Geriatric issues in patients with or being considered for implanted cardiac rhythm devices: a case-based review
Michael A Chen
Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748, Seattle, WA 98104, USA
Keywords: Arrhythmias; Cardiac implanted electronic devices; Older adults; Palliative care; Syncope
1 Introduction
Virtually all cardiovascular diseases including arrhythmias, valve disease, coronary artery disease and heart failure(HF) are increasingly common with advancing age.[1]Age and disease-related changes in the heart including fibrosis in the atrial and ventricular myocardium and conduction system, scar tissue from myocardial infarction or other cardiomyopathic processes, increased inflammatory cytokines and changes to ion channels are just some of the factors that predispose older adults to arrhythmias.[2]These factors make many older adults potential candidates for cardiac implanted electronic devices (CIEDs).This paper presents several common scenarios regarding older adults with cardiac disorders who already have (or may be considered for) CIEDs and reviews the cardiac, electrophysiologic and geriatric issues that should be addressed in these situations.
2 Case 1
A 78 year-old man with an ischemic cardiomyopathy(left ventricular ejection fraction (LVEF) 33%) and sinus node dysfunction who had a primary prevention dual-chamber implanted cardioverter defibrillator (ICD) placed 5 years ago (he later became pacemaker dependent) presents after a recent diagnosis of stage IV colon cancer with widespread metastases to liver and bone.After appointments with Oncology and Palliative care, a collective decision was made to forgo any cancer treatment (e.g., chemotherapy, radiation,surgery) in favor of comfort-oriented measures.On the advice of his oncologist he is in clinic to discuss management of his ICD.
Although it would appear obvious that patients with significant cardiovascular disease, especially those requiring CIED therapy, should have discussions with their providers regarding advanced care planning, evidence suggests that these discussions often do not occur.For example, in one study of 51 patients with an ICD and multiple medical problems nearly all of whom had either living wills (88%)or health care proxies designated (98%), communication with their providers regarding ICD deactivation only occurred in 10 and 23%, respectively.[3]As a result, five of the nine patients who died during the study experienced frequent shocks at the end of life.In another study of 125 ICDs explanted post-mortem, ventricular tachyarrhythmias were identified in the last hour of life, with 24% having had arrhythmic storm and 31% having had a shock in the last 24 hours of life, although arrhythmia was deemed to be the cause of death in only 13%.Although 52% of patients had“do not resuscitate” orders, 65% still had tachytherapies programmed “on” in the 24 hours prior of their lives.[4]While some have interpreted this as inconsistent decision-making, some patients may feel that an arrhythmia that may be terminated with a shock and that would be acceptable to them, while they would want to forgo a full resuscitation, with cardiopulmonary resuscitation.Beginning February 15, 2018, every patient receiving an ICD for primary prevention under Medicare has been required to have an encounter for shared decision-making using an evidencebased decision tool.[5]This discussion is meant to include the information that the device can be deactivated.[6]This critically important discussion is best initiated early and revisited at regular intervals or when there are changes in health status or goals of care.
The ethical principle of autonomy underlies the ethical and legal basis which supports patients’ (who have decisional capacity), or their legally designated surrogate, and after an informed discussion) right to refuse/decline any medical or device treatment including those that may be considered life-sustaining such as dialysis in a patient with end-stage renal disease.Furthermore, this right, upheld by the UScourt system, holds even if the patient is not “terminally ill,”and is supported by medical society guidelines.[7-12]
Case conclusion: after a discussion of his options, the patient requested that his ICD’s tachy-therapies be disabled,but wished the pacing function to remain active and his device was reprogrammed accordingly.
3 Case 2
An 83 year-old woman with a history of intermittent complete heart block status post dual chamber pacemaker 10 years ago presents to clinic after a 1.5 year absence.Her husband reports that her dementia has progressed, and she is now dependent on him for all of her activities of daily living(ADLs).She rarely remembers who he is and often asks him for his name.He has hired a caregiver to help and give him breaks.Although they never have completed an advanced directive, he reports that in many prior conversations she had stated that she would never want to be supported“artificially” or be on any “machines,” and thus he would like her pace-maker (PM) “turned off” to let “nature take its course.” The patient’s cardiologist is uncomfortable with complying with the request.
As noted, professional societies have issued guidelines which state that patients have the right to request withdrawal of any therapy, including those provided by CIEDs,and furthermore, that if the practitioner’s (e.g., physician,other clinician or Industry-Employed Allied Professional(IEAP) personal or professional values does not allow them to comply with such a request, they cannot be compelled to do so, but they have an obligation to arrange for another care provider who is willing to carry-out the patient’s request to do so.[8,13-14]
Case conclusion: Patient referred to another Cardiologist who makes the programming changes.From a practical point of view, once the decision has been made to discontinue therapies, a programmer can be used to disable the various functions such as anti-tachycardia pacing, or shocks,or setting the pacemaker mode to a sensing only mode (e.g.,OOO, DOO, VOO) or the output may be programmed well below the output threshold.A few devices may have an“off” setting.For an ICD, if a programmer is not available, a magnet placed over the generator will deactivate tachycardia therapies (although back-up pacing will still be active).
4 Case 3
A 77 year-old man with moderate dementia (Alzheimer’s type) was noted to have a heart rate of 40 beats/min on intake vital signs at his geriatrician’s office who then referred the patient to cardiology.His medications include Donepezil which the provider notes in the referral has been associated with bradycardia.Upon questioning, the patient reports a subtle decrease in his exercise tolerance over the last few months, especially with hills.His 12-lead ECG is notable for sinus bradycardia at 43 beats/min, and an incomplete right bundle branch block (RBBB).
