Preparation and Properties of Fluorene and Fluorine Functional Polyimide Films
YU Zhaopeng,WANG Jian,ZHAO Shengjie,WANG Chao,LI Delun,AN Haoyuan
(1.Beijing Institute of Petrochemical Technology,School of Materials Science and Engineering, Beijing 102617, China; 2.The Research Group of the School of Materials Science and Engineering,Beijing Institute of Petrochemical Technology, Beijing 102617, China)
Abstract:In order to study the effect of introducing fluorene group,fluorine atom and flexible ether bond on the properties of polyimide (PI) films,PI films were prepared by homopolymerization or copolymerization of 9,9′-bis (4-amino,3-trifluoromethylphenoxyphenyl) fluorene,o-phenylenediamine (OPDA),biphenyltetracarboxylic acid(BPDA),3,3′,4,4′-benzophenone tetracarboxylic acid (BTDA),2,2′-bis [4-dicarboxyphenoxyphenyl] propane dianhydride (BPADA),and the effect of fluorene groups and fluorine atoms on the solubility,thermal and electrical insulation properties of PI films were studied. The results show that with the introduction of fluorene group and fluorine atom,the solubility and thermal properties of PI are improved,and PI has good electrical insulation performance.
Key words:polyimide; fluorene group; fluorine atom; solubility; thermostability; electrical insulation
1 Introduction
Polyimide (PI) is a kind of high performance polymers with repeating units in the molecular chain with the characteristic structure of an imide ring. The rigid imide structure gives polyimide unique properties,such as excellent high temperature resistance,corrosion resistance,environmentalstability and good mechanical properties[1-2],these properties have enabled polyimide to be widely used in aerospace,mechanical,microelectronic packaging,and solar cells.
Although polyimide has many advantages,but its unique rigid skeleton structure and strong intermolecular force,it is usually insoluble and difficult to process and form[3]. Intermediate polyamic acid and intermediate processing are used. Due to instability,volatile small molecule (H2O) byproducts and other problems will occur in the processing process[4].So at present,structural modification is widely used to improve the solubility and processability of polyimide.In recent years,fluorene group substituted polyimide has gradually attracted extensive attention of researchers. Introducing fluorene group into polyimide can improve the heat resistance,solubility and processability of polyimide.At the same time,the large free volume and fused ring structure of fluorene group are beneficial to increase the distance between molecular chains,reduce the force between molecular chains and improve the melt fluidity[5].The soluble polyimide can be dissolved in an organic solvent under the condition of complete imidization,and only the solvent needs to be removed in the application process,thus greatly improving the processability of the polyimide and expanding the application range of the polyimide.
The noncoplanar biphenyl structure and steric hindrance effect limit the free movement of the polyimide molecular chain after introducing fluorenyl groups into the polyimide molecular chain,which keeps the polyimide molecular chain rigid. But the polyimide containing fluorene is brittle and the film-forming property is poor.However,introducing flexible groups such as ether bonds into the polyimide backbone can increase the flexibility of the molecular chain.Recent studies have shown that the simultaneous introduction of fluorenyl and ether bond into the main chain of polymerization can improve its solubility,thermal and mechanical properties.
As we all know,fluorine has been widely used in drugs,refrigerants and solvents[6]. Among polymer materials,fluoropolymers represented by polytetrafluoroethylene have become a kind of important materials and have been widely used in the field of industrial technology. For polyimide (PI),the introduction of fluorine also brings remarkable effects,so the research on fluorine-containing polyimide has been going on for a long time since the mid-1960s.
The introduction of fluorine-containing groups,such as trifluoromethyl or hexafluoropropyl,into polyimide polymers can increase the distance between their molecular chains and reduce the intermolecular force,thus dissolving into various organic solvents. The introduction of fluorine atoms into the molecular structure of PI can improve the solubility and processability of PI,while the introduction of fluorine atoms can also give it high transparency,low dielectric constant,low water absorption and low crystallinity[7-9].This is because fluorine atoms have a series of unique physical and chemical properties,such as small atomic radius and C-F bond length close to C-H bond.The interaction between nucleus and electron is strong,and the molar polarizability of C-F bond is low,which is equivalent to that of C-H bond.Fluorine has the largest electronegativity among all elements,and the C-F bond energy is large.9,9′-bis (4-amino,3-trifluoromethyl phenoxyphenyl) fluorene (6F-BAOFL) is a new fluorene-containing diamine monomer. Its large free volume and fused ring structure endow PI with excellent solubility,heat resistance and optical properties.
