Horn of plenty: Value of the international registry for pediatric chronic myeloid leukemia
Meinolf Suttorp, Markus Metzler, Frédéric Millot
Meinolf Suttorp, Pediatric Hemato-Oncology, Medical Faculty, Technical University Dresden,Dresden D-01307, Germany
Markus Metzler, Pediatric Oncology and Hematology, Department of Pediatrics and Adolescent Medicine, University Hospital Erlangen, Erlangen D-9105, Germany
Frédéric Millot, Inserm CIC 1402, University Hospital Poitiers, Poitiers F-86000, France
Abstract Chronic myeloid leukemia (CML) in minors is a rare disease which can be effectively treated by tyrosine kinase inhibitors (TKIs) since the year 2000. A majority of pediatricians will encounter one or two CML patients in the course of their careers and will typically have to rely on written information along with their own intuition to provide care. Knowledge of response to TKIs and of agespecific side effects has an impact on the design of pediatric CML trials in many ways aiming to contribute toward greater predictability of clinical improvements.Information from a registry on a rare disease like CML offers the enormous benefit of enabling treating physicians to interact and share their collective experience. The International Registry on Pediatric CML (IR-PCML) was founded at Poitiers/France almost 10 years ago. Since then, the number of collaboration centers and in parallel of registered patients continuously increased (> 550 patients as of December 2019). Ideally, from a given treatment center in a country data are transferred to a national coordinator who interacts with the IR-PCML. In the sense of quality assurance, the registry can offer dissemination of knowledge on state-of-the-art diagnostics (including reference appraisal), optimal treatment approaches, and follow-up procedures within a network that is exerting its strength via participation. With continuous growth during the recent years, very rare subgroups of patients could be identified (e.g., CML diagnosed at age < 3 years, children presenting with specific problems at diagnosis or during course of treatment) which had not been described before. Publications coming from the IR-PCML disseminated this useful information derived from patients who robustly participate and share information about their disease, among themselves and with their caregivers and clinicians. Patient input driving the collection of data on this rare leukemia is the basis for the considerable success of bringing new therapeutics into clinical use.
Key words: Pediatric chronic myeloid leukemia; International registry; Rare disease;Collaboration and data exchange
RATIONALE OF A REGISTRY FOR PEDIATRIC CHRONIC MYELOID LEUKEMIA
Pediatric chronic myeloid leukemia (CML) is a very rare disease. Its incidence increases with age: CML is exceptionally rare in infancy, occurs in 0.7 per million children 1 to 14 years of age per year, and in 1.2 per million adolescents per year. As a result, only limited expertise on pediatric CML exists[1]. Even in larger pediatric hemato-oncology units only one case per annum of CML will be diagnosed while smaller centers are faced with a diagnosis of pediatric CML only every 3 to 5 years.This rarity represents a major obstacle to the continuation and updating of the necessary knowledge of pediatricians involved in the management of CML.
With the beginning of this millennium the major change in the therapeutic approach to CML by the introduction of the tyrosine kinase inhibitor (TKI) imatinib also had an impact on CML data collection. Before the year 2000, children were treated by allogeneic stem cell transplantation and registered in the EBMT or IBMTR registry[2-4]. Given the efficacy of TKI in adult CML, TKI nowadays are regarded as the established first-line treatment also in pediatric patients[5-7].
Facing the new scenario it was rapidly recognized that the so far limited experience with imatinib based on small-sized pediatric phase I or phase II trials did not suffice the need of generating solid-based data on all aspects of pediatric CML -from diagnostic features of this leukemia in minors to assessment of long term efficacy and childhood-specific TKI side effects. It had to be acknowledged that only a registry would allow the continuous and long-term recruitment of all pediatric CML patients,including documentation of defined but also unexpected events and in particular a centralized monitoring of all treatment related aspects in newly diagnosed patients.Such an infrastructure cannot be established by clinical trials with selected entry and stopping criteria and limited by a defined follow-up period.
To enable a world-wide registration of all pediatric patients with this rare subtype of leukemias, an international registry for pediatric CML was established on Jan 1st,2011 under the umbrella of the International BFM Study Group[8]. This international registry complements and combines data of pediatric CML patients recruited for consecutive studies on a national basis[9-19].
The aims of the International Registry on Pediatric CML are: (1) To describe the characteristics of CML in a large cohort of patients less than 18 years of age; (2) To describe the treatment policies; (3) To identify prognostic factors in this age group; (4)To determine prognostic scoring systems in this population in order to optimize individual treatment choices; and (5) To determine side effects and long-term effects of treatments -mainly the TKI effects- on growth and development of a pediatric population.
