ARNI and SGLT2i: a promising association to be used with caution
Andrea Herbst, Francesco Orso,#, Marta Migliorini, Simona Virciglio, Silvia Tognelli, Viola Camartini,Alessandra Pratesi, Francesco Fattirolli,, Niccolò Marchionni, Andrea Ungar,, Samuele Baldasseroni
1Heart Failure Clinic, Division of Geriatric Medicine and Intensive Care Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy
2Department of Experimental and Clinical Medicine, University of Florence, Italy
3Department of Cardiothoracovascular Medicine, Azienda Ospedaliero-Universitaria Careggi, Italy
Keywords: Chronic heart failure; Sacubitril/valsartan; SGLT2i
During the last years, several pharmacological treatments have significantly improved outcome of patients with heart failure with reduced ejection fraction (HFrEF) particularly by inhibiting the renin-angiotensin-aldosterone system(RAAS) and by blocking the sympathetic system.[1,2]More recently, it was demonstrated that a further positive neurohormonal modulation of RAAS and of the natriuretic peptides pathway with Sacubitril/Valsartan (Sa/Va), a first in class dual-acting angiotensin receptor blocker and neprilysin inhibitor (ARNI), was proven to be safe and effective in improving prognosis in the PARADIGM-HF trial.[3]Furthermore, novel promising therapeutic strategies focused on management and treatment of comorbidities (e.g., iron deficiency, diabetes mellitus) have shown benefit in HF patients.[1]Particularly, in type 2 diabetes mellitus patients,sodium-glucose co-transporter 2 inhibitors (SGLT2i) have shown to improve HF outcomes.[4-6]The DAPA-HF and the EMPEROR-Reduced trials demonstrated that, compared to placebo, dapagliflozin and empagliflozin significantly reduce the risk of worsening HF and cardiovascular death among patients with HFrEF, regardless of the presence of diabetes.[7,8]On the basis of these evidences, these drugs,which were born as glucose lowering drugs will soon become one of the fundamental columns in the pharmacological treatment of HFrEF together with ARNIs, Betablockers (BBs) and Mineralcorticoid Receptor Antagonists(MRAs) and will be strongly recommended in the next coming HF guidelines.Particularly fascinating will be the association between the two classes of drugs which more recently have proved to improve significantly outcomes in HFrEF patients: ARNIs and SGLT2i.In both DAPA-HF and in EMPEROR-Reduced trials, sub-group analysis showed that the effects of dapagliflozin and empagliflozin on prognosis were independent and not influenced by treatment with Sa/Va at baseline.[7,8]Nevertheless it should be noted that only a minority of patients were treated with a SGLT2i and an ARNI, 250 (10.9%) and 340 (18.3%) respectively,with a total number of less than 600 patients treated with both drugs, which, at present, represent the only experience we have with this association.[7,8]Solomon,et al.[9]analysed outcomes and safety of patients enrolled in the DAPA-HF trial according to baseline treatment with Sa/Va and found that all measures of safety, including adverse events related to volume depletion and worsening renal function, which were remarkably similar in patients who received dapagliflozin or placebo, were also similar in the dapagliflozin group whether patients were taking Sa/Va or not.
