one of the two pregnant patients with low-risk PTC under AS experienced tumor growth during pregnancy, and again reached the initial size 6 mo after childbirth[8].
In conclusion, although some PMCs can become enlarged during pregnancy,women who plan their pregnancy should be classified as appropriate for AS, since rescue surgery after childbirth can solve this situation[42].
Insulin resistance
Similar to the effects of TSH on follicular cells, insulin serves as a thyroid proliferation factor[58]. Dr. Rezzónicoet al[58]were the first to observe that patients with insulin resistance (IR) presented with higher incidence of thyroid nodules and larger thyroid glands. Following the same line of research, Pitoiaet al[59]studied the effects of IR in a cohort of 171 patients with PTC. With a median follow-up of 5.5 years, patients with IR had a higher frequency of structural incomplete response at the end of follow-up.One of the hypotheses proposed to explain this phenomenon is the potential overexpression of insulin receptors on neoplastic cell surfaces, which may transmit monogenic signals of insulin-like factor type I (IGF-1) that is locally produced by thyroid cancers[51]. This is a potent mitogen and antiapoptotic factor that plays an important role in early stages of thyroid carcinogenesis[53,58]. Similarly, other authors demonstrated that metformin treatment in patients with diabetes mellitus and thyroid cancer is associated with higher rates of oncological remission and survival[60]. The impact of metformin treatment in patients with insulin resistance under AS has not been established, and is currently the subject of further investigation.
Molecular profile
Several studies have determined that theBRAF V600Emutation is present in more than 50% of PMCs[13,61]. Considering the low rate of tumor progression seen with AS,isolatedBRAF V600Emutations are not considered to be a reasonable factor for cataloguing a patient as inappropriate for observation[7]. Conversely, it is different for tumors that harbor a combination ofBRAFand telomerase reverse transcriptase(TERT) mutations or multiple driver mutations (e.g.,BRAF V600E+TERTorRAS+TERT), since these are associated with poorer prognoses in PTC patients[7,40,62]. Some authors recommend excluding patients with high-risk molecular profiles from AS[7].Furthermore, Yabutaet al[63]showed that the incidence ofBRAFmutations in PMC surgical samples that are resected based on the presence lymph node metastases,tumor enlargement, and other non-progression-related reasons were very similar(80%, 70% and 64%, respectively). Meanwhile, TERT promoter mutations were not found in 15 PTMCs that showed disease progression under AS. Therefore, in light of the non-aggressive natural history of PMC, the cost-effectiveness of molecular analysis to determine AS necessity remains unclear.
Ultrasonographic features
Some findings in the ultrasonographic pattern of PMCs during AS might predict disease progression and could be considered as a prognostic tool[64,65]. Fukuokaet al[64]examined calcification patterns and tumor vascularity by ultrasound of 480 PMCs in 384 patients, with a mean follow-up of 6.4 years. This study found that lesions with rich vascularity at the last follow-up had a significant increase in maximum diameter.On the other hand, multivariate analysis showed that strong calcification and poor vascularity were significantly correlated with non-progressive disease, and occurred in a time-dependent manner during observation. Furthermore, this good prognostic pattern was mostly observed in older patients in accordance with data provided by the authors mentioned above[47,64]. In contrast, Ohet al[65]reported a direct correlation between larger calcification size evidenced by ultrasound and higher rates of lymph node metastasis. From 379 patients with PTMC who underwent thyroidectomy,calcifications were detected in 203 (53.5%) and central neck node metastasis was observed in 119 (31.3%). Among the latter, 38.8% of patients presented microcalcifications, while 44.1% exhibited macrocalcifications in the preoperative ultrasound imaging (P= 0.008)[65]. More research is required to clarify the conflicting aforementioned data in order to draw definitive conclusions.
NON-BIOLOGICAL ASPECTS OF AS
Acceptance by most patients of carrying out AS is typically difficult in clinical practice, even despite broad evidence regarding the safety and superiority of this approach in the management of low-risk PTC. In a study conducted in the United States that involved 10795 eligible patients, only 15.5% accepted AS[66]. Additionally,partial results from a multicenter prospective study carried out in South Korea(MaeSTro) showed that only 34% of 439 patients with PMCs, to whom AS was offered, ultimately opted for immediate surgical intervention[67]. As mentioned in the Argentine experience, the AS patient acceptance rate was only 25%[8]. Finally,although AS began at Kuma Hospital in 1993, the proportion of patients who chose this alternative was only 30% until 1997. While the level of acceptance has increased over time, only about 15% of current selected patients choose immediate surgery[68].
This implementation barrier reflects the confluence of multiple factors from the patient and medical team. Under such a scenario, two contrapositive lines of thinking that strongly influence decision-making in health were described[69]: (1) "Maximalist,believer and technologist": Under theleitmotiv"the more you do, the better you do",the patient/scientist that embraces this belief usually makes more radical health decisions. Patients typically prefer the most aggressive treatments, rely on the expertise of the medical team and current technologies, and are convinced that surgery will improve their prognosis with minimal adverse events. On the other hand, the "maximalist" scientist is typically not in favor of less invasive therapeutic options, and has a distrust of medical guidelines that offer a more conservative approach[7,42,68,69]; and (2) "Minimalist, doubter and naturalist": Patients and doctors who are in favor of observation until therapeutic intervention is strictly necessary,following the premise "less is more". "Minimalist" patients are interested in alternative therapies that include a wide use of natural products and physical activity. Generally,these patients are concerned about potential risks and undesired effects of treatment over the reached benefits. Considering this point-of-view, the medical team feels comfortable with offering a less aggressive approach, likely because it relies on scientific evidence in favor of observation while taking into account their own values and beliefs[7,69].