Patients less than 18 years of age with newly diagnosed CML (confirmed by Philadelphia positive and/or BCRABL positive) are eligible whatever the phase of the disease, the type of treatment or their enrollment status in a clinical study.Retrospective and prospective data are collected from patient flow charts and/or existing databases.
HISTORY AND DEVELOPMENT OF THE REGISTRY
Since the foundation of the registry almost 10 years ago the number of collaboration centers and countries and in parallel the number of registered patients has been continuously increasing (Figure 1). As of 1st of November 2019, so far 15 countries have enrolled a total of 565 patients (Figure 2).
The median age of the total cohort (58% boys, 42 % girls) is 12.2 years (range, 8 mo to 18 years) and was diagnosed with CML in chronic phase (92.3%) or in advanced phases (7.7%). All these patients have been diagnosed after the year 2000 and were registered prospectively and retrospectively. Another 5 countries have started negotiations and signed precontracts to become full member of the registry in the near-by future.
STRUCTURE AND LEGAL STATUS OF THE INTERNATIONAL REGISTRY FOR CML IN CHILDREN AND ADOLESCENTS
The registry is governed by a steering committee consisting of national member group representatives with Millot F (Poitiers/France) as Chair. The data base of the registry is located in the Clinical Investigation Center (CIC) of the University Hospital of Poitiers (France) which is the International Central Data Center (ICDC) of the study in charge of centralizing the data. The study was accepted by the French ResearchHealth Authorities (CCTIRS) in October 2010 (Certificate number 09.401bis) and by the National Ethic Committee (CNIL) in December 2010 (Authorization number 910031).A National Coordinating Center and a leader is identified in each participating country. Each National Coordinating Center is responsible for the collection of data in its country. Each National Coordinating Center forwards the data to the ICDC (CIC INSERM 802, Poitiers, France). The ICDC will provide a financial compensation per patient to support the organization of the data collection in each participating country at registration and €100 upon reception of at least one year’s follow-up data).
The ICMLPed study is open to inclusion of patients from national groups that are authorized by their own health authorities to collect and transfer data to the ICDC. On demand the necessary documents will be sent to each national representative (contact:f.millot@chupoitiers.fr). Registry results and future projects are regularly discussed and analyzed at international meetings (SIOP-E, ASH, EHA, Annual John Goldman Conference on CML).
As outlined in Figure 3 the organizational structure for reporting data to the registry is highly flexible. Ideally data are transferred from the treating centers to a national coordinator who interacts with the International Pediatric CML Registry(Figure 3, country 1). Alternative reporting modes are also possible (Example country 2, Figure 3) including a report directly to the International Pediatric CML Registry(Example country 3, Figure 3). In countries without a national coordinator on pediatric CML, all treatment centers may report directly to the International Pediatric CML Registry (Example country 4, Figure 3). Other scenarios comprise patients enrolled into a trial on pediatric CML (Example countries 5 and 6, Figure 3). The basic data (e.g., characteristics at diagnosis; stage of disease) from these patients can be passed on by the chairman of the trial to the International Registry.
By this highly flexible approach the pediatric CML registry is analogue to other pediatric registries for rare hematological diseases which are acting successfully on a national basis,e.g. National Centre of Reference for Autoimmune Cytopenia in Children (CEREVANCE) in Bordeaux/France[20,21], the German PID-NET registry serving as the first national registry of patients with primary immunodeficiencies[22,23],or on an international basis (e.g., Intercontinental Cooperative Immune Thrombocytopenic Purpura Study Group[24], Severe Chronic Neutropenia International Registry[25]).
