Gastrointestinal and hepatic manifestations of COVID-19 infection: Lessons for practitioners
Syed B Pasha, Ahmed Swi, Ghassan M Hammoud
Syed B Pasha, Department of Internal Medicine, University of Missouri, Columbia, MO 65212, United States
Ahmed Swi, Ghassan M Hammoud, Division of Gastroenterology and Hepatology, University of Missouri School of Medicine, Columbia, MO 65212, United States
Abstract
Key Words: COVID-19; Severe acute respiratory syndrome; Coronavirus; Angiotensin converting enzyme; Gastrointestinal; Liver
INTRODUCTION
The pathogen responsible for a mysterious cause of pneumonia, linked to a seafood market in Wuhan, China, first reported in early December 2019, was later identified as a novel coronavirus by the Chinese Center for Disease Control (CDC)[1,2]. Based on phylogenetics, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses named it the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the disease was later named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO)[3-5]. Within months, the disease spread across the globe like wildfire infecting many and was declared a pandemic by the WHO on March 11, 2020[6].
This is not the first time that a CoV has caused a global health crisis. Just within the last two decades, the SARS- and MERS-CoVs had been responsible for two major viral outbreaks, causing the death of 774 and 838, respectively[7-9]. This number in comparison to the ongoing pandemic appears to be miniscule. Within a period of nine months, the SARS-CoV-2 has managed to infect more than 28 million people and has nearly claimed a million lives[10]. The ongoing pandemic has left the global economies shattered and health care systems exhausted. The current pandemic has reached a level not seen since the Spanish flu of 1918 and will go down the history books as the worst healthcare crisis experienced by mankind in the 21stcentury.
Early in the pandemic, COVID-19 was thought be an exclusively respiratory illness. However, as the number of cases grew and reports of numerous extra pulmonary and atypical presentations emerged, it became apparent that COVID-19, is much more than a simple case of viral pneumonia.
Typical symptoms of COVID-19 include fever, cough, dyspnea, fatigue which are accompanied by radiographic evidence of pneumonia[11]. Additionally, a wide range of extra-pulmonary symptoms, including gastrointestinal (GI) and hepatic dysfunction are frequently reported. The presence of viral RNA in fecal samples from COVID-19 patients also raise concern for fecal-oral transmission in addition to the already established droplet and airborne routes[11].
GI symptoms of COVID-19 may not only precede the typical respiratory manifestations but can sometimes be the only presenting symptom[12]. Interestingly, the first reported case of COVID-19 in the US developed GI symptoms such as vomiting and diarrhea prior to the onset of respiratory symptoms[11]. The predominant focus on the respiratory aspect of the disease can allow these atypical presenters to slip through the cracks. This can potentially result in significant exposure and increased risk of transmission in unsuspecting healthcare workers (HCWs). Wanget al[13]’s study had particularly highlighted this concern. They reported isolated GI symptoms in around 10% of cases and mentioned of one patient who was overlooked and admitted under a surgical specialty. This single patient was inadvertently responsible for infecting more than 10 HCWs[13].
Thus, it is important for clinicians, especially gastroenterologists to be aware of these atypical and often missed presentations of COVID-19. This will not only serve to mitigate the spread but also help reduce clinician’s anxiety during this pandemic.
VIROLOGY OF SARS-COV-2
Before moving on to these extra-pulmonary manifestations of COVID-19, a brief overview of SARS-CoV-2 would better serve the ensuing discussion. Ranging from 60 to 140 nm in diameter, coronaviruses are one of the largest, single-stranded RNA viruses known. Around 39 different types of coronaviruses have been identified since they were first discovered[11,14-16].
Most of them are zoonotic and their natural hosts include mammals and birds[17-20]. They are responsible for a multitude of diseases involving respiratory, enteric and other organ systems in their hosts[21,22]. Majority of coronaviruses that are capable of infecting humans have been thought to originate from an inter-specie crossover event between animals and humans[2].
SARS-CoV-2 is the seventh and the newest member of human coronaviruses, which also include OC43-, 229E-, NL63-, HKU1-, SARS- and MERS -CoVs[23,24]. The former four are endemic and have been responsible for common seasonal upper respiratory tract illnesses, while the latter two and the SARS-CoV-2, cause severe pneumonia outbreaks which can lead to multi organ failure and death[25].
Like most viruses, there are five key steps in the life cycle of coronaviruses. These include attachment, penetration, biosynthesis, maturation and release (Figure 1)[26]. Attachment and penetration are crucial steps which rely on receptor recognition and subsequent downstream processes allowing virus to gain entry into host cell (Figure 2)[27]. The structural transmembrane glycoprotein called Spike (S) is important for this process. Functionally it consists of two subunits, S1 and S2, which after a host protease dependent activation, allows the virus to bind to its specific receptor and the host-virus membrane fusion to take place[26]. In the case of SARS-CoV-2, the target receptor is the Angiotensin- converting enzyme 2 (ACE2) receptor and the host protease required for S glycoprotein activation is identified as a transmembrane serine protease called TMPRSS2[28-33].
The degree to which the virus specific receptor is expressed on a host cell determines viral tropism[34]. The high expression of SARS-CoV-2’s target receptor, the ACE2 in lungs and distal airway makes them an easy target for SARS-CoV-2 and explain the predominant respiratory nature of the disease[35]. However, this ACE2 receptor is not exclusive to pulmonary tissues and is widely expressed across a broad range of human tissues. They are abundant throughout the glandular epithelial cells of the GI tract, cholangiocytes, proximal renal tubules among many others[12,36-38]. A lot of these cell types also have high co-expression of TMPRSS2, which makes them a target for SARS-CoV-2[39,40].
Another important concept is the affinity of a virus for its specific receptor. This not only is an important determinant of its successful replication, but also demonstrates a correlation with transmissibility and disease severity. The evidence for this comes from an observation made during the 2002-03 SARS pandemic. Compared to the earlier 2002 phase, a lower pathogenicity and transmissibility was seen with the 2003 re-emergence phase which also co-related with a lower viral affinity for its receptor[27]. The role of host proteases such as the TMPRSS2 is also important in this regard. Not only can they facilitate viral penetrance but can also enhance it. This can allow the virus to infect cells with relatively low ACE2 expression, which would otherwise not be possible[30]. The binding affinity of SAR-CoV-2 is 10 to 20-fold greater than that of SARS-CoV[41]. This can not only explain its higher transmissibility but also the involvement of multiple organ systems in COVID-19.
PATHOPHYSIOLOGY OF COVID-19
The innate immune system consists of epithelial cells, dendritic cells and macrophages[35]. These cells not only serve as the first line of defense, but by the process of antigen presentation also activate the adaptive arm of immune system. Effectors cells of adaptive immunity are CD4 and the CD8 T-Cells. By activating BCells, the CD4 T-cells also activate humoral immunity, while CD8 T-cells directly kill the virus infected cell[26]. This is body’s natural immune response to any foreign pathogen. However, in a subset of COVID-19 patients, overactivation of this normal immune response can occur. This results in severe manifestations of the disease including acute respiratory distress syndrome and multi-organ failure. Higher levels of various pro-inflammatory cytokine, including IL-6, IL-10, granulocyte-colony stimulating factor, monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha and tumor necrosis factor (TNF) - alpha is often seen in such patients. This phenomenon is also called ‘cytokine storm’[42-44]. The levels of these proinflammatory cytokines correlates with disease severity[26]. Thus an aberrant immune response is the underlying mechanism for severe COVID-19.