Alzheimer’s disease is the most common cause of dementia, affects over five million Americans; approximately 1/3 of seniors.[15]The pathophysiology of Alzheimer’s disease involves cholinergic neurons and levels of acetylcholine in the brain, and thus acetylcholine has been a target for therapy.[16]There are three acethylcholinesterase inhibitors(AChEI) available for the treatment of dementia: donepezil,galantamine and rivastigmine.Based on studies that showed modest improvements in scores of cognitive function, each has been FDA approved to slow the progression of cognitive decline in patients with mild to severe Alzheimer’s disease.Adverse events associated with the medications are generally related to overstimulation of the central and peripheral cholinergic system which is found throughout the body.The most commonly associated symptoms are nausea,vomiting, diarrhea, weight loss and bradycardia.[17]
The available data on the cardiovascular effects of AChEI are mixed, but some concerning results have been reported.An administrative database study found that dementia patients on AChEI had 1.4 times the odds of having bradycardia (vs.patients not on these medications), and there was a dose-dependent increase in risk for patients on donepezil.[18]In a population-based analysis in Ontario,Canada, after controlling for time to hospitalization, patients receiving AChEIs had an increased risk of hospitalization for syncope (HR = 1.76), bradycardia (HR = 1.69), pacemaker insertion (HR = 1.49) and falls (HR = 1.18).[19]Other studies have also reported that the use of these medications is associated with increased risks of heart block, sinus bradycardia and syncope.[20-22]Given that the population of patients who are on these medications is older and may be vulnerable to age-related changes that can predispose to orthostasis (and therefore syncope) or have pre-existing cardiovascular disease which may exacerbate any tendency towards bradycardia (e.g., sinus node dysfunction, heart block) or interact with other medications that patients may be on (i.e., beta blockers, calcium channel blockers, antiarrhythmic medications), the concerns over these agents seem well founded.In fact, the package insert for donepezil states:“…because of their pharmacologic action, cholinesterase inhibitors may have vagotonic effects on the sinoatrial andatrioventricular nodes.This effect may manifest as bradycardia or heart block in patients both with and without underlying cardiac conduction abnormalities.Syncopal episodes have been reported in association with the use of ARICEPT”.
When a patient presents with symptomatic (or asymptomatic bradycardia) and is on one of these agents, one might be tempted to simply stop the medication and see if the bradycardia resolves or improves, but this approach should not be undertaken lightly.Although AChEIs are not considered to be disease modifying agents, they have been shown to provide some clinical improvements.And while at some point the disease is expected to progress and providers may elect to stop the medication due to concerns regarding polypharmacy and diminishing benefits, there is data that suggests that patient’s cognitive and neuropsychiatric conditions may deteriorate after they are stopped, and some have reported withdrawl-like symptoms.[23,24]Thus, simply stopping the agent may not be a good option for patients.
Therefore, in consultation with the provider who prescribes the AChEI and the patient (or their surrogate) medication review and lowering the dose of other agents that might be contributing to bradycardia, consideration of stopping the AChEI, or consideration of pacemaker placement are potential strategies to deal with symptomatic bradycardia.
Case conclusion: the patient (with input from his wife)elected to remain on Donepezil and although it was difficult to determine if the bradycardia was caused by the medication, the patient did appear to have symptomatic bradycardia and so a dual chamber pacemaker was placed.
5 Case 4
A 79 year-old man with O2dependent chronic obstructive pulmonary disease (on 3L O2by nasal cannula), diabetes mellitus, stage IV chronic kidney disease, ischemic CM status post multivessel percutaneous coronary intervention(PCI) three years ago on maximum tolerated guideline directed medical therapy has an LVEF that increased from 22% to 28% was referred to electrophysiology for consideration of primary prevention ICD placement.
The incidence of sudden cardiac death increases with age,although the percentage of sudden versus all-cause deaths decreases as age increases.[25]In the amiodarone trialists meta-analysis database, 51% of deaths were sudden in those< 50 years old, but this decreased to 26% in those ≥ 80 years of age.[25]As in younger patients with sudden cardiac death(SCD), those with ventricular tachycardia (VT) or ventricular fibrillation (VF) had better survival.In one analysis, patients ≥ 80 years old who had VT or VF had better survival to discharge than younger patients who had suffered a pulseless electrical activity (PEA) arrest.[26]
ICDs are highly effective in preventing arrhythmic SCD and are widely used, particularly in the HF population.Eligible patients are described in major society guidelines, and the indications regarding implanted device therapies including ICDs, pacemakers and cardiac resynchronization therapy (CRT) are similar in older as they are for younger adults.[27]This is despite the fact that patients > 80 years of age have been poorly represented in most of the trials the recommendations are based on.Nonetheless, these devices are commonly placed even in that age group.For example,in the 2013 NCDR ICD Registry report, 17.9% of ICD recipients were ≥ 80 years old with 23% having undergone generator replacement.[28]Unsurprisingly, the mortality in octogenarians with ICDs is high.In one study, primary and secondary prevention ICD recipients at least 80 years old had 2-year mortality rates of 40%-50% following their first appropriate shock.[29]
The Evidence Review Committee of the 2017 ACC/AHA/HRS Guideline for the Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death completed a systematic evidence review to evaluate the impact of ICD implantation for primary prevention in older patients and patients with significant comorbidities (including diagnoses such as renal disease,chronic obstructive pulmonary disease, atrial fibrillation and other forms of heart disease).[30]They determined that neither age nor co-morbidities should exclude patients from receiving an otherwise indicated ICD, if “meaningful survival of greater than one year is expected.” They did note that the available data could have been biased due to patients who were the most frail or otherwise poor candidates for an ICD being screened out.[27]