RUNNING A REGISTRY VS PERFORMING A STUDY/TRIAL -PROS AND CONS
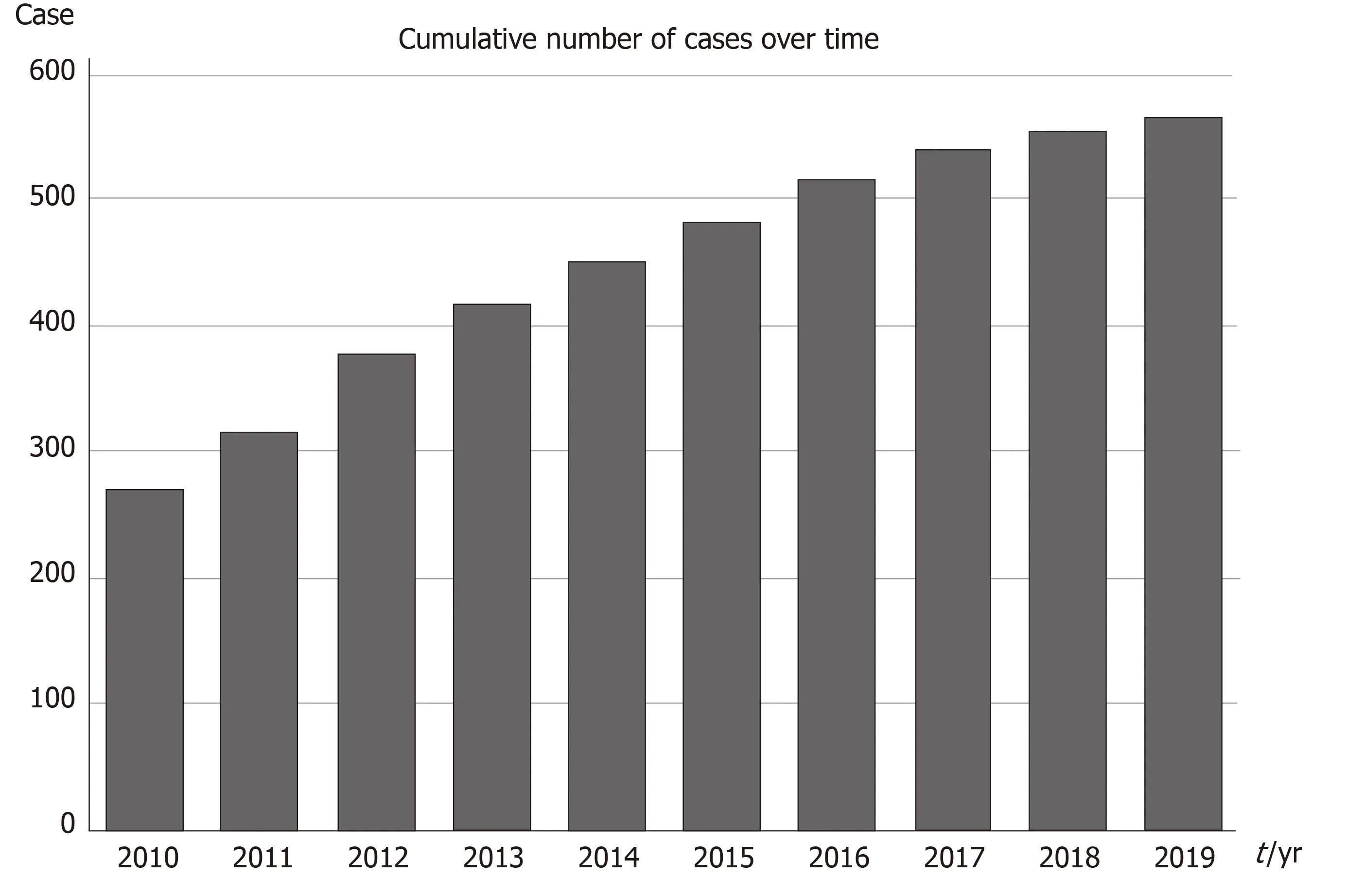
Figure 1 Cumulative number of cases of pediatric chronic myeloid leukemia enrolled into the registry annually.
Unfortunately, new legislation at least in Europe has impeded establishment of new trials or their conduct. In parallel to the licensing of the first TKI imatinib (although not linked to this event) the European Clinical Trials Directive (EUCTD) was promulgated in 2001. This directive claimed the intention, allegedly, of improving the protection of patients and the reliability of research reporting and of harmonizing and increasing the competitiveness of European clinical research. In addition, the European's Good Clinical Practice Directive 2001/20/EC, effective since 2004, was meant to harmonize the conditions for clinical trials across Europe, but, instead, the challenge of initiating and running multinational, non-commercial clinical trials has become greater than ever[26]. The directive and consecutive national acts of the European countries increased the responsibilities of the research sponsor and imposed a variety of other requirements regarding documentation of responsibilities,patient information, pharmacovigilance, and retention of documentation. Institutions participating in existing non-commercial academic clinical trials are struggling to cope with increased administrative and financial burdens and consumption of researcher time. As a problematic result few new studies are initiated any more, and recruitment to trials is rapidly falling as institutions reduce the numbers of trials they are able to offer as a consequence of increases in costs and bureaucracy that can be attributed directly to this directive[27,28].