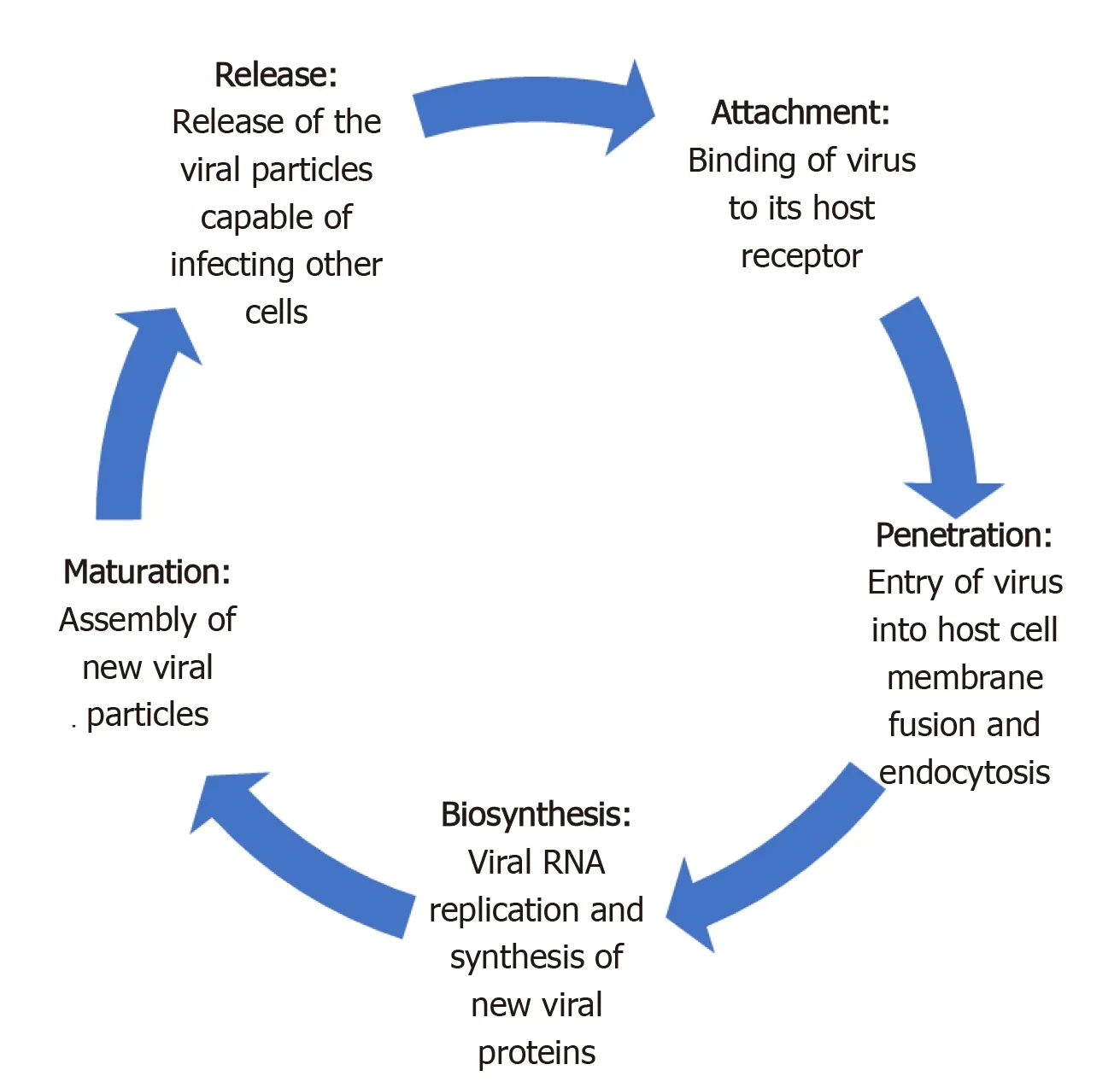
Figure 1 Five steps of severe acute respiratory syndrome coronavirus-2 life cycle.

Figure 2 Entry of viral particle in the host cell. SARS-Cov-2: Severe acute respiratory syndrome coronavirus-2.
MORBIDITY AND MORTALITY OF COVID-19
Case fatality rate (CFR), which is a crude way to assess mortality has demonstrated tremendous regional and demographic variability for COVID-19[45]. While a mild disease phenotype is reported for the majority (80%), epidemiological studies have indicated a higher mortality in the elderly (> 60 years)[46].
Initial studies from China estimated a CFR between 1.36% and 33% and reported majority (80%) of deaths in the elderly[42,47-50]. The official numbers later released by the Chinese government reported it at 3.17%[51]. The US CDC not only reported variable CFRs across different age groups but also found a positive correlation between older age and rates of hospitalization[46]. CFRs ranged from 0 in < 19 years to up to 10%-27% in > 85 years[46]. Similarly, the rates of hospitalization also ranged between 2%-3% in <19 years to greater than 31% in > 85 years[46]. The worldwide CFR for COVID-19 for all age groups currently stands at approximately 3% but can range from as high as 12% in Italy to < 0.1% in Singapore[45].
Some authors have attributed the observed regional differences in mortality to their respective demographic characteristics. For example, the abnormally high CFR reported from Italy was thought to be related to its relatively older population[26]. While the morbidity and mortality of COVID-19 is almost certainly higher in older age groups, it is important to be aware that severe disease can occur in any age group[46].
Compared to its predecessors, the SARS-CoV (approximately 10%) and MERS-CoV (34%-37%), the mortality rate for COVID-19 appears to be substantially low[45,52,53]. But longer incubation period and sustained viral shedding during pre-symptomatic phase have enabled a much more efficient spread for SARS-CoV-2[53,54]. The estimated reproduction number for SARS-CoV-2 ranges from 2.24 to 3.58, which is higher than that of its predecessor[55,56]. The mean incubation period for SARS-CoV-2 is 5.2 days (95%CI: 4.1-7.0)[57], but the incubation period can generally range from 1 to 14 d[16,56,58].
TRANSMISSION
A direct person-to-person transmission by respiratory droplets is the predominant route of transmission for COVID-19[59]. This can occur during coughing, sneezing, or normal breathing. Droplets generatedviathese routes can travel up to six feet and can remain suspended in air for up to three hours[60]. Aerosol transmission during aerosol generating procedures, such as endotracheal intubation and nebulization can also occur[61]. Indirect transmissionviafomites is another potential mode of transmission. Viral particles can remain viable on different surfaces (plastic, steeletc.) for up to a few days[62]. Viral particles have also been detected in samples from toilet bowl and sink used by COVID-19 patients[63]. A cluster of COVID-19 cases associated with a shopping mall in Wenzhou, China was thought to be linked to a common restroom[64].