Data for ICDs in older adults are somewhat mixed.In one analysis of pooled data from five primary prevention ICD trials, they were associated with a reduction in mortality in patients ≥ 75 years of age (HR = 0.54 (95%CI: 0.37-0.78)).[31]A more recent trial in patients with nonischemic cardiomyopathy, however, failed to show a survival benefit in primary prevention ICDs, including in older adults, although SCD was reduced by 50%.[32]
Multiple studies have shown increased procedural complications in older adults receiving CIEDs.[33-36]For example, in one study of 150,264 patients who received primary prevention ICDs (from January 2006 to December 2008)were followed for a primary end point of any adverse event or in-hospital mortality, and secondary end points includingmajor adverse events, minor adverse events, and length of stay.61% of the sample was ≥ 65 years old.About 3.4% of the entire cohort had a complication or death after implantation.Any adverse event or death occurred in 4.5% of patients who were at least 80 years old, whereas that rate was 2.8% in patients less than 65 years (OR = 1.15; 95% CI:1.01-1.30).[35]
On the other hand, in a population-based cohort study in Denmark, all patients (n= 5918) who had a CIED implanted or revised (May 2010 to April 2011) were studied for complications.562 patients (9.5%) experienced at least one complication, and while the risk of any complication was higher if the patient was female, underweight, implanted at a low-volume center, or placed by a low-volume operator, received a dual-chamber ICD or a CRT-D device,underwent system upgrade or lead revision or underwent an emergency, off-hours procedure, patients > 80 years had a 20% lower rate of complications than those < 80 years.In particular, fewer lead-related re-interventions occurred.The authors hypothesized that this may have been a result of a higher tolerance to accept suboptimal lead function, a higher implantation rate of simpler CIED types, or because the older patients were less active and thus put less strain on the implanted leads.[37]This study is more the exception than the rule however, as noted.
In deciding whether to place an ICD, factors to take into consideration are that patients ≥ 80 years of age, with an LVEF < 30% and renal dysfunction have been shown to have only a 1.5 year mean survival after ICD implantation.[38]Moreover, in that age group, those with an LVEF ≤ 20% is the strongest predictor of 1-year mortality (38.2%).[39]The severity of HF may also have an impact.In a recent posthoc analysis of 81,492 Medicare patients with HFrEF and an LVEF ≤ 35% from the NCDR ICD Registry who had a first-time primary prevention ICD or CRT-D device placed(between 2010 and 2014), the group with severe HF (Class IV and other signs of severe disease,n= 3,343) was compared to patients with more mild HF (NYHA Class II symptoms,n= 19,424).[40]All-cause mortality at 30 days was 3.1% in the advanced HF groupvs.0.5% in the mild group(P< 0.001).This corresponds to a 22% risk of death for the severe HF group and a low median survival rate of 3.5 years.For comparison, in NYHA Class II control group patients enrolled in 4 ICD primary prevention trials (MADIT-I,MADIT-II, DEFINITE, SCD-HeFT), 3 year morality was 10%-11%, whereas in Class III patients the sudden death morality rate was 11%, and the non-sudden death mortality rate was 25%.[41]In the current study, the main predictors of all-cause mortality were: NYHA class IV symptoms, ischemic heart disease, and diabetes.In the severe HF group, the in-hospital periprocedural complication rate was 3.74%vs.1.10% in the mild group (P< 0.001).The most common adverse events were: in-hospital death (1.82%) and resuscitated cardiac arrest (1.05%).Patients with NYHA class IV symptoms, ischemic heart disease, or diabetes had a higher risk of mortality.
Thus, the use of primary (and in some cases secondary)prevention ICDs in the very elderly needs to take into account multiple factors including: life expectancy, comorbidities (cardiac and non-cardiac) their tolerance of procedural risks and the possibility of inappropriate shocks in the context of their overall goals of care as evidenced by advanced directives, or prior discussions with their health care team and surrogate decision-makers.
Frailty is a specific condition that may influence the decision to place an ICD in a patient who otherwise meets standard criteria.A study of 83,792 Medicare patients from the National Cardiovascular Data Registry ICD Registry(NCDR-ICD) who underwent first primary prevention ICD implantation between 2006 and 2009 helps speak to this issue.[42]Medicare analytic files were used to determine the prevalence of frailty, dementia, and other conditions prior to ICD implantation, as well as collecting 1-year mortality data.Ten percent of the sample had frailty and 1% had dementia.Overall 1-year mortality was 12% but was 22% for patients with frailty and 27% for patients with dementia.Several patterns of multi-morbidity were associated with high 1-year mortality rates: dementia with frailty (29%), frailty with chronic obstructive pulmonary disease (25%), and frailty with diabetes mellitus (23%).These patterns were present in 8%of the cohort.These data suggest that frailty (and dementia)should be considered in clinical decision-making and guideline development.
CRT is usually used for the treatment of HFrEF and dyssynchrony manifest as a prolonged QRS with a left bundle branch (LBBB) morphology.The treatment is associated with an antiarrhythmic effect in patients whose left ventricular end systolic volume improves.In a sub-study of the MADITCRT trial there was a 20% reduction in ventricular arrhythmias for every 10% improvement in LV end systolic volume,a finding that extended to patients over 80 years old.[43]Of note, procedural complication rates when compared to younger patients were not increased.[44,45]In the Swedish Heart Failure Study, while 37% of patients over 80 years met the indications for CRT, only 4% received such a device.[46]In patients who do not wish to have or who may not be appropriate for an ICD, a CRT-PM (without ICD) may be implanted to improve functional outcomes and quality of life.
Case conclusion: there was some consideration given to placing a bi-ventricular pacing device (without a defibrillator) as this has been utilized in older adults in whom a defibrillator has been declined or not felt to be appropriate, but who otherwise meet indications for a bi-ventricular pacemaker.[33]After a joint discussion of the risks and benefits no device was placed.