New drugs and new strategies for treatment of CML typically emerge from adult hematology because the ethical principle is generally accepted that any new knowledge which can be generated from adult patients should not be depicted from underaged patients. Therefore, studies fulfilling all the requirements of the EUCTD and typically sponsored by pharmaceutical companies will be performed in pediatric CML patients if at all with a considerable delay. As of note, while imatinib was licensed for adults in the year 2001 and with a delay of “only two years” for children the 2ndgeneration TKIs nilotinib and dasatinib, however, were licensed for adults in 2009/2010 and in 2017/2018 for children, respectively. Again, the even smaller number of recruitable patients failing first line TKI treatment requires that such trials can be performed only on an international basis[29,30]. Performing academical trials with licensed drugs in a low number of minors suffering from a rare disease presently is almost impossible due to administrative regulations and associated financial burdens[31]. The same limitations hold true when optimally a trial should be performed in a randomized fashion. While homogenous cohorts in pediatric CML may be stratified at diagnosis, results after randomization cannot be expected before the end of decade-long recruitment periods[18].
In contrast to the problems outlined above, a registry has the advantage that patients -once an indication for a defined therapeutic intervention emerges- may be enrolled into a randomized trial while all other patients representing the majority will remain in the registry. This approach guaranties that at the time of randomization consistent data exist[32]thus allowing upscale analysis (e.g., by making use of a biometric multi-state model allowing comparison of different treatment results simultaneously)[33]. In addition, the description and validation of risk factors will allow and improve the possibility of patient stratification. By such means clinical studies adhering to all the criteria of the EUCTD can be restricted to defined cohorts of patients (e.g., trials on efficacy of stopping TKI-treatment)[18,34].
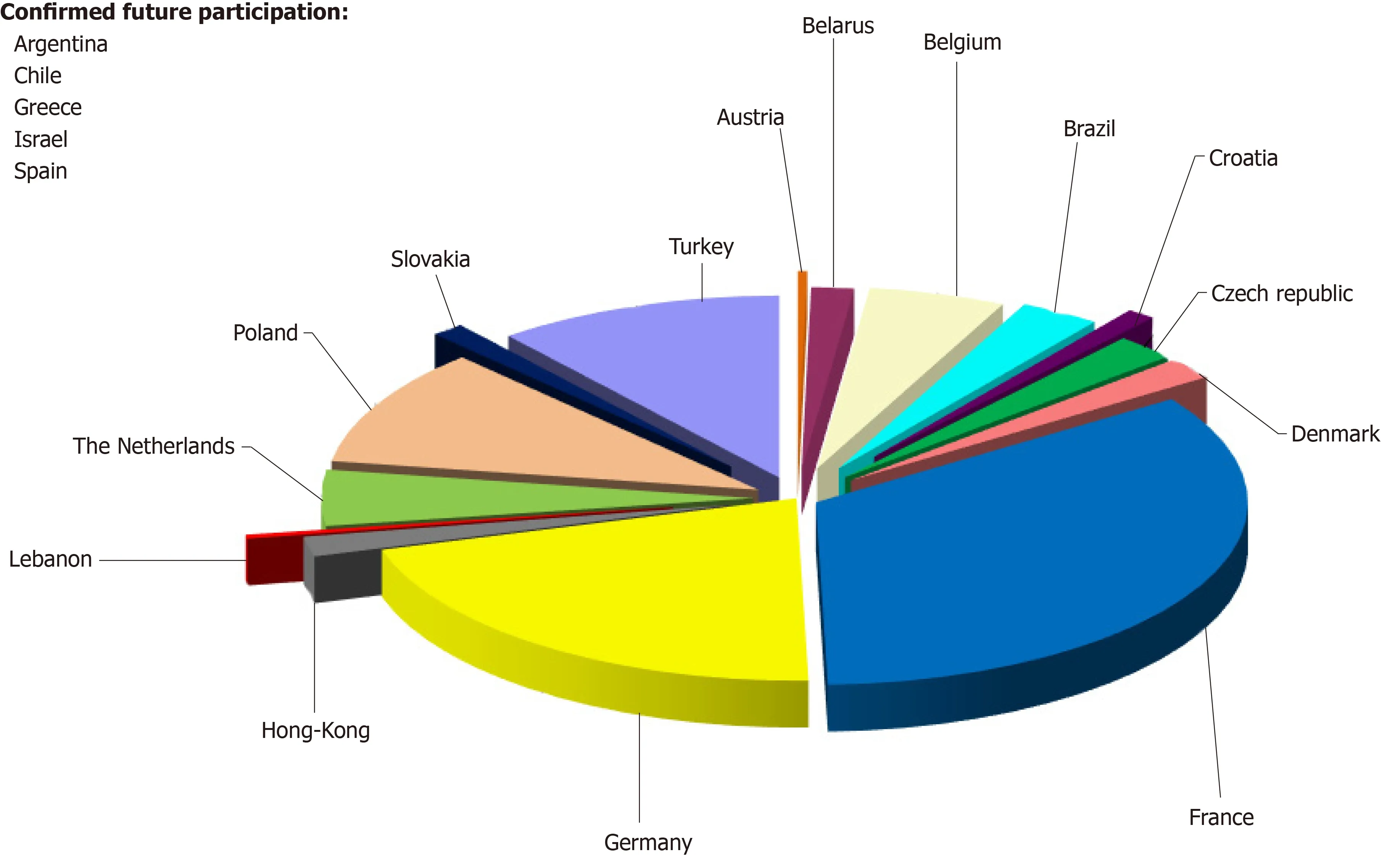
Figure 2 Pie chart showing the proportion of patients enrolled into the registry by each participating country. Another five countries have started negotiations and signed precontracts to become full member of the registry in the near-by future.