In this project,6F-BAOFL( 9,9′-bis (4-amino,3-trifluoromethylphenoxyphenyl) fluorene) containing flexible ether bond,rigid fluorenyl and fluorine atom,which was polymerized with different dianhydride monomers ODPA(o-phenylenediamine),BPDA(biphenyltetracarboxylic acid),BTDA(3,3′,4,4′-benzophenone tetracarboxylic acid ) and BPADA(2,2′-bis [4-dicarboxyphenoxyphenyl] propane dianhydride) respectively. A series of PI films with different structures were obtained by two-step method[10]using the same molar ratio of diamine and dianhydride. The solubility,thermal properties and dielectric properties of the PI were tested. The effects of monomer reactivity and molecular structure on PI performance were studied.
2 Experimental part
2.1 Main Raw Materials
Diamine: 6F-BAOFL,98.0%,powdersJiangsu Yongxing Chemical Co. LTD,vacuum dry for 12 h before use;
Dianhydride: OPDA,≥98.0%,powder,Zouping Mingxing Chemical Co.LTD;
BPDA,98%,powders,Changzhou Wujin Linchuan Chemical Co. LTD;
BTDA,99%,powdes,rFusman Technology (Beijing) Co.LTD;
BPADA,≥ 99 %,powders,Shanghai Synthetic Resin Research Institute Co. LTD;
all the above drugs were dried in vacuum for 12 h before use.
Solvent: N-methyl-2-pyrrolidone(NMP),99.5%,liquid,Jiangsu Jiaren Chemical Co. LTD.
2.2 Experimental Instruments
The magnetic stirrer is Ronghua 79-1 magnetic heating stirrer;
Seiko DSC 6300 and TG/DTA 6300 thermal analyzers for thermal performance testing;
Waveform generators AFG-2225 and TH2828 precision LCR digital bridges for electrical insulation detection.
2.3 The synthetic route of polyimide
Fig.1 shous the sketch of synthetic route.
2.4 Preparation of PAA Solution
First,diamine monomer 6F-Baofl was added to a 250 mL three-necked flask equipped with a magnetic agitator at room temperature,and then the diamine monomer was fully dissolved in the solvent NMP. While stirring,the diamine monomer was added to make it completely dissolved in the above solution.The reaction temperature was controlled at 0~5 ℃ by ice water bath. After 24 hours of reaction,a transparent and viscous PAA solution was obtained. Table 1 shows the PI system synthesized in the experiment. Fig.2 shows the preparation process of the experiment.
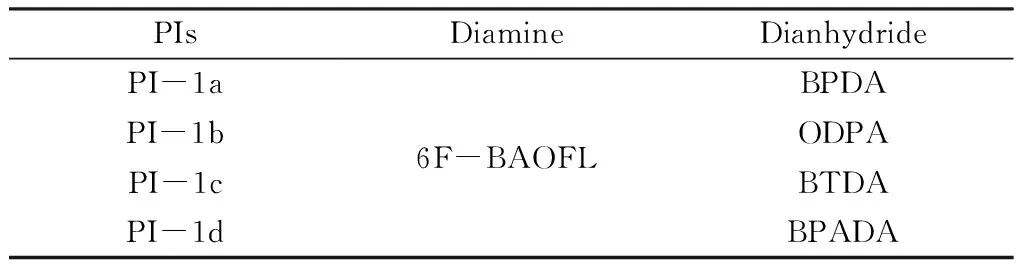
Table 1 PI System Synthesized in this Experiment
2.5 Preparation of PI Film
The PAA solution was uniformly coated on the clean glass plate,and the thickness of the film was controlled by the diameter of copper wire on the glass rod. Then the film was put into a vacuum oven for thermalimide. After natural cooling,the film was removed,and four kinds of PI films were obtained,which are dried in vacuum for later use. Fig.3 shows the coating process and the Fig.4 shows the bubble file process.
2.6 Test Instruments and Characterization
(1)Solubility test is carried out according to the following steps: 3 g of fully dried PI resin and 17 mL of corresponding solvent are mixed to prepare a 15% (w/v) solid content solution,then let stand for 24 h in a dryer and observe the dissolution.If the solution system is uniform and transparent,and there is no gel,delamination and other phenomena,it is considered to be dissolved.The same method is used to determine whether it is partially dissolved or insoluble.