6 Case 5
A 77 year-old man with severe aortic stenosis had a transcatheter aortic valve replacement (TAVR) placed which was complicated by complete heart block (CHB).
Aortic stenosis is the most common acquired valve disease in the United States, and since its introduction in 2002,TAVR has emerged as a commonly utilized, less invasive alternative to surgical aortic valve replacement.The aortic root complex is closely related to the atrioventricular (AV)node and distal conducting fibers, making compression,disruption or peri-procedural edema possible during a valvular procedure.[47]Surgical valve replacement is associated with a 3%-6% risk of AV block, whereas TAVR is associated with even higher rates.[47,48]In the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy registry, a new pacemaker or ICD was placed in 8.8% of the 26,414 patients.[49]Risk factors for the development of AV block necessitating device therapy include pre-existing RBBB and the use of specific types of prostheses.[50,51]Depending on the type of prosthesis placed and the population studied, the need for a pacemaker ranges from 1.8% to as high as 42.5%.[52,53]The larger profile,self-expanding (as opposed to balloon-expandable) Core-Valve which is implanted deeper into the LVOT tends to have a higher incidence.In a review of 35,500 TAVR cases performed between 2012 and 2014, the overall incidence of complete heart block was 10.4%, with the rate increasing over time from 8.4% to 11.8%.[54]Those with CHB had higher in-hospital mortality than those without it (5.9%vs.4.2%, adjusted OR: 1.32; 95%CI 1.12-1.56;P= 0.001).Unsurprisingly, CHB is associated with both longer lengths of stay and higher costs.
There is also evidence from a meta-analysis that new onset LBBB after TAVR was associated with an increased need for pacemaker implantation (RR = 2.18; 95% CI:1.28-3.70).[55]Obviously, if a patient has a pre-existing complete RBBB, developing a LBBB can result in CHB.The development of LBBB with TAVR is also associated with an increased risk of cardiac death (RR = 1.39; 95%CI:1.04-1.86) at one year of follow-up.[55]On the other hand,all-cause mortality rates after TAVR for patients who requiredvs.those who did not require a pacemaker were similar in another registry (883 patients (FRANCE-2)).[56]Interestingly, the rate of pacemaker implantation after a valve-in-valve TAVR appears to be lower than after initial TAVR placement.[57]Predictors of the need for a permanent pacemaker in literature reviews are noted in Table 1.
Of note, not all patients will ultimately “use” their permanent pacemaker long-term.For example, in one single center prospective study of patients who received a PM after TAVR, of the 167 patients, only 44% were pacemaker dependent.[58]There is also a rate of late developing heart block.In one single-center trial of patients discharged from 2016-2018 who received ambulatory rhythm monitoring,10% of patients were found to have new heart block requiring a permanent PM greater than two days after TAVR placement.Predictors for this outcome were hypertension and RBBB.However, the sensitivity of RBBB for delayed AV- block was 27% (and the specificity 94%).[59]Clearly, a better ability to predict the development of AV-block would be useful.
Case conclusion: The temporary pacemaker which had been placed at the time of TAVR was replaced with a permanent dual chamber implanted system.
The type of device chosen is individualized to eachpatient’s individual circumstances.For example, in this case,the patient was in normal sinus rhythm at baseline, but developed CHB, therefore a dual chamber device could have been chosen.Alternatively, if the patient was in chronic atrial fibrillation, a single lead right ventricular pacemaker could have been considered.Increasingly, leadless pacemakers are being placed for patients for whom a single chamber device is appropriate or adequate.These are small,self-contained devices which are placed percutaneously and provide RV only pacing (possible modes include VVI,VVI-R, VOO, OVO or “off”), see Figure 1.Studies of their safety and efficacy are favorable and have improved over time.[60]Advantages of these devices include their placement procedure being less invasive and shorter (thus requiring less intensive sedation), and being less prone to infection (no subcutaneous pocket is needed) and that they are associated with fewer complications.[60,61]These advantages and others may be particularly applicable to elderly patients.For example, elderly patients with dementia may not be able to remember their activity restrictions and inadvertently stress the lead of a transvenous system or forget what the“lump” and incision on their chest is and pick at it.They may also be at increased risk for deeper procedural sedation and after a leadless device placement they may be less likely to need pain medication which could further alter their sensorium.Probably due to these benefits there is data that hospital lengths of stay are shorter in patients when they are placed compared to a traditional pacing system.[62]Although seemingly a technology primarily for patients without an indication for a dual chamber device such as chronic atrial fibrillation, in patients who are at particularly high risk for the placement of a transvenous system or who are less likely to need frequent pacing and/or who may not be very active,they may be a favorable option.