Nevertheless, the limited number of patients with this association and the highly selected nature of clinical trials patients should still recommend caution.Furthermore, the mean age of patients enrolled in these trials (just above 65 years) is significantly lower compared to the one of patients in clinical practice and this might rise some concerns with the use of these drugs in older patients even if age focused sub-group analysis carried out from trials with ARNIs and SGLT2i are somehow reassuring.In this regard, Jhund,et al.[10]analysed the efficacy and safety of Sa/Va according to age highlighting that the benefit of Sa/Va over enalapril was consistent across the age categories studied and that the effect of Sa/Va on primary outcome and its components as well as quality of life even in the oldest patients (≥ 75 years,18.6%) seemed qualitatively and quantitatively similar to those observed in younger patients.Also, apost hocanalysis of the DAPA-HF trial confirmed that efficacy and safety of dapagliflozin were consistent across the spectrum of age studied including individuals ≥75 years of age (24.6%).Although adverse events and study drug discontinuationincreased with age, neither were significantly more common with dapagliflozin in any age group.[11]
Furthermore, although even if it has been shown that both ARNIs and SGLT2i have long term nephroprotective evidences,[8-14]we currently lack extensive data on safety and tolerability of this promising association in real world patients regarding its effect on renal function.In the PARADIGM-HF trial, patients treated with Sa/Va were more likely to have symptomatic hypotension than those in the enalapril group, nevertheless an increase of serum creatinine level up to 2.5 mg/dL or more, and a serum potassium level of more than 6.0 mmol/L were less frequently reported in the Sa/Va group and fewer patients in this group stopped the study drug due to an adverse renal event.[3]These results were consistent across all spectrum of age.[10]
Data from clinical trials and post-marketing surveillance have addressed the safety profile of SGLT2i.[4-6,15]The most common side effects reported were genital mycotic infection, urinary tract infection and volume depletion (broadly including hypotension, syncope, and dehydration).Treatment with SGLT2i is associated with sustained lowering of systolic (4 to 6 mmHg) and diastolic (1 to 2 mmHg) blood pressure, due to plasma volume reduction and direct effects on vascular function, thereby causing orthostatic hypotension especially among the elderly.[16]
Less common were acute kidney injury, hypoglycaemia and ketoacidosis.[4-6,15]About kidney injury, some possible causes have been reported: (1) SGLT2i cause osmotic diuresis with an increased risk of hyperosmolarity and dehydration; (2) exchange of urinary glucose for uric acid leads to uricosuria and tubular injury via crystal-dependent and independent pathways; and (3) fructose generation and metabolism are responsible for local inflammation and tubular injury.[17]
Nevertheless, recently two SGLT2i trials were prematurely interrupted due to clear benefit on renal outcomes.The CREDENCE trial which had enrolled more than 4000 type 2 diabetes mellitus patients with albuminuric chronic kidney disease (CKD) showed clear benefit of canagliflozin vs.placebo in reducing the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine, or death from renal or cardiovascular causes.[13]Similarly, in the DAPA-CKD trial, which included more than 4000 diabetic and non-diabetic patients with an estimated glomerular filtration rate (eGFR) of 25-75 mL/min, dapagliflozin showed a significant reduction of the primary composite endpoint of sustained decline in eGFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes.[14]Furthermore, this nephro-protective effect of SGLT2i was proven also in HF trials.In the DAPA-HF trial of relevance was the renal safety profile in the elderly group in which the association with RAAS inhibitors and diuretics (both prescribed in more than 90% of patients) could have raised renal safety concerns which were not confirmed in the trial.On the contrary, particularly in the older group, dapagliflozin showed to have renal protective effects compared to placebo with a significantly lower incidence of serious renal adverse events.[11]
Nevertheless, it should be considered that both Sa/Va and SGLT2i share a similar effect on renal function on initiation with a significant drop in eGFR, compensated in the longterm, as above reported, by a slower decline in eGFR.Data from the PARADIGM-HF trial had previously shown that also Sa/Va initiation is associated with a mild worsening of renal function.A 2-5 mL/min per 1.73 m2decrease in eGFR(due to reduced transglomerular pressure) after initiation of Sa/Va is a known and reversible effect.[12,18]A similar pattern of eGFR variation has been observed with SGLT2i with a modest decline in eGFR (3 to 4 mL/min per 1.73 m2)is expected after initiation, but SGLT2i result in long-term reno-protection and reduced albuminuria.[16]The above described drop in eGFR might not be clinically relevant in case of single administration, but may have a significant clinical impact if Sa/Va and SGLT2i are initiated simultaneously.Furthermore, looking at the probable scenario that will characterize the next HF Guidelines, we will probably have recommendation to treat HFrEF patients with BBs,ARNIs, MRAs and SGLT2i in order to significantly improve prognosis.It should be noted that in the DAPA-HF trial this combination was used in only 131 patients and even if the benefit and safety (volume depletion and adverse renal events) of dapagliflozin were not influenced by this background therapy[19]it is clear that the number of patients treated with this “future guideline recommended therapy” is limited.Moreover, even if in the DAPA-HF trial, the benefit of dapagliflozin on efficacy endpoints was consistent regardless of diuretic use and diuretic dose,[20]in clinical practice renal function and volemic status should be assessed prior to SGLT2i initiation and then monitored regularly for an early recognition of signs and symptoms of hypovolemia(e.g., orthostatic hypotension) particularly in patients with impaired baseline eGFR, concomitant RAAS blockade and on high dose loop diuretics.In fact, if we imagine the next guidelines scenario diuretics, which are currently prescribed to about 90% of HF patients in order to relieve symptoms of congestion,[21]will act together with Sa/Va, MRAs and SGLT2i at different levels of the nephron modulating renal function and volemic status with possible and non-completely predictable interactions (Figure 1).