During the SARS outbreak of 2003, the SARS-CoV particles were detected in the GI biopsy and stool specimens, indicating potential for fecal-oral transmission and GI troposim[65]. Based on shared homology, the same was speculated for SARS-CoV-2, which was afterwards confirmed by the presence of fecal viral RNA and nucleocapsid proteins in the cytoplasm of GI epithelial cells on autopsy[66,67]. Around 36% to 53% of stool samples can test positive for SARS-CoV-2 viral RNA[38]. The stool sample from the first US case of COVID-19 also tested positive for the viral RNA[68].
Patients with GI symptoms can have a 70% higher chance of testing positive on the nasopharyngeal swabs, suggesting a higher overall viral burden[69]. Stool samples can turn positive around 2-5 d after a positive respiratory specimen and can remain positive despite a negative respiratory specimen in up to 23% to 82% of cases[38]. The reason for this can be a longer survival or persistent viral replication in the GI environment[38,68].
A study from China reported positive stool samples in more than half (53%) of the cases, which can remain positive up to 12 d. Around 23% of these samples can continue to test positive despite a negative respiratory test[67]. Another study reported 53% of stool samples testing positive on RT-PCR. But reported lower fecal viral load compared to respiratory samples[70]. Zhanget al[71]also demonstrated presence of viral particles in oral and anal swabs. While it can be argued that positive RT-PCR does not necessarily mean a live virus capable of causing infections, Wanget al[72]actually found live SARS-CoV-2 virus in stool samples.
In summary, droplet and aerosol routes are still responsible for bulk of transmission but fecal-oral transmission can be another possibility. Gastroenterologists who can encounter patients with GI symptoms in their practice should be aware of the risk of such transmissions and adopt necessary precautions.
GI SYMPTOMS OF COVID-19
GI symptoms such as diarrhea, anorexia, nausea, vomiting and abdominal pain are commonly reported in COVID-19[25,73]. The retrospective study by Guanet al[48]which included 1099 COVID-19 cases from 552 centers across China, reported nausea, vomiting in 5% and diarrhea in 3.8% cases. Another study reported at least one GI symptom in 32.5% of cases. These included anorexia (56.7%), diarrhea (37.8%), nausea (16.5%), abdominal pain (10.4%) and vomiting (7.9%)[39]. A cross-sectional study found GI symptoms in more than half (50.5%) of the cases on initial presentation. The severity of these symptoms correlated with the overall disease severity and significant liver function abnormalities[74]. The retrospective study by Luoet al[75]which included 1141 cases found at least one GI symptom in 16% of cases on initial presentation. These included anorexia (98%), nausea, vomiting (66%), diarrhea (37%) and abdominal pain (25%). Jinet al[76]’s study which was the first COVID-19 study outside Wuhan reported the prevalence of GI symptoms at 11.4%. Diarrhea, at 8.14% was the most common of these symptoms[76].
A single-center case series from Flushing, New York, including 892 cases, reported GI symptoms in a quarter. Diarrhea (19.8%) was the most common, followed by nausea (16.6%), anorexia (11.8%), vomiting (10.2%) and abdominal pain (7.8%)[77]. Another retrospective study from New York which included 1059 patients, reported diarrhea in 22% of cases. Nausea, vomiting and abdominal pain were reported in 16%, 9% and 7%, respectively[78]. A multicenter, retrospective cohort study, from Massachusetts reported at least one GI symptom in up to two-thirds (61.3%) of cases. These symptoms included anorexia (34.8%), diarrhea (33.7%) and nausea (26.4%). Around 20% experienced isolated GI symptoms, while 14% developed these symptoms prior to the onset of other disease manifestations[79]. An outpatient questionnaire based study by Sierpińskiet al[80]which included 1942 cases with mild to moderate disease reported at least one GI symptom in 53.6%. These included Anorexia (47%) and diarrhea (24%).
A meta-analysis of 60 studies including 4243 cases from 6 countries, found pooled prevalence of GI symptoms at 17.6%. Anorexia (26.8%) was most common, followed by diarrhea (12.5%), nausea/vomiting (10.2%) and abdominal pain/discomfort (9.2%)[3].
GI symptoms are also seen in pediatric cases. A retrospective study including 20 pediatric cases from Wuhan, reported GI symptoms, such as diarrhea and vomiting in 15% and 10% of cases, respectively[81].
In addition to GI symptoms, gross and microscopic changes of the GI tract are also seen on autopsy of COVID-19 patients. Segmental dilation and stenosis of small intestine, along with mucosal shedding and necrosis is reported on autopsy, while inflammatory infiltrates and interstitial edema, consistent with a colitis/enteritis like picture is seen on histology[38,67]. Macroscopic abnormalities of GI tract can also be evident on imaging. Luiet al[82]reported bowel wall thickening involving any portion of the bowel, as well as ileus, ascites, pneumatosis intestinalis and pneumoperitoneum on abdominal imaging.
We have summarized the most frequently reported GI symptoms across different studies in Tables 1-4.
PROPOSED MECHANISM FOR GI SYMPTOMS
There are several proposed mechanisms for the GI manifestations of COVID-19 (Figure 3). Subjective and nonspecific symptoms such as anorexia and nausea are difficult to accurately characterize and can be over-reported. Objective symptoms, such as diarrhea and vomiting are speculated to be either a result of direct viral attack or due to an adverse drug reaction. An overwhelming systemic inflammatory response commonly seen in severe cases can also produce these symptoms.
The structural similarities and inter-specie immunologic cross-reactivity between animal and human coronaviruses have indicated a zoonotic origin for most human coronaviruses[54]. Some of these inherently have the ability to cause gastroenteritis in their animal hosts[18,20,54]. Some of the endemic human coronaviruses, such as OC43 and 229E have antigenic similarities to these animal coronaviruses[54,138,141]. It is possible that the recombination events resulting in these inter-specie barrier crossover can allow some of them to retain their enteric infectivity[54]. This can be a possible scenario in SARS-CoV-2, whichviathe ACE2 receptor can infect and exert a direct cytopathic effect on enterocytes. Fecal leukocytes and occult blood which can be seen in other cases of viral diarrhea is reported in 1.7% and 5.2% of fecal samples, respectively[38]. Increased inflammatory infiltrates and interstitial edema of the GI tract indicating active inflammation is also observed on histology[67]. The presence of an infectious SARS-CoV-2 virion in stool, and persistent fecal viral RNA shedding which in some cases, can outlast respiratory shedding points towards viral replication in GI tract[67,72]. Together, these findings can support a direct viral attack on enterocytes resulting incellular dysfunction and increased permeability which in turn can lead to malabsorption, culminating in diarrhea[73]. It is also a possibility that viral involvement of upper GI glandular epithelium produces nausea and vomiting in a similar way.