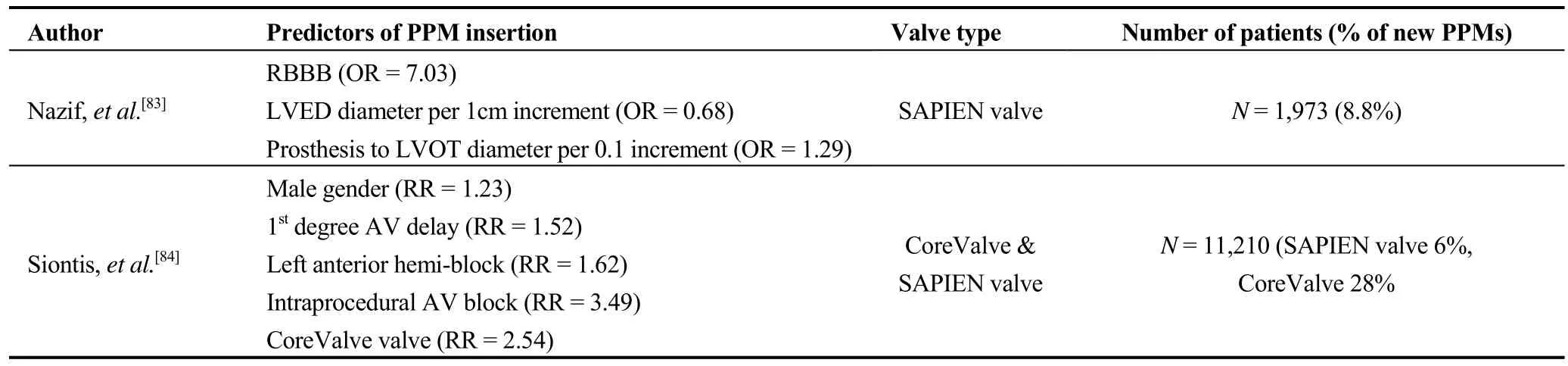
Table 1.Predictors of permanent pacemaker implantation following TAVR.
7 Case 6
67 year-old woman with hypertension, coronary artery disease, status post a non-ST segment elevation myocardial infarction and percutaneous coronary intervention to the right coronary artery and heart failure with a preserved ejection fraction with occasional exacerbations (generally when she has atrial fib), also has paroxysmal atrial fibrillation that is poorly tolerated and she is unable to tolerate sufficient doses of AV nodal blockers to achieve rate control when she has atrial fibrillation.She had a significant drop in her DLCO on Amiodarone (which had only been somewhat successful at reducing her episodes of atrial fibrillation), and thought not to be a candidate for other agents because of her other heart disease.She has a friend who had complications during ablation, so declines this treatment and was therefore referred for atrioventricular nodal (AVN) ablation and PM placement.
As described in current atrial fibrillation management guidelines, AVN ablation with PM placement is a treatment reserved for patients for whom pharmacologic rate control is unsuccessful or not tolerated, including those with tachycardia medicated cardiomyopathy and/or intolerable symptoms from their arrhythmia or the medications used to control it.[63]The procedure appears to be a successful treatment.In a meta-analysis of 21 studies with 1181 patients there were significant improvements in all 19 different clinical outcomes (see Table 2).[64]
The benefits may be due to improved LV function, theslower and more regular ventricular rate or a combination of these.[65]There does not, however, appear to be an effect(either positive or negative) on mortality.[64,66]
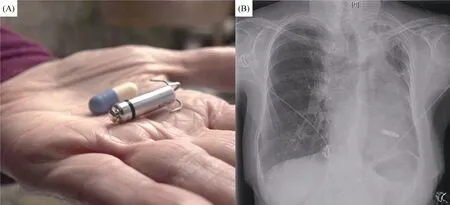
Figure 1.Panel A shows a Medtronic Micra leadless pacemaker next to a standard medication capsule.Panel B shows a chest x-ray in a patient with the same model device.
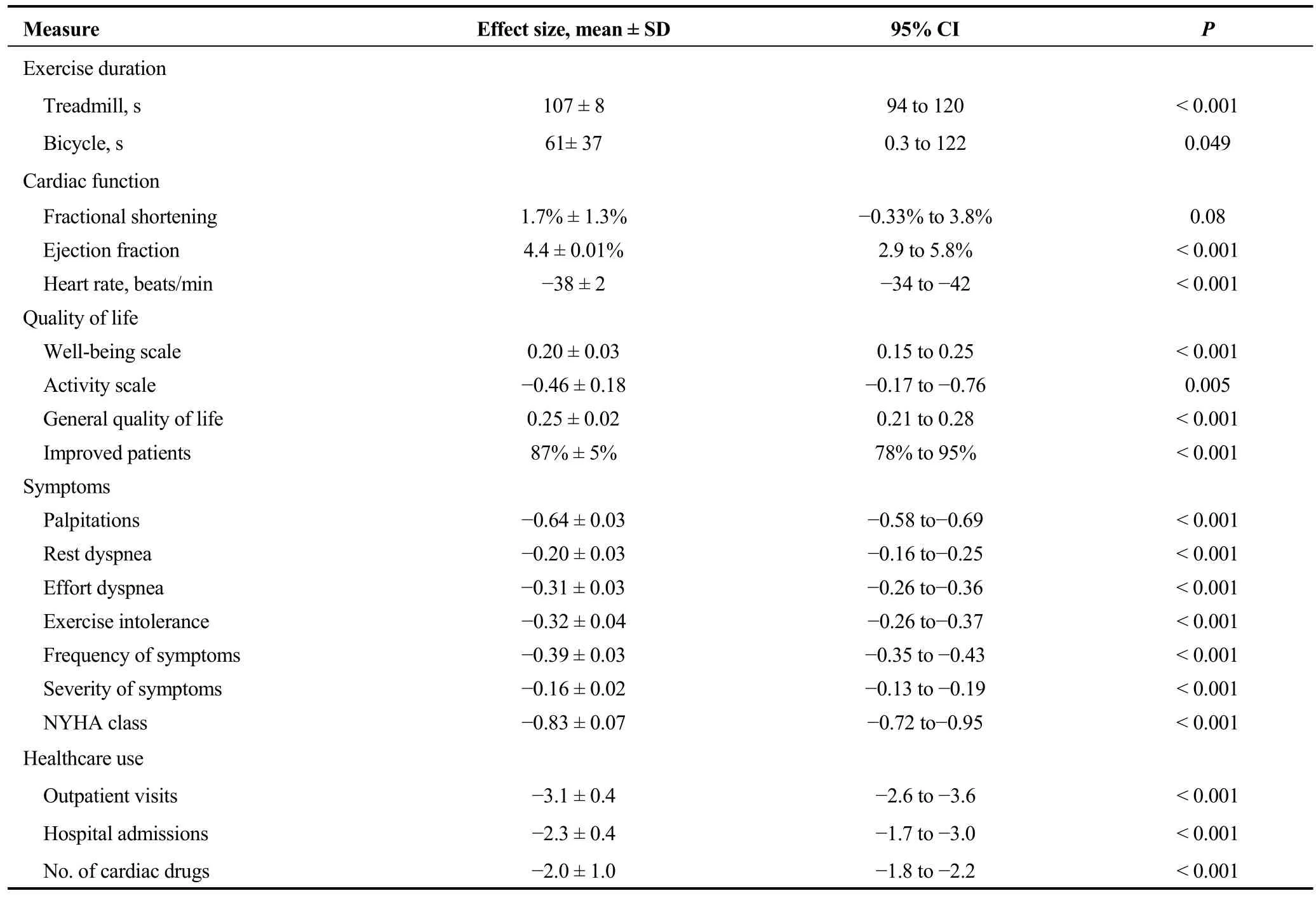
Table 2.Measures of clinical outcome after treatment.Adapted from Wood, et al.[64]
Since AVN ablation renders patients PM dependent, a device must be placed prior to or at the time of the ablation.If the patient has chronic AF, a single chamber PM is indicated,and it is usually programmed with rate-responsiveness (e.g.VVI-R).If the patient’s AF is paroxysmal, then a dual chamber device is usually placed to maintain A-V synchrony during periods when the patient is in sinus rhythm.These devices are usually programmed DDD-R with mode switching, to prevent excessively fast-pacing when patients are in atrial fibrillation.Two trials comparing single and dual chamber pacing with mode switching in patients with paroxysmal AF showed improved symptoms and quality of life compared to either a single chamber PM or a dual chamber device without mode switching.[67,68]
Chronic RV pacing causes the RV to be activated before the LV and the intraventricular septum prior to the LV lateral wall resulting in ventricular dyssynchrony.This dyssynchrony can, in turn, lead to systolic dysfunction, decreased exercise capacity and functional status, HFrEF and increase mortality.[69,70]Because of this and data that those with LV dysfunction improve their ejection fractions with upgrading to a CRT, such devices are often implanted prior to AV nodal ablation.[71]This can be done with or without an atrial lead depending on whether the AF is chronic or paroxysmal.Such an approach is supported by the results of two randomized trials.