Clinical trials with clear entry criteria, limits on inclusion, and statistical considerations allow processing of therapeutic questions with the associated necessary security for the patients enrolled. On the other hand, only a registry with broadly formulated definitions (e.g., all stages of CML) will guarantee quality assurance for all pediatric patients, descriptions of newly identified subgroups, and specific patterns of response. Running the International registry on pediatric CML has resulted in first recommendations for treatment of rare cohorts[5,30], translational experimental research[35,36], and also long-term follow-up of individuals having received new drugs in studies fulfilling all the criteria of the EUCTD[37-39]. In the sense of quality assurance, a registry can offer dissemination of knowledge on state-of-theart diagnostics (including reference appraisal), optimal treatment approaches[1,5,30,40,41]and follow-up procedures within a network that is exerting its strength via participation. This holds especially true in very rare diseases like pediatric CML.
Long-term follow-up is a peculiar issue in pediatric oncology given the situation of treating not-outgrown organisms[38]. Especially the latter point cannot be expected from trials with a recruitment and follow-up period of several years only. Resistance to treatment possibly resulting in recurrent relapses or progression in individual patients will make the course of CML rather complex as well as individual in a given patient, thus requiring individual counselling based on assessable data[1]. Emerging treatment options like third and fourth generation TKIs not yet licensed at minor age require a close collaboration with colleagues from internal medicine[42-44]. To optimize the “real world” treatment, all the necessary information from complex individual cases can only be coordinated and integrated by a registry.
PUBLICATIONS EMERGING FROM THE REGISTRY (FULL PAPERS AND CITABLE ABSTRACTS)
Clinical and biological data at diagnosis and interim results regarding cytogenetic responses, survival and prognostic factors were presented regularly at international annual meetings of the American Society of Hematology as Posters[45-50]or oral presentations[51-53]. Thus, the registry has contributed considerably to generating and distributing data on the special role of pediatric CML which differs in biological features from adult CML[11,47].