Table 1 Prevalence of common gastrointestinal symptoms reported by coronavirus disease 2019 patients in studies from Wuhan, China, n (%)
Adverse drug reactions can be another possible explanation. Some of the antivirals, antibiotics and immunomodulators, commonly prescribed to COVID-19 patients are known to cause diarrhea. Compared to earlier studies, a higher prevalence of GI symptoms was reported in later studies. This temporal variation also happens to coincide with a more widespread use of these medications[38]. Hypoxia induced necrosis and cellular injury resulting in enterocyte dysfunction is another possibility[38].
HEPATOBILIARY MANIFESTATIONS OF COVID-19
Hepatic dysfunction, as evidenced by abnormal laboratory tests is frequently seen in COVID-19. Histological changes including lobular and portal inflammation have also been reported[142]. Most cases of COVID-19 associated hepatic dysfunction are mild and transient, but in a small subset of patients a higher degree of dysfunction and hepatic enzyme derangements can be seen[143].
One of the earliest studies from Wuhan, reported aspartate transaminase (AST) elevation in 37% of cases[42]. This number was even higher in critically ill [62%vs25% intensive care unit (ICU)vsnon-ICU cases, respectively][42]. Another study from Wuhan, reported liver function test (LFT) abnormalities in 43% of cases. AST elevations were more common than alanine aminotransferase (ALT) (35%vs28%), while total bilirubin was elevated in only 18%[1]. Though most cases had only mild to moderate degree of LFT derangements, a case of severe liver injury with profound ALT and AST elevation was also reported[1]. Guanet al[48]’s analysis of data reported tothe Chinese National Health Commission found AST, ALT and total bilirubin elevation in 22.2%, 21.3% and 10.5% of cases, respectively. LFT derangements were more commonly seen in severe cases (AST 39.4%vs18.2%: ALT 28.1%vs19.8% and T. Bili 13.3%vs9.9%; Severevsnon-severe, respectively)[48]. Data from 56 COVID-19 patients admitted to the People’s Liberation Army Hospital in Beijing reported LFT abnormalities in 28.6% of cases[144].
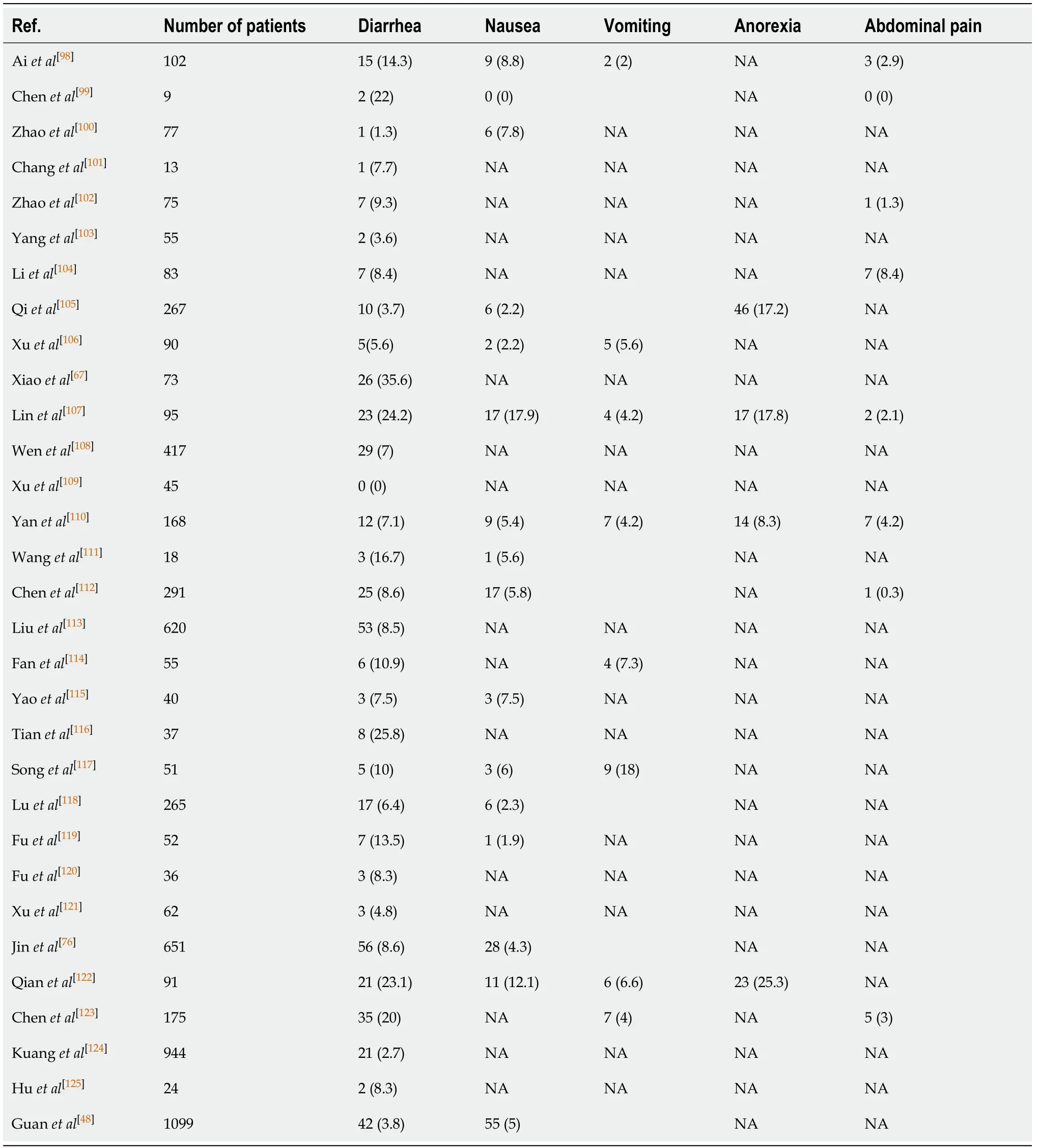
Table 2 Prevalence of common gastrointestinal symptoms reported by coronavirus disease 2019 patients in Chinese studies outside Wuhan, n (%)
A study from New York, reported mild (2-5 × ULN) AST and ALT elevation in 13.8% and 11.5% cases on admission. Severe (> 5 × ULN) AST and ALT elevations were seen in 2.8% and 1.9%. 4.3% had abnormal bilirubin (> 1.2 mg/dL), 11.9% had abnormal alkaline phosphatase and 24% had an INR > 1.13[77]. LFT abnormalities, especially AST elevations were associated with severe disease and higher mortality[77].The strength of this study was that it looked at the prevalence of LFT derangements on admission. This can help mitigate the effects of confounders such as medications and other factors.

Table 3 Prevalence of common gastrointestinal symptoms reported by coronavirus disease 2019 patients in studies from United States, n (%)
Like adult cases, pediatric cases have also demonstrated hepatic dysfunction. A study from Wuhan reported ALT elevation in a quarter of pediatric cases[81].
We have summarized studies which have reported hepatic dysfunction in Table 5.