Thus, the decision to adopt AS does not elude these two archetypes, which is clearly exhibited in a modern field of research that uses surveys to evaluate patients with PMCs who were offered AS[69]. This study showed that "minimalist" patients accepted AS because they considered PMC as a common, indolent, and low-risk disease. They were aware that future surgical approaches might be required, and expressed concern about possible negative changes in quality-of-life due to surgery and/or hormonal replacement[69]. Conversely, “maximalist” patients chose the immediate surgical option as the only possible alternative, expressing a rush to undergo intervention. These individuals perceived PMC as a life-threatening disease that could be cured by surgery, and expressed anxiety about the development of local and/or distant metastases during follow-up[69,70]. These causes were reported to be the main sources of worry in recent research, which described the experiences of patients with low-risk PMCs under an AS program[71].
Occasionally, patients and doctors differ in their approach to decision-making.Thus, despite emphasizing the benefits of AS, the Argentine experience showed that most of the appropriate candidates for AS selected immediate surgery[8]. Moreover,10% of patients abandoned the observation due to anxiety after obtaining conflicting opinions from other maximalist physicians[8]. Sometimes clinicians may have difficulty communicating the benefits and risks of treatment options. For example,two qualitative studies showed that patients with PMCs who chose surgery were not informed about the advantages of AS[72,73]. They also showed that 50% of doctors were opposed to this approach, while the other 50% were open to AS, but did not routinely offer this alternative[73]. These findings suggest that deficient communication is a relevant cause of limited AS implementation[72].
Finally, patients are strongly influenced not only by their own values but also by their family and friends. Thus, their influence can determine the acceptance and permanence of AS[7].
In conclusion, each patient will process the information provided by the attending physician through their own decision-making constructs, based on their own values,beliefs, and preferences.
CONCLUSION
The acquired knowledge from worldwide studies that were performed over two decades demonstrated the safety and superiority of AS over immediate surgery in patients with low-risk papillary thyroid carcinomas. The accumulated evidence allowed for the recognition of the natural history and biological behavior of these tumors, which in most cases exhibited an indolent course. Thus, during follow-up,these tumors evolve with no size changes, very slow growth rates, and even decreased diameters. Similarly, the few patients who experienced progression and underwent rescue surgery did not exhibit distant metastases, potentially fatal recurrences, or death from thyroid cancer-related causes.
The incidence of adverse events, such as recurrent laryngeal nerve paralysis,hypoparathyroidism, and thyroid hormone replacement, is higher in patients who undergo immediate surgery, even when surgeries are performed by expert thyroid surgeons. In addition, according to different regions, the medical costs of surgery and its postoperative management were calculated to be several times higher than those of AS.
Importantly, sophisticated imaging technologies and reliable prognostic biomarkers would be essential to avoid the under-diagnosis of high-risk PMC patients who are not eligible for AS. Thus, a proper selection of candidate patients is key for the success of AS. This is based on the characteristics of the patient, tumor and medical team, following specific selection models, and considering several progression predictors. In the first place, converse to clinical papillary carcinomas, the estimated probability of low-risk PMC progression during life is inversely proportional to age at diagnosis. On the other hand, although a small proportion of PMCs could grow during pregnancy, rescue surgery after childbirth could be a valid option. Further investigation is needed regarding the impact of other factors (i.e. TSH levels, insulin resistance, ultrasound, and molecular patterns).
Despite encouraging results, acceptance of AS in clinical practice remains limited. It is likely that a "maximalist" environment, the impossibility of front toward the emotional burden of cancer diagnosis, and difficulties in managing information are confluent factors. In emerging countries, socio-economic and cultural phenomena could hinder AS implementation, such as inhomogeneous access to multidisciplinary health teams, high quality neck ultrasound, and resistance to change by some physicians. However, the experience in our institution demonstrates that AS can be easily implemented in centers experienced in the management of patients with thyroid cancer. Finally, patients are strongly influenced by family, friends, and their own values. Each patient will process the information provided through their own decision-making constructs, which are based on their own beliefs and preferences. In addition, effective communication between doctor and patient is an essential tool to improve acceptance.
Given the high prevalence of papillary thyroid microcarcinomas in the general population, AS constitutes a safe and feasible alternative in properly selected patients.This is due to the indolent course and excellent observational outcomes of these carcinomas, even in tumors between 1-2 cm.
 World Journal of Clinical Oncology2020年6期
World Journal of Clinical Oncology2020年6期
- World Journal of Clinical Oncology的其它文章
- Angioimmunoblastic T-cell lymphoma accompanied by pure red cell aplasia: A case report
- Horn of plenty: Value of the international registry for pediatric chronic myeloid leukemia
- Lingual lymph nodes: Anatomy, clinical considerations, and oncological significance
- Role of microRNA dysregulation in childhood acute leukemias:Diagnostics, monitoring and therapeutics: A comprehensive review
- Preoperative markers for the prediction of high-risk features in endometrial cancer
- National Comprehensive Cancer Network guidelines compliance of a sarcoma service: A retrospective review