Figure 1.Sites of action of disease modify in treatments and diuretic therapy at level of nephron.AT1: angiotensin II type 1; K+:potassium; Cl-: chloride; MRA: mineral corticosteroid receptor antagonist; Na+: sodium; NP: natriuretic peptide; SGLT2: sodium-glucose co-transporter 2.
Here we present a case of volume depletion and acute kidney injury in a HFrEF patient treated with loop diuretics,Sa/Va, MRA after introduction of an SGLT2i (empagliflozin).We believe that this case may help physicians in the management of comprehensive HF therapies.
A 68-year-old man was first evaluated at our outpatient HF clinic in February 2017.He was affected by ischemic HFrEF diagnosed two years earlier, arterial hypertension,type 2 diabetes, first-degree obesity and mild to moderate renal impairment with an eGFR of 55 mL/min per 1.73 m2calculated with CKD-EPI.In addition, he had undergone implantation of biventricular implantable cardioverter defibrillator (BIV-ICD) a year earlier.After having undertaken decongestant therapy for the presence of signs and symptoms of HF, treatment was optimised, according to current guidelines, with the introduction of low dose Sa/Va (24/26 mg b.i.d.) that was progressively up titrated to maximum dose (97/103 mg b.i.d.) with a clinical improvement and the possibility to reduce furosemide to 50 mg/day.On May 2019, he was clinically stable with a good pressure profile(125/70 mmHg), and good renal function which had also slightly improved with eGFR 66 mL/min per 1.73 m2.Therefore also in view of a sub-optimal glycaemic control(HbA1c 63 mmol/mol), we decided to optimise HF and diabetes therapy with empagliflozin 10 mg; in consideration of its glucosuric, natriuretic and diuretic effects we further reduced furosemide to 25 mg/die.A clinical follow-up was scheduled at two months.Two weeks later he contacted us complaining of fatigue associated with hypotension and significant weight loss, so we further reduced diuretic (furosemide 25 mg, one tablet three times week) and Sa/Va doses (49/51 mg b.i.d.) and scheduled a clinical control after two weeks.At the moment of visit, he was symptomatic for fatigue and nausea, had experienced a weight loss of 7 kg,was hypotensive with a sitting blood pressure of 90/60 mmHg and presented hyperkaliaemic metabolic acidosis(pH =7.29, K+ 5.8 mEq/L).Therefore, he was hospitalised and blood tests showed pre-renal worsening renal function(creatinine = 1.98 mg/dL, eGFR of 34 mL/min per 1.73 m2).Consequently Sa/Va, empagliflozin, metformin and potassium canreonate were withdrawn.He was then treated with hydration and intravenous bicarbonate therapy and was discharged at home after five days with demonstration of renal function improvement (eGFR of 42 mL/min per 1.73 m2and serum potassium 4.5 mEq/L) on bisoprolol 5 mg and low dose furosemide (25 mg/die); linagliptin was prescribed for diabetes.One month later, after a further improvement of renal function (eGFR of 54 ml/min per 1.73 m2), Sa/Va(24/26 mg BID), potassium canrenoate (25 mg/die) and metformin (1000 mg/die) were reinstated.Further treatment modifications are shown in Table 1.Since discharge he refused to repeat a trial of any SGLT2i.
Waiting to have more real world data in relation to all these associations, it would be useful, in order to prevent complications and not to waste the potential benefits of this highly promising association, to take in consideration the following advices: (1) short-term clinical control and blood tests including renal function should be scheduled after initiation of these therapies or after an up titration; (2) patients should be educated on body weight and blood pressure self-monitoring and to alert the caring physician in case of significant changes; (3) medical therapy should be reviewedwith dose adjustment (and in selected cases temporary withdrawal) of diuretics; (4) metformin dose adjustments (or withdrawal) should be discussed with diabetologists to lower the risk of metabolic acidosis; (5) simultaneous initiation of SGLT2i and Sa/Va should probably be avoided preferring a close but progressive approach; (6) clinical re-assessment including renal function in presence of possible volume depletion conditions such as fever or diarrhoea; and (7) consider seasonality, preferring un titration or initiation of these therapies during milder climates and re-evaluating always diuretic therapy during summer.