In the Post AV Nodal Ablation Evaluation (PAVE) Trial,184 patients with chronic AF who were refractory to medical rate-controlling therapy were randomized after AVN ablation to standard RV pacing or biventricular pacing (also called cardiac resynchronization therapy (CRT)).[72]After 6 months, CRT patients had greater increases in 6-minute walk distance, peak O2 consumption and exercise duration when compared to RV pacing (31%vs.24% improvement).The improvement in 6-min walk distance was limited to patients who had an LVEF ≤ 45% or were experiencing NYHA Class II or III symptoms (83% of the patients).CRT patients had higher LVEFs than those who received RV pacing.
In the BLOCK HF (Biventricular Versus Right Ventricular Pacing in Heart Failure Patients With Atrioventricular Block) trial patients with advanced AV block and an LVEF < 50% had improved outcomes when treated with a biventricular pacemaker compared with those randomized to RV apical pacing.[73]The primary outcome was the time to all-cause mortality, an urgent visit for HF requiring IV therapy, or a 15% or more increase in the LV end-systolic volume index.Of 918 patients enrolled, 691 underwent randomization and were followed for an average of 37 months.The primary outcome occurred in 55.6% of the RV-pacing group,vs.45.8% of the BiV-pacing group.Patients in the BiV-pacing group had a significantly lower incidence of the primary outcome over time than did those assigned to RV pacing (HR = 0.74; 95% CI: 0.60-0.90);results were similar in both the pacemaker and ICD patients.
The usefulness of AV Nodal ablation in patients with HFrEF and atrial fibrillation is also supported by an observational study which suggested that AVN ablation plus CRT may significantly improve survival compared to CRT alone.[74]In this study, of the 1285 patients who received CRT, 243 were in AF.Rate control was achieved by medical therapy in 55 patients and in the other 188 AV nodal ablation was required.Patients who received CRT devices achieved Bi-V pacing ≥ 85% of the time.During a median follow-up of 24 months, morality was lower in patients with AV nodal ablation as compared with the medications only group (4.3%vs.15.2%; adjusted HR 0.26 for all-cause mortality and 0.15 for HF mortality).
Current guidelines support the use of CRT in patients with AF who have an EF ≤ 35% on GDMT if the patient requires ventricular pacing or meets CRT criteria and if AVN ablation or pharmacologic rate control allows near 100% ventricular pacing.[75,76]
Although rare, ventricular fibrillation and sudden death rates appear to be increased after AVN ablation.For example,in a review of 334 patients after AVN ablation, 2.7% suffered sudden death.Four took place within 4 days of the procedure,3 more within 3 months, and 2 were late.[77]This increased risk may be due to several factors including comorbid heart disease, post-procedural sympathetic nervous system activation, prolongation of the action potential, and repolarization abnormalities secondary to bradycardia or a combination of these.[77,78]Pacing at a rate of 90vs.a rate of ≤ 70 beats/min was evaluated in a study of 235 patients.Those whose devices were programmed ≤ 70 beats/min had a 6% rate of ventricular fibrillation, whereas those programmed at 90 beats/min for three months had no VF.This may be because a reduction of sympathetic activity.[78]Therefore, most electrophysiologists program the HR higher early after AVN ablation.