PROPOSED MECHANISM FOR HEPATIC DYSFUNCTION IN COVID-19
There are many possible explanations for COVID-19 associated hepatic dysfunction. SARS-CoV-2 can directly infect hepatocytes and cholangiocytes, which also harbor the virus specific ACE2 receptors (Figure 4)[145]. Expression profiling have indicated a 20-fold higher expression of ACE2 in cholangiocytes, compared to hepatocytes[145]. Inaddition to maintaining normal function of biliary system, cholangiocytes also play an important role in liver regeneration and immune response. Direct involvement of these cells can cause liver injury as manifested by impaired hepatic transaminases[145]. Liver biopsy on autopsy of COVID-19 patients show micro vesicular steatosis as well as lobular and portal inflammation[142]. Although non-specific, these findings can represent direct hepatic injury from SARS-CoV-2 infection. However, no viral inclusions have been detected to date in hepatic or biliary tissue and the pattern of LFT derangement seen is mostly hepatocellular rather than cholestatic, which is expected in biliary and cholangiocyte involvement[145]. This indicates that the liver involvement in COVID-19 is not a likely consequence of direct liver injury.
Indirect liver injury from overwhelming release of inflammatory cytokines (cytokine storm) or ischemia is a more likely explanation[144]. AST elevation is more commonly seen than ALT which is in contrast to the pattern of liver injury typically seen in viral hepatitis[147]. Preferential AST elevations are more commonly seen with toxic and ischemic liver injury which involves zone 3 hepatocytes[147]. A higher prevalence of LFT abnormalities in severe cases of COVID-19 also supports this finding[77]. Overwhelming release of inflammatory cytokines such TNF-α, interferon-λ and various interleukins such IL-2, IL-6, IL-7, IL-8 can lead to global tissue damage and multiorgan dysfunction including the liver[145].
Liver injury seen in COVID-19 can also be drug induced. A wide range of medications can cause hepatotoxicity. These can range from commonly taken OTC acetaminophen to antiviral drugs currently under investigation for COVID-19. A summary of these drugs will be mentioned in Table 6.
Due to high worldwide prevalence of chronic liver disease (CLD), it is possible that hepatic dysfunction seen with COVID-19 is a consequence of pre-existing liver disease exacerbation[143]. Patients with CLD can have interruptions in their ongoing treatment resulting in exacerbations. Corticosteroids and immunomodulators which are increasingly being used to treat COVID-19 can also exacerbate underlying viral hepatitis. Singhet al[148]did a comparative study of patients with and without preexisting CLD. Their study, which included 2780 patients, reported similar elevations in ALT, AST, GGT, Alkaline Phosphatase and total bilirubin in both groups, suggesting COVID-19 associated hepatic injury as a more likely reason for LFT derangements[148].
CHRONIC LIVER DISEASE AND COVID-19
Data on the effects of COVID-19 on pre-existing liver disease, at present is limited. Most studies have not specified the prevalence of chronic liver disease in their COVID-19 cases. The few studies that have, report it between 1.25% to 11%[145]. 2.1% of 1099 COVID-19 cases included in Guanet al[48]’s study had pre-existing chronic hepatitis B which was associated with worse outcomes. In Singhet al[148]’s study, 9% of COVID-19 patients also had pre-existing liver disease. At 42%, nonalcoholic steatohepatitis was the most common liver condition, while cirrhosis was present in 1.8% of cases. The authors also compared laboratory and clinical characteristics of patients with preexisting liver disease to those without. The results demonstrated a significantly higher mortality rate with chronic liver disease (RR 3.0, 95%CI: 1.5-6.0;P= 0.001) and cirrhosis (RR 4.6, 95%CI: 2.6-8.3,P< 0.001), irrespective of the cause[148]. However, liver function derangements from baseline were seen in both groups and the overall prevalence of hepatic dysfunction during the disease course did not appear to be different either[148].
Another study found a significantly higher risk of disease progression, LFT derangement and prolonger viral shedding in patients with co-existing nonalcoholic fatty liver disease[149]. Impaired hepatic immune system in these patients was speculated to be a reason[149]. A case series from Northern Italy did not find any COVID-19 related complications in the majority of Autoimmune liver disease patients[150]. Another Italian study did not find any difference in disease course between patients with autoimmune hepatitis (AIH) on immunosuppressive therapy and who are those not[151].
To address the current scarcity of data for CLD patients, two international registries have been created. These are the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion (SECURE-Cirrhosis) and European association for the study of liver (EASL) supported COVID-HEP registries. According to the combined weekly update (released on August 25th, 2020) a 31% mortality rate is seen in CLD patients with cirrhosis compared to 7% in those without. In liver transplant recipients, the mortality rate is 18%[152].
社会经济的快速发展,特别是“健康中国”的有效推进,体育作为特殊的身体练习项目之一,既能满足人们的健康需要,也能提升人们的生活品质,体育健身健康逐渐成为人们生活的必需.习近平在党的十九大报告中指出:中国特色社会主义进入新时代,我国社会主要矛盾已经转化为人民日益增长的美好生活需要和不平衡不充分的发展之间的矛盾 [1].健康与养生已成为人们对美好生活的基本需求之一,满足人民对体育健康和养生的需求已成为一种社会责任.从民族传统武术文化传承的视角,探索和构建民族传统养生与健康高素质人才的培养模式,是一个全新且具挑战性的尝试.