(2)Differential Scanning Calorimetry (DSC) and Thermogravimetric analysis(TGA) were measured by Seiko DSC 6300 and TG/DTA 6300 thermal analyzers respectively. The heating rate was set at 10 ℃/min and the test environment was nitrogen atmosphere.The glass transition temperature (Tg) is determined by the DSC curve of the second scan.
(3)Electrical insulation test,using waveform generator AFG-2225 and TH2828 precision LCR digital bridge to test,the film thickness is 0.25 mm,and the test is carried out at frequencies of 20,100,500,1 000,5 000,10 000,50 000,100 000,500 000 Hz and 1.000 000 MHz.
3 Results and discussions
3.1 Film Forming and Solubility of PI
PI-1a,PI-1b,PI-1c,and PI-1d have good film-forming properties.The film-forming properties and solubility of the four PI systems are shown in the following Table 2.
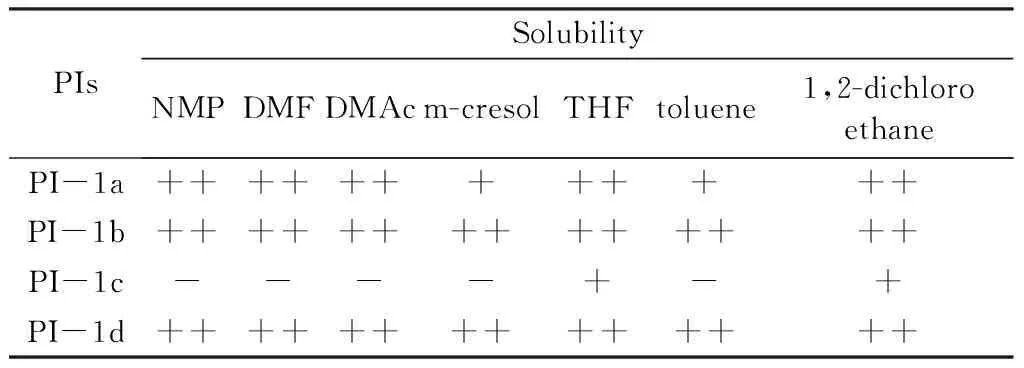
Table 2 Solubility of PI System
From Table 2,it can be seen that the PI-1c system synthesized by 6F-BAOFL and BTDA is only partially dissolved in tetrahydrofuran and 1,2-dichloroethane. The remaining three PI films show good solubility in organic solvents. The improvement in solubility is mainly due to the introduction of trifluoromethyl and groups,which increases the distance between molecular chains and weakens the intermolecular interaction force.
3.2 Thermal Properties of PI Films
Since differential scanning calorimetry (DSC) was established in 1963,this method has been playing an important role in the research and characterization of polymer materials. Although DSC is only one of many thermal analysis methods,the development trend of polymer thermal analysis in recent years shows that DSC has become the main method of polymer thermal analysis.The DSC test of this experiment was carried out in a nitrogen environment,scanning at a heating rate of 10 ℃/min,quenching to below 100 ℃,and then performing a second scanning at a heating rate of 10 ℃/min.The glass transition temperature of PI is determined by the second DSC scanning curve,and the result is shown in Fig.5.
In Fig.5,DSC curves of all samples show baseline shift to endothermic direction without melting endothermic peak,indicating that PI films prepared in this experiment are amorphous polymers[10].
As we can see from the picture that the Tg of PI-1a is 313 ℃,the Tg of PI-1b is 291 ℃,the Tg of PI-1c is 299 ℃,and the Tg of PI-1d is 255 ℃.