Of note, indications for anticoagulation remain unchanged after an AV nodal ablation with pacemaker placement.
Case conclusion: AV nodal ablation is performed with/simultaneous placement of a bi-ventricular pacemaker.Initial programming was for a HR 90 bpm, which was reduced on a subsequent visit.
8 Case 7
A 74 year-old man with a history of HTN presents after a second episode of syncope.The first was 8 months prior and had been attributed it to dehydration.Two weeks ago when he was talking on the phone with a friend and he passed out in a chair mid-sentence, without prodromal symptoms.He saw his PCP the next day, had borderline abnormal orthostatic vital signs and was referred to Cardiology.His physical exam was unremarkable and his 12-lead ECG showed sinus rhythm and bi-fascicular block (BFB) (specifically, left anterior fascicular block and RBBB).
Syncope is a broad topic with a multitude of potential causes, and a difficult problem, particularly in older adults.The lifetime risk of syncope is near 40%, and the prevalence increases with age, exceeding 20% in those > 75 years old.[79]The reasons for this increase include age-related impairment in baroreceptor function, diastolic left ventricular dysfunction, a tendency towards intravascular volume depletion, an age-related increase in arrhythmias and polypharmacy.
Multiple guidelines for the work-up and management of syncope have been published, as have risk scores and protocols meant to help triage and direct care decisions, including whether to admit a syncope patient to the hospital.[80-81]Guidelines suggest factors that point towards a cardiac etiology of syncope (Table 3)[81]as well as ECG findings that specifically suggest an arrhythmic cause of syncope (Table 4).[80]These include findings such as non-sustained ventricular tachycardia, pathologic q-waves,long or short QT interval, Brugada syndrome, or epsilon waves suggestive of arrhythmogenic right ventricular cardiomyopathy.The list also includes another set of common findings, which was seen in this case: BFB.BFB is any combination of left anterior or posterior fascicular block and RBBB.
The workup of syncope focuses on a thorough history and physical exam (including orthostatic vital signs), and strategic testing that usually includes a 12-lead ECG.Anechocardiogram can be useful to rule out or further evaluate a cardiac structural abnormality such as hypertrophic obstructive cardiomyopathy, impaired left ventricular systolic function or a valvular abnormality such as aortic stenosis.When an arrhythmic cause is suspected, rhythm monitoring(24-48 h Holter, 1-2 week patch monitors, month long monitors or longer term (months to years) implanted loop recorders) can be considered.Electrophysiologic study may be useful in some situations, and guidelines allow for the placement of a pacemaker in certain cases.
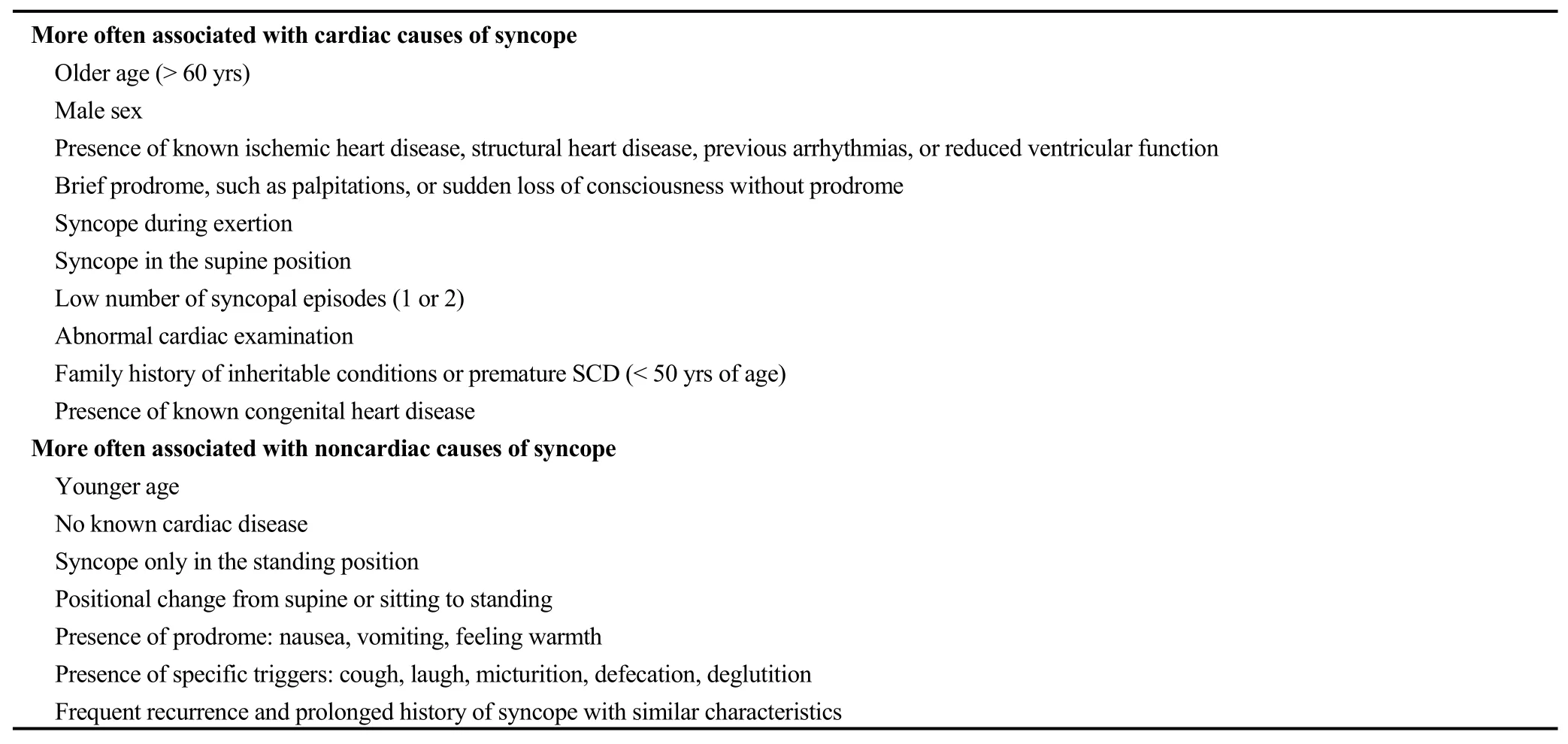
Table 3.Historical characteristics associated with increased probability of cardiac and noncardiac causes of syncope.Adapted from the 2017 ACC/AHA/HRS Guideline for the evaluation and management of patients with syncope.[81]

Table 4.Clinical features suggesting cardiovascular syncope.