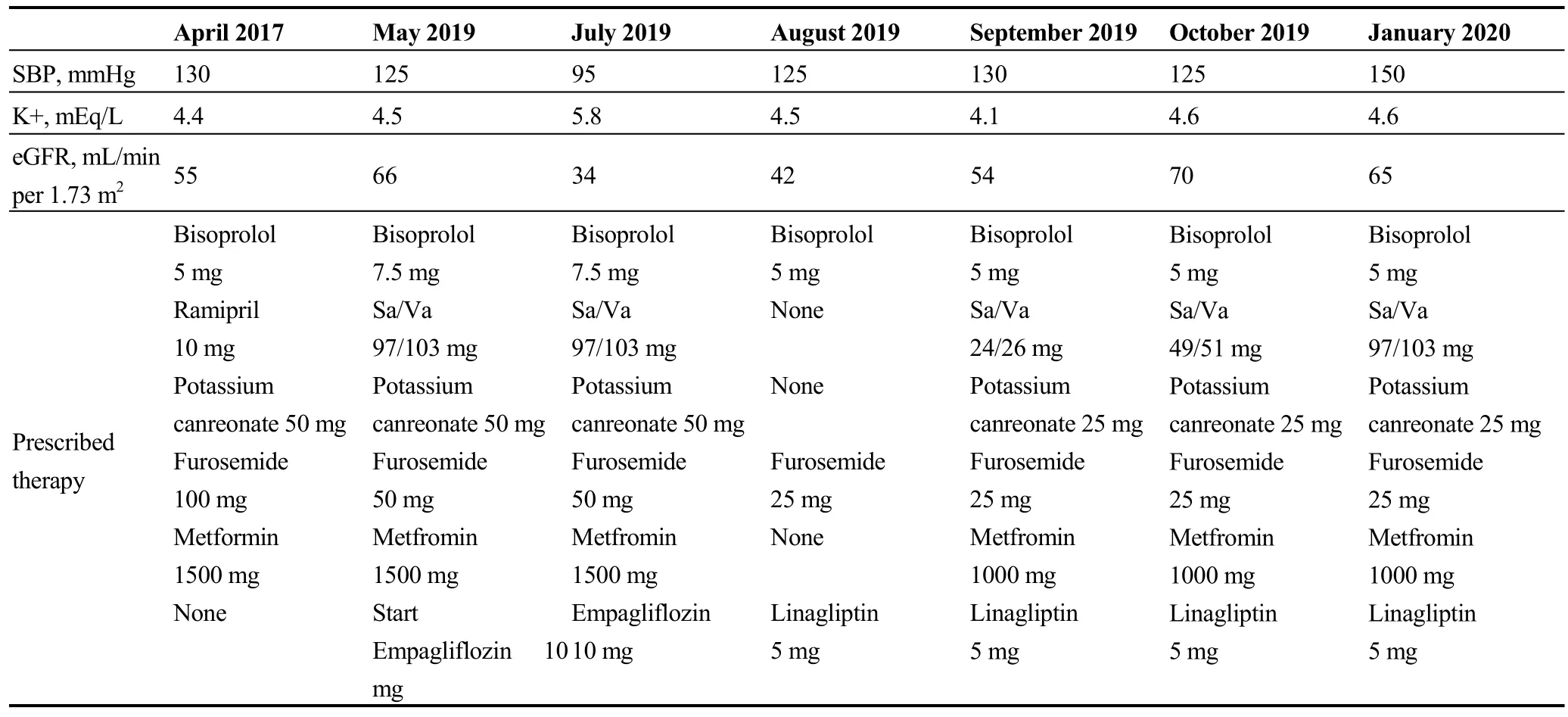
Table 1.Trend of renal function, potassium and pressure in relation with therapy.
 Journal of Geriatric Cardiology2020年11期
Journal of Geriatric Cardiology2020年11期
- Journal of Geriatric Cardiology的其它文章
- Incident frailty and cognitive impairment by heart failure status in older patients with atrial fibrillation: the SAGE-AF study
- Impact of proton pump inhibitors on clinical outcomes in patients after acute myocardial infarction: a propensity score analysis from China Acute Myocardial Infarction (CAMI) registry
- Comparison of low-density lipoprotein cholesterol/high-density lipoprotein cholesterol and total cholesterol/high-density lipoprotein cholesterol for the prediction of thin-cap fibroatheroma determined by intravascular optical coherence tomography
- Plasma levels of Elabela are associated with coronary angiographic severity in patients with acute coronary syndrome
- Gender differences in clinical outcomes of acute myocardial infarction undergoing percutaneous coronary intervention: insights from the KAMIR-NIH Registry
- Ablation strategies for arrhythmogenic right ventricular cardiomyopathy:a systematic review and meta-analysis