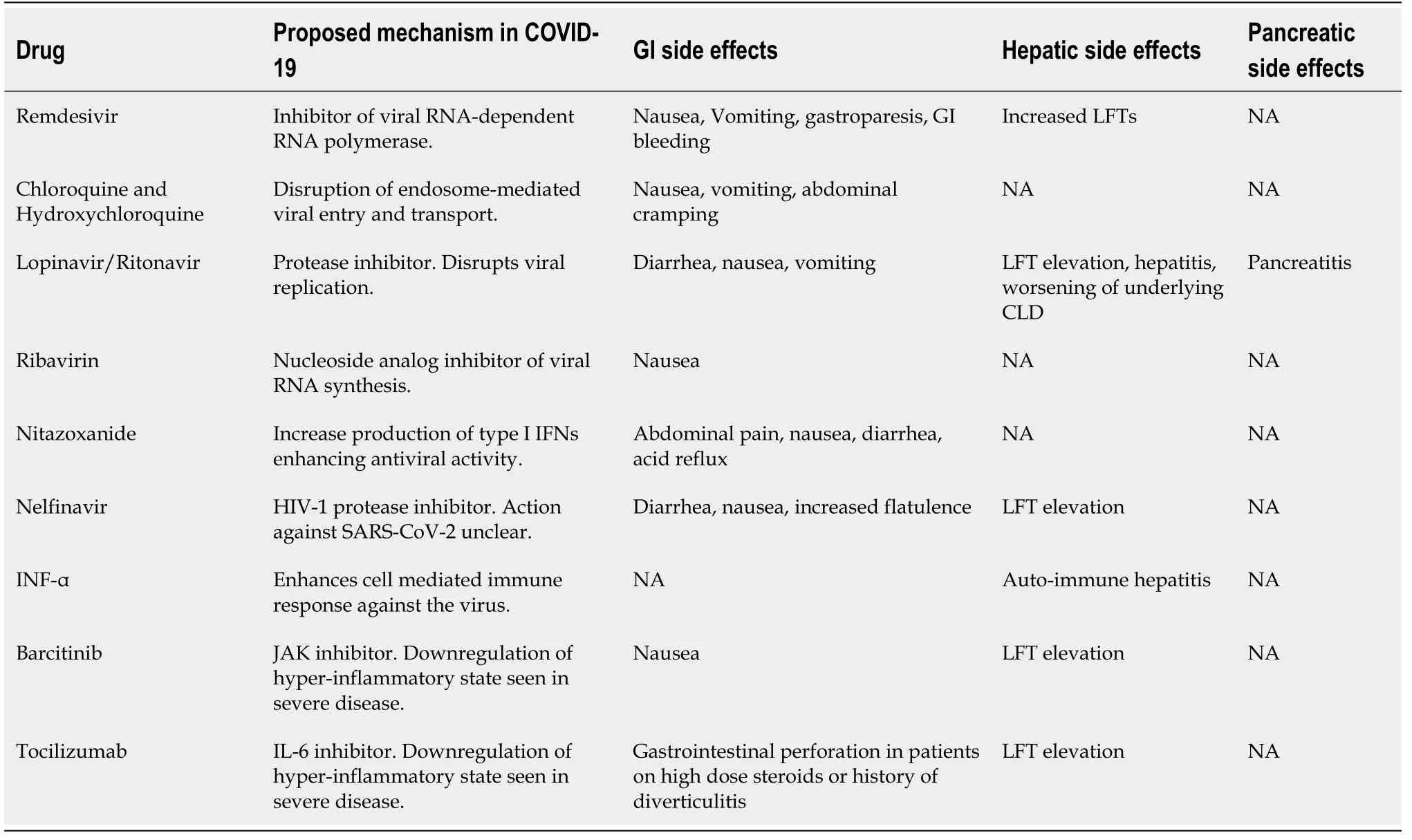
Table 6 Commonly administered pharmacotherapy for coronavirus disease 2019 and their gastrointestinal adverse effects[162]
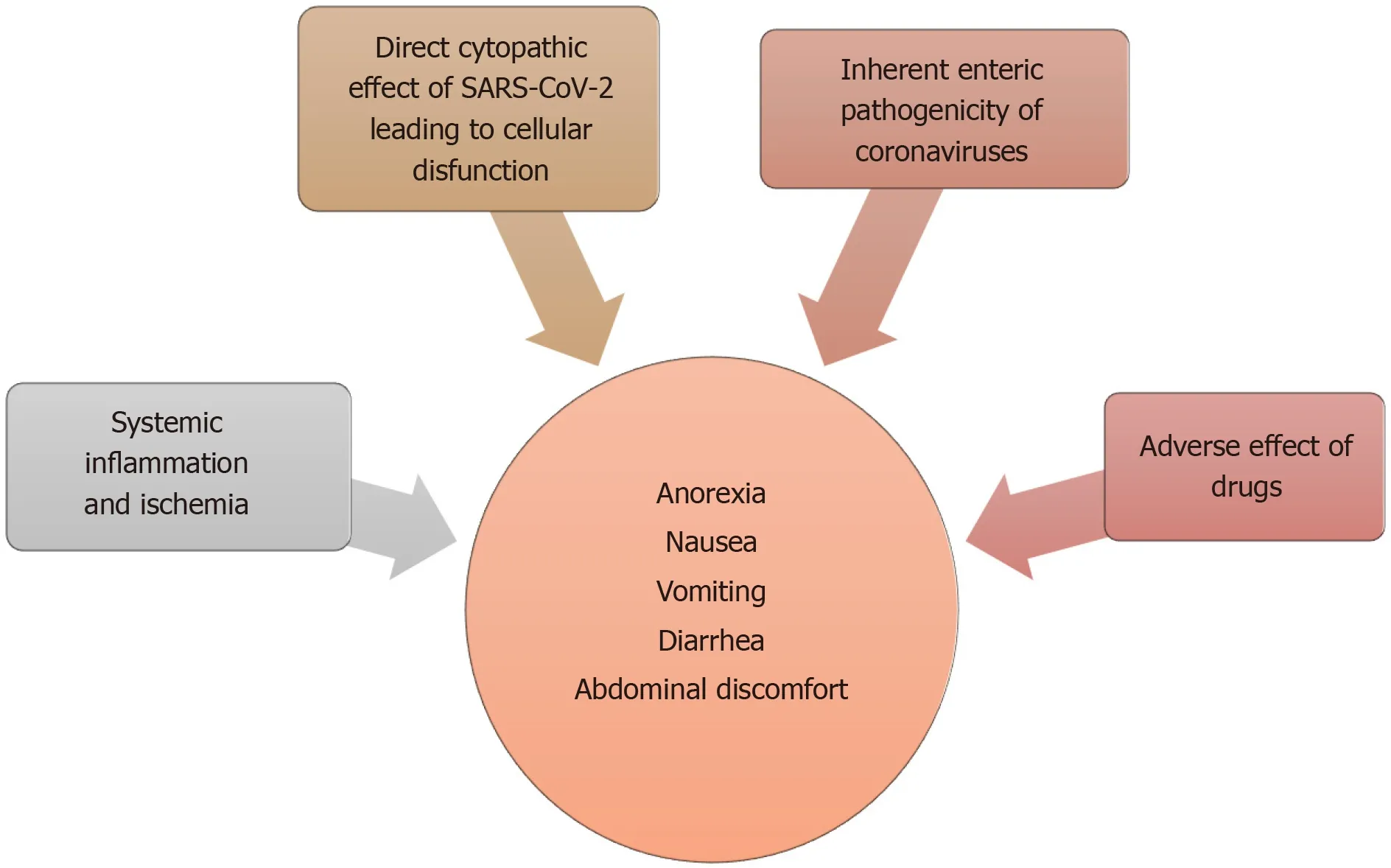
Figure 3 Summary of proposed mechanisms for gastrointestinal manifestations of coronavirus disease 2019.
To summarize, an overall higher COVID-19 related mortality is seen with preexisting liver diseases, especially cirrhosis. However, autoimmune hepatitis appears to be an exception to this rule.
PANCREATIC MANIFESTATION OF COVID-19
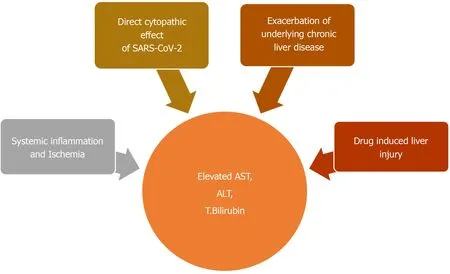
Figure 4 Summary of proposed mechanisms for Hepatobiliary dysfunction seen in coronavirus disease 2019.