Fig.6 is TGA curve of PI system. As can be seen from Fig.6,no obvious weight loss occurred in PI system before 500 ℃. When the weight loss of the four PI systems is 5% and 10%,the corresponding temperatures and the residual weight percentage of PI at 700 ℃ are respectively:
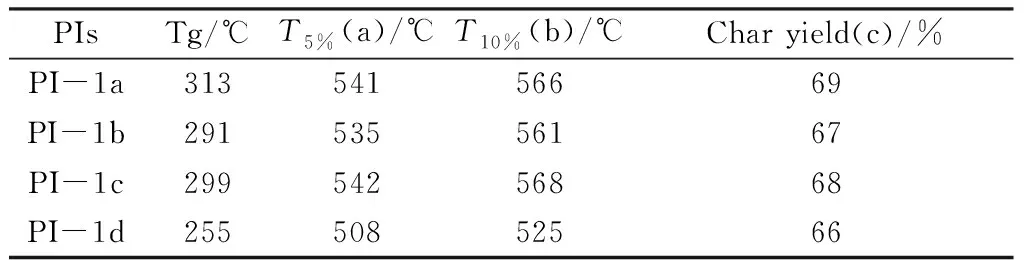
Table 3 The thermal properties of PI films
As it shown in Table 3,6f-baofl base PI that the Tg value is in the range of 255~313 ℃,and the Tg value of PI obtained by polymerization with BPDA is the highest,while the Tg value of PI obtained by polymerization with BPADA is the lowest. The Tg value of PI is related to the molecular weight of the material and the rigidity of the monomer. The higher the molecular weight of PI,the greater the rigidity of the monomer,and the higher the corresponding Tg value. The molecular weight of PI obtained by the polymerization of these four dihydride monomers and diamine should be BTDA>BPDA>ODPA>BPADA. Meanwhile,the rigid ordering of the dihydride monomer is BPDA>BTDA~ODPA>BPADA. Therefore,the Tg value of PI obtained by BPDA polymerization is the highest,while the Tg value of PI obtained by BPADA polymerization is the lowest. The experimental results are consistent with the theoretical analysis.
It can be seen from the table that the weight loss of PI is 5% and 10%,which the corresponding temperatures are higher,above 493 ℃ and 513 ℃ respectively. The residual weight percentage of resin reached or exceeded 59% at 700 ℃. The introduction of fluorene group significantly increases the rigidity of PI molecular chain that made it difficult to move even at high temperatures. Therefore,the thermal stability of PI is improved.
3.3 Electrical Insulation Performance Test:
We selected polyimide system synthesized by 6F-BAOFL and ODPA to test its electrical insulation stability. The test results are shown in Table 4.
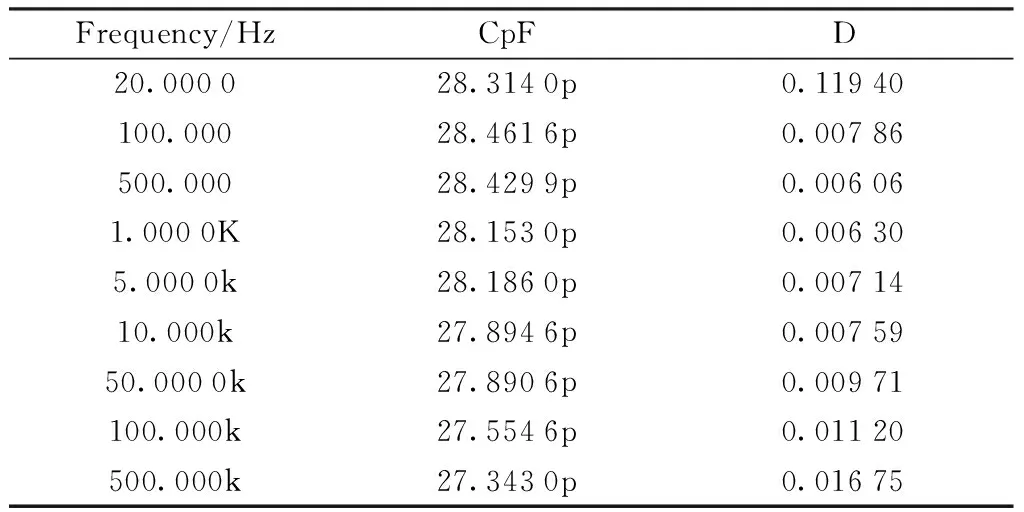
Table 4 Electrical Insulation Performance Test of PI-1B
Results analysis: the PI film made of 6F-BAOFL and ODPA has good electrical insulation performance and it was stable in a wide frequency range.
4 Conclusion
Experimental results proved that the introduction of ether bond increased the flexibility of molecular chain.The large volume and thick ring structure of the new fluorene group were helpful to increase the distance between molecular chains,reduce the intermolecular forces and improve the melting fluidity. Moreover,fluorine atoms introduced into the structure of PI have unique physical chemical properties,which improved the solubility and processability of PI.
Therefore,the introduction of the three (fluorene group,fluorine atom and flexible ether bond) made the insoluble polyimide into soluble polyimide,and made the electrical insulation of polyimide stability.The introduction of fluorene group increased the Tg of polyimide and increased the rigidity of PI molecular chain,which made it difficult to move at high temperature,so improved thermostability of PI.