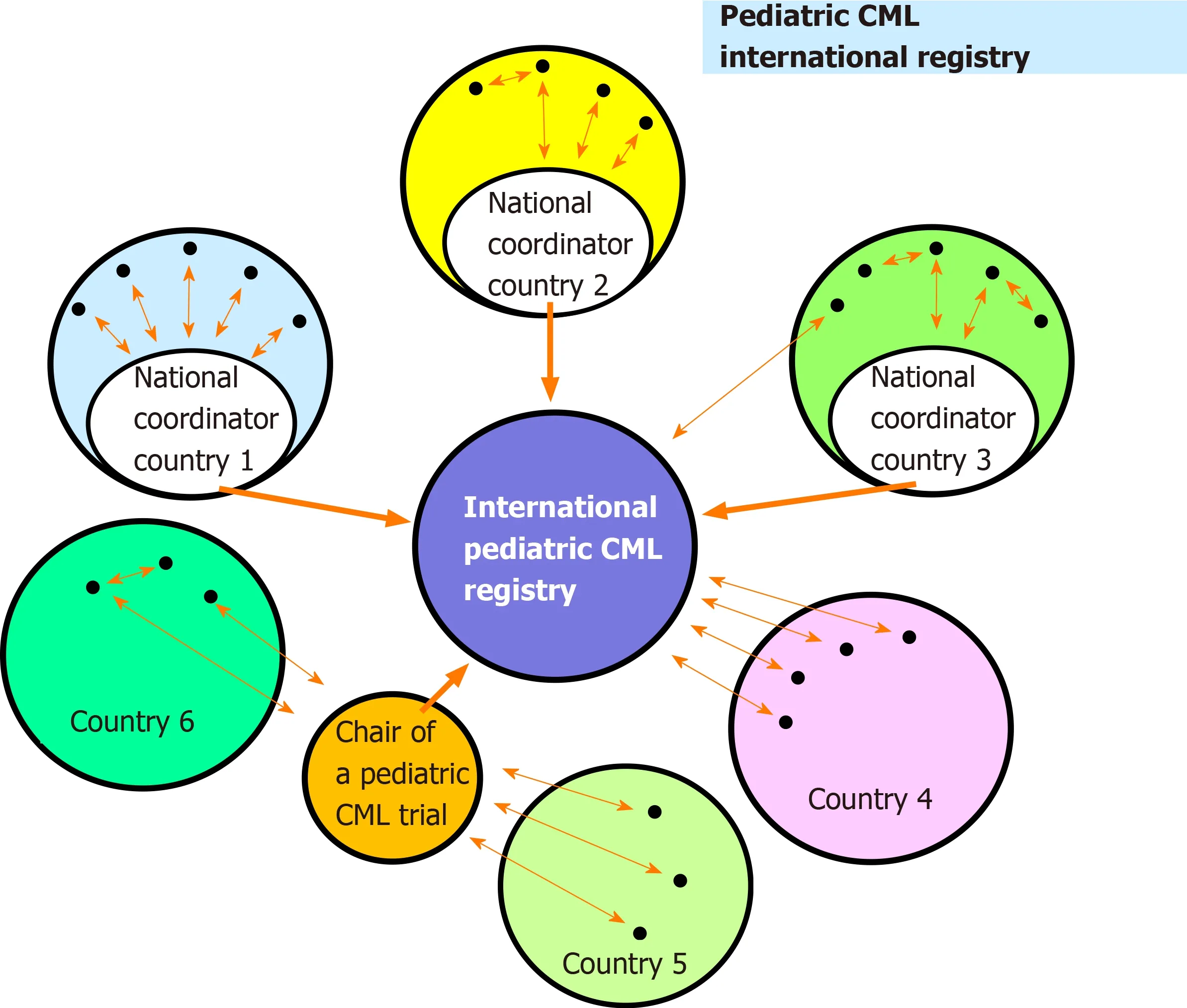
Figure 3 Schema of the possible ways how individual treatment centers may interact with the registry. CML:Chronic myeloid leukemia.
Figure 4 schematically demonstrates how collaboration in managing a rare disease like pediatric CML allows to extract out of a registry sufficient data. Thus, in the recent years out of a bigger cohort of pediatric patients with CML, subgroups were identified (e.g., patients diagnosed with CML at a very young age of < 3 years[45],patients presenting with specific problems at diagnosis such as rheological[10]or hemostaseological problems[50,54]). These cohorts were analyzed descriptively as a first step. Small in number, pediatric cohorts with specific problems have not been described in the worldwide CML literature on the basis of a homogenous treatment approach[34,55-58]. Also the registry has been and still is of great support in identification of risk parameters (e.g., advanced phases of CML[53,59], hemostaseological complications associated with high platelet counts[50,54], and relevant interventions(e.g., experience with off label use of 3rdgeneration TKI[57]), and generated data are helpful with the stratification of individual patients[37,53,56,57,59]. In addition, a correlation of clinical data with identified molecular markers is fostered by a clinical registry allowing the collection of biological specimens from the participants within the network[35,36,39,60].
Last but not least, once the registry had become large enough in size (> 200 patients) it became a “vein of gold” or “horn of plenty” for researchers engaged and willing to dedicate their time and efforts to “data mining” and analysis of specific or even rare features of pediatric CML (Figure 4).