Although not as common as the other extra-pulmonary organs, pancreatic involvement can also be seen in COVID-19. A few cases of possible COVID-19 induced pancreatitis have been reported[153,154]. A Chinese and an Italian study also found some form of pancreatic injury in 17% and 8.5% of cases, respectively[155,156].
PROPOSED MECHANISM FOR PANCREATIC INJURY
ACE2 receptor is also found in pancreas, especially its islet cells[157]. Pancreatic islet cell injury and ensuing, acute diabetes was previously seen with SARS infection[157]. The SARS-CoV virus was detected in pancreatic cells, indicating direct viral attack[158]. Like its predecessor, SARS-CoV-2 can also involve the pancreas[159]. The two-thirds of cases reported by Wanget al[155]developed hyperglycemia, which can be a consequence of endocrine pancreas involvement. Pancreatic injury can also be a part of the multi organ failure seen in severe COVID-19[155,156,160]. An English study from Liverpool reported 5 COVID-19 related cases of metabolic-pancreatitis. All these patients were overweight or obese and had hypertriglyceridemia and glucose intolerance. Transient metabolic distress induced by COVID-19 was hypothesized to cause pancreatitis in these cases[161]. Dehydration and drug induced pancreatic injury are other possibilities (Figure 5)[156].
COVID-19 PHARMACOTHERAPY AS A POTENTIAL DRIVER OF GI, HEPATIC AND PANCREATIC MANIFESTATIONS
Many of the commonly encountered extra-pulmonary manifestations of COVID-19 can be an adverse reaction to frequently prescribed drugs. We will summarize some of the well-known GI and hepatic side effects of these commonly administered drugs (Table 6)[162].
MANAGEMENT OF GI SYMPTOMS
GI symptoms associated with COVID-19 are mostly mild to moderate in severity. Diarrhea seen in these patients is typically low volume and does not cause severe dehydration[74]. Management of these patients should involve adequate hydration and correction of electrolyte imbalances. Anti-emetics can also be administered for nausea and vomiting, provided there are no contraindications. Anorexia is a nonspecific symptom and is likely due to severe inflammation. Enteral nutrition should be encouraged whenever possible. A dietician consultation can also be beneficial for these patients.
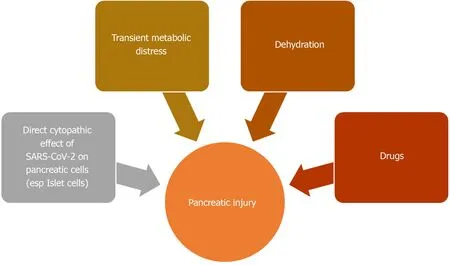
Figure 5 Summary of proposed mechanisms for pancreatic injury seen in coronavirus disease 2019.
MANAGEMENT OF LIVER INJURY IN COVID-19
COVID-19 associated liver injury is mostly transient and is likely indirect, which could be related to hypoxia, systemic inflammatory response, and drugs. Therefore, management of liver injury in COVID-19 should revolve around correction or removal of underlying etiology. Correction of hypoxiaviaoxygen supplementation or mechanical ventilation, renal replacement therapy for cytokine storm and restoration of effective intravascular volume in cases of shock can often correct liver injury[145]. Prompt identification and discontinuation or dose reduction of drugs responsible for drug induced liver injury is also important. Hepato-protective anti-inflammatory drugs such as L-ornithine-L-aspartate can also be used as adjuvant treatment in severe cases[145].
MANAGEMENT OF CHRONIC LIVER DISEASE DURING PANDEMIC
Significantly higher COVID-19 associated mortality is seen in cirrhotics[163]. Data from international registries have also pointed towards a higher mortality in these patients[152]. Therefore cirrhotics and CLD patients warrant special attention and for this reason three liver associations have provided recommendations for management of CLD during the pandemic (Table 7)[164].
The EASL[165], the American Association for the Study of Liver Diseases (AASLD)[166]and three European referral centers have also made recommendation for the management of AIH which are summarized in Table 8[167].
At present, the data on the safety of corticosteroids or other immunosuppressive medications is limited. Given the incomplete understanding of the effect of immunemodulating therapy on COVID-19, as well as the effect of COVID19 on immunosuppressed patients, the AASLD and EASL have provided guidance on the use of these drugs which is summarized in Table 9[168].
MANAGEMENT OF CHRONIC GI DISORDERS DURING PANDEMIC
Management of chronic GI disorders such as inflammatory bowel diseases (IBD) which require close follow ups and often require immunosuppressive medications is also challenging. Despite its limitations, virtual platforms, such as telehealth offer a safe and convenient alternative to in-person visits and can be an effective platform for triaging. This can minimize potential exposure to COVID-19 and can determine need for in-person visits and urgent interventions. In situations where no alternative to in-person evaluation exists, precautions including social distancing, proper hand hygiene, wearing of facemasks at all times and symptoms and exposure screening prior to entry in the healthcare facility is recommended[169].
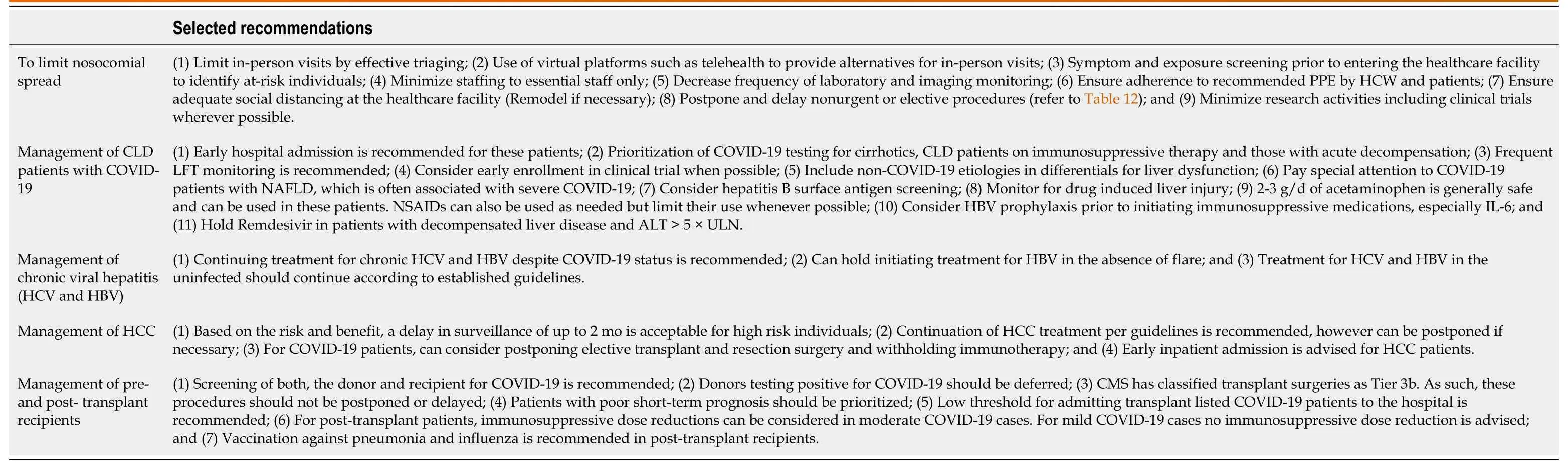
Table 7 Selected American Association for the Study of Liver Diseases, Asian Pacific Association for the Study of the Liver, and European Association for the Study of the Liver recommendations for liver disease management during the coronavirus disease 2019 pandemic[164]
Another problem gastroenterologists face during the pandemic is to determine whether to continue chronic immunosuppressive treatments that are often required for these conditions. Many of these medications carry an increased risk of infections which range between 0.5% to 30%[169]. Societies like American Gastroenterological Association (AGA), European Crohn’s and Colitis Organization and International Organization for the Study of IBD have provided recommendations regarding their use during the pandemic which will be summarized in Table 10[169].