Pacemaker placement for syncope in patients with chronic BFB when syncope has not yet been demonstrated to be responsible and other causes have been excluded (specifically ventricular tachycardia) was included in the 2012 ACCF/AHA/HRS Update to the Guidelines for devicebased therapy of cardiac rhythm abnormalities as a Class IIarecommendation (Level of Evidence: B),[75]and was reinforced in the most recent syncope guidelines[81]which appeared to raise the recommendation to a Class I level while citing the PRESS (Prevention of syncope through permanent cardiac pacing in patients with bifascicular block)study.[82]PRESS was a multicenter, prospective, randomized, single-blinded study designed to evaluate the impact of pacemaker placement on symptomatic events in patients with BFB and syncope of undetermined origin.Fifty two of the 101 patients’ pacemakers were programmed DDD with a lower rate of 60 ppm and 49 patients’ devices were programmed with backup pacing (DDI) with a lower rate of 30 ppm.The end point consisted of (1) syncope, (2) symptomatic presyncopal episodes associated with a device intervention (ventricular pacing), and (3) symptomatic episodes associated with intermittent or permanent atrioventricular block (of any degree).
Subjects were eligible to participate if they had ECG documentation of BFB defined as complete LBBB or complete RBBB with left anterior hemiblock or left posterior hemiblock and had experienced at least one episode of syncope in the prior six months.In addition to an ECG all patients underwent Holter monitoring, tilt table test, carotid sinus massage, and an electrophysiological study (EPS), to rule out any possible pre-existing cause of syncope.Patients were excluded if the cause of syncope was identified: (1)vasovagal syncope, (2) carotid sinus syndrome, (3) persistent or permanent AF, (3) sinus node dysfunction or brady-tachy syndrome, (4) second or third degree AVB,diagnosed at ECG or during EPS, (5) spontaneous or inducible sustained ventricular tachycardia, and (6) minimal nocturnal heart rate < 35 beats/min documented on Holter monitoring.Echocardiography was also performed and patients with significant structural heart disease (ejection fraction < 40%) were also excluded.
At 2 years the primary endpoint was observed in 23 patients, with a significantly lower incidence in the DDD 60 group (HR = 0.32; 95% CI: 0.10-0.96;P= 0.042).In addition, a reduction of any symptoms, associated or not with device intervention, was better in the DDD 60 compared with the DDI 30 group (HR = 0.4; 95% CI: 0.25-0.78;P=0.0053).14 patients developed other rhythm diseases and met Class I indication for pacing.The annual incidence of rhythm disease development was 7.4%.
The conclusion of the study was that in patients with BFB and syncope of undetermined origin (after work-up as described), the use of a dual chamber pacemaker programmed to DDD 60 led to a significant reduction of syncope or symptomatic events associated with a cardioinhibitory origin, compared with DDI 30 programming.Symptoms associated with new onset rhythm disease were found in 15% of the population at 2 years.
Despite excluding patients found to have an alternate likely cause of syncope (with EPS, TTT, Holter, and Echo)the patients in the PRESS study had an annual incidence of 7.4% of indications for pacing.This coupled with a clinically significant reduction of symptomatic episode with a dual chamber pacemaker suggests, they argue, that in a patient with a history of sudden syncope, empirically placing a PM could be appropriate.They further note that in this older age group there was a 25% prevalence of atrial fibrillation which they assumed would require the use of medications(AV nodal, or other anti-arrhythmics) that may impair intraventricular conduction in BFB patients possibly providing further indications for pacing.
Case conclusion: in this patient a DDD pacemaker was placed.
9 Summary
Older adults are frequently candidates for and recipients of CIEDs.This article presented several common clinical situations related to CIEDs of older adults, their surrogates and their health care providers may encounter, and reviewed the associated geriatric issues.
 Journal of Geriatric Cardiology2020年11期
Journal of Geriatric Cardiology2020年11期
- Journal of Geriatric Cardiology的其它文章
- Incident frailty and cognitive impairment by heart failure status in older patients with atrial fibrillation: the SAGE-AF study
- Impact of proton pump inhibitors on clinical outcomes in patients after acute myocardial infarction: a propensity score analysis from China Acute Myocardial Infarction (CAMI) registry
- Comparison of low-density lipoprotein cholesterol/high-density lipoprotein cholesterol and total cholesterol/high-density lipoprotein cholesterol for the prediction of thin-cap fibroatheroma determined by intravascular optical coherence tomography
- Plasma levels of Elabela are associated with coronary angiographic severity in patients with acute coronary syndrome
- Gender differences in clinical outcomes of acute myocardial infarction undergoing percutaneous coronary intervention: insights from the KAMIR-NIH Registry
- Ablation strategies for arrhythmogenic right ventricular cardiomyopathy:a systematic review and meta-analysis