FUTURE TASKS AND OUTLOOK
Although major success has been achieved at the registry’s 10thanniversary many issues still remain to be addressed and managed in the future. With the use of imatinib now for about 20 years it must be remembered that the median age of diagnosis of pediatric CML from a cohort with an upper age limit of 18 years is 12.5 years[12,18]. Current data on stopping TKI demonstrate that only a small proportion of patients will benefit from that attempt and only if preceded by several years of TKI treatment resulting in sustained deep molecular remission[34,61,62]. This makes a close cooperation necessary with physicians and hematologists treating adolescent and adult patients with CML. However, adolescent patients with cancer reside in a "noman's land" between the worlds of pediatric oncology and that of "adult" medical oncology. As compared to younger and older patients, adolescents and young adults are under-represented on clinical trials. Thus, physicians -inadequately trained or reluctant to care for adolescents- have important responsibilities. If 15- to 19-year-olds are diagnosed with CML and are treated at adult facilities they are treated according to their type of leukemia but not according to their age. This solution is probably appropriate for the leukemic cell clone, but not necessarily for the patient.Adolescents are neither old children nor young adults, and are complicated individuals, with unique socio-psychological problems and needs, that may be addressed only by dedicated professionals, adequately trained and supported[1,63]. The ultimate challenge is the development of a new discipline, adolescent/teenage and young adult oncology, devoted to the care of these patients. To follow a given patient during his/her transition to adult hematology, data from the existing registries should be linked. This concerns: (1) A link to the EBMT-SCT Registry to follow those patients who ultimately will fail TKI treatment and require stem cell transplantation;(2) A link to adult studies on TKI where former pediatric patients may be enrolled;and (3) An approach to get information on long-term side effects observed in former pediatric patients. Involving the patients‘ representatives from layman organizations and spreading information on the registry may be helpful to achieve these goals[64].
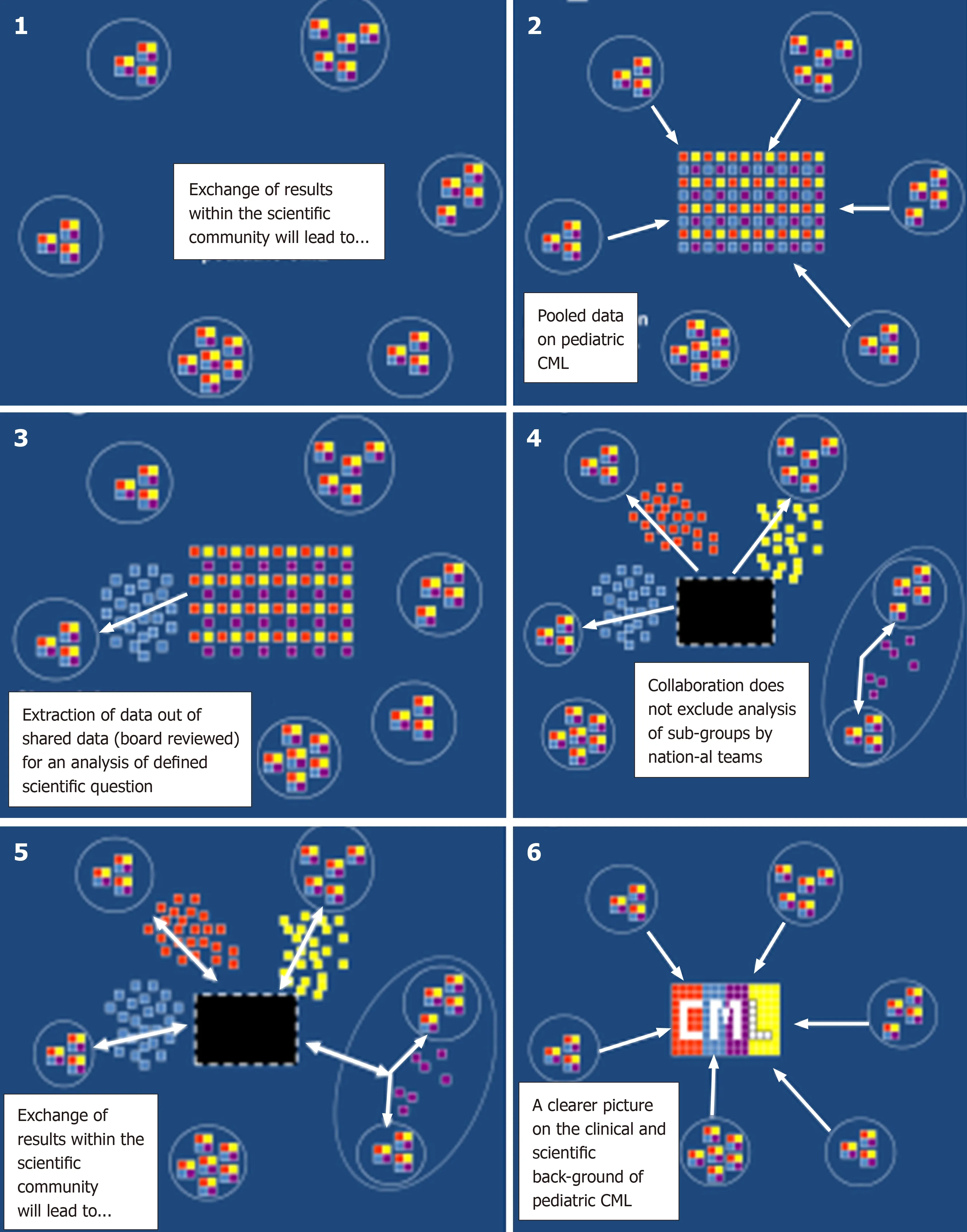
Figure 4 Cartoon demonstrating that with a rare disease only collaboration allows to analyze a sufficient number of patients. Different features of the disease (represented by different colors of the squares) can be analyzed by individual participants contributing to the registry. CML: Chronic myeloid leukemia.
From a global viewpoint, children with CML and their families face major inequity between countries in terms of access to innovative drugs[65]. New drug development pathways could efficiently address the challenges with innovative methods and trial designs, investment in biology and preclinical research, new models of partnership and funding including public-private partnerships and precompetitive research consortia. Data from the registry can provide sound evidence on the numbers of patients in need of better treatment approaches than first line imatinib but also ongoing and increased collaboration between pediatric CML cooperative groups worldwide must be fostered.
Even though the cohort of patients failing first line imatinib treatment is small, but to improve regulatory requirements, initiatives and incentives should urge pressure of politicians that can better address the legislative needs and hurdles. Increased cooperation between all stakeholders-academia, parents' organizations and advocacy groups, regulatory bodies, pharmaceutical companies, philanthropic organizations,and government- will be essential and should have the registry in focus[66].
The presently most burning unanswered questions on adult as well on pediatric CML patient management are: (1) How to predict more reliably treatment failure at the time of diagnosis; and (2) How to select the appropriate frontline TKI to achieve an optimal outcome. Molecular mechanisms resulting in resistance to TKI treatment are largely uncharacterized and besides unravelling BCR-ABL1 kinase domain mutations there are no other routine molecular testing strategies[67]. As shown in other malignancies, next-generation sequencing technology has enabled the incorporation of somatic mutation screening in diagnosis, classification, and prognostication[68].Genomic studies have the potential to lay the foundation for improved diagnostic risk classification according to clinical and genomic risk, and to enable more precise early identification of TKI resistance.
First promising results have shown in Ph-negative and Ph-positive clones of CML patients BCR-ABL1-independent mutations, which may be involved in the pathogenesis, clonal evolution, and progression of CML and may be considered as important cofactors in the clonal evolution of CML[69]. In a small cohort of 21 children and young adults investigated so far, six patients harbored mutations in the ASXL1 gene at diagnosis[70].
CONCLUSION
Future studies will analyze the frequency of BCR-ABL1-independent mutations (e.g.ASXL1) in larger cohorts of pediatric CML patients and correlate findings with followup data and prognosis. Thus, as a new task the pediatric CML registry should be extended also to store biological materials for further analysis (Biobanking).
ACKNOWLEDGEMENTS
The authors are very thankful to the staff of the registry and all collaborators from many treatment centers for continuously processing the data and ensuring the quality of the registry.
 World Journal of Clinical Oncology2020年6期
World Journal of Clinical Oncology2020年6期
- World Journal of Clinical Oncology的其它文章
- Angioimmunoblastic T-cell lymphoma accompanied by pure red cell aplasia: A case report
- Active surveillance in low risk papillary thyroid carcinoma
- Lingual lymph nodes: Anatomy, clinical considerations, and oncological significance
- Role of microRNA dysregulation in childhood acute leukemias:Diagnostics, monitoring and therapeutics: A comprehensive review
- Preoperative markers for the prediction of high-risk features in endometrial cancer
- National Comprehensive Cancer Network guidelines compliance of a sarcoma service: A retrospective review