Table 8 Expert guidance for management of autoimmune liver disease during coronavirus disease 2019 pandemic[168]

Table 9 Expert guidance on immunosuppression for liver disease in the setting of coronavirus disease 2019[168]
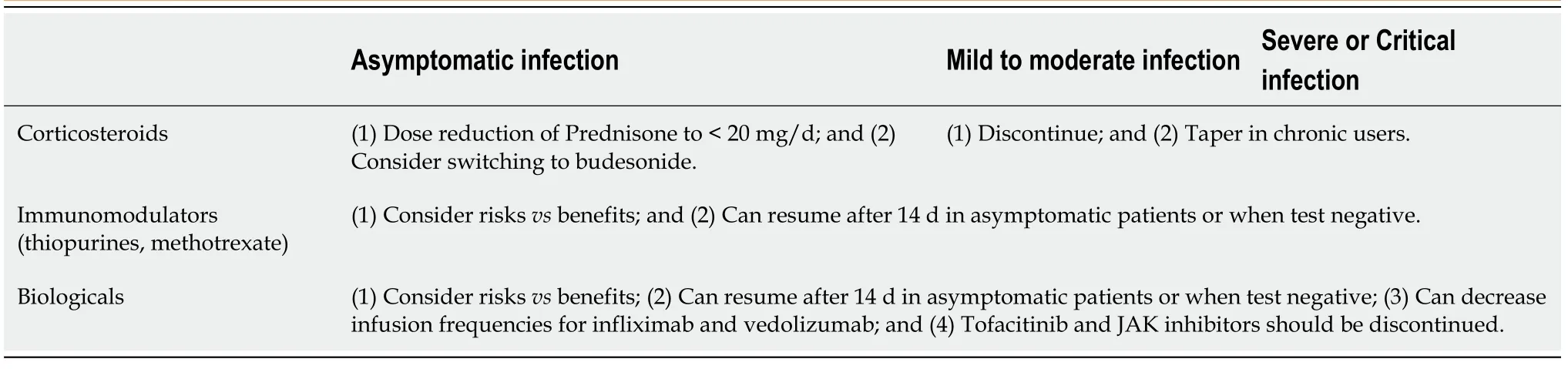
Table 10 Recommendations on use of immunosuppressive therapies in inflammatory bowel diseases[169]
GENERAL RECOMMENDATION FOR GASTROENTEROLOGISTS AND HEPATOLOGISTS
The ongoing pandemic has put an immense strain on healthcare systems and created and overwhelming demand for critical supplies. Judicious use of available resources and ensuring safety of HCWs who are constantly exposed, with consequently higher rates of infections is essential for optimum functioning of healthcare machinery during the pandemic[170,171]. The US CDC has issued interim guidelines for infection prevention and control which is summarized in Table 11[172].
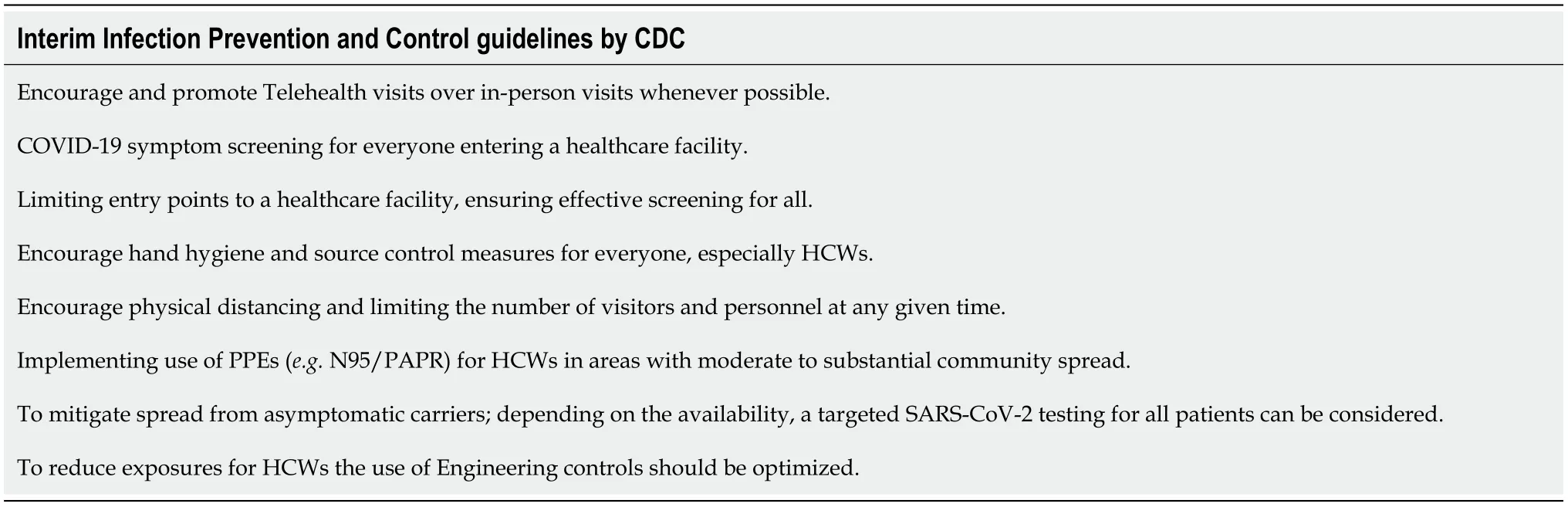
Table 11 Interim infection prevention and control guidelines by Centers for Disease Control and Prevention[172]
RECOMMENDATIONS FOR ENDOSCOPIES
To mitigate the spread and to conserve limited resources, Joint Gastroenterology Societies, the Centers for Medicare and Medicaid Services, the US surgeon General and American College of Surgeons have all proposed delaying non-urgent and elective procedures[173-176]. AGA recommends a review of any planned procedures by trained medical personnel to determine urgency of a procedure[171]. AGA and AASLD have also provided an outline for triaging[166,171]. Though this approach is quite reasonable; the ensuing diagnostic and therapeutic delays is another challenge that needs to be addressed. Therefore, gradual, and stepwise resumption of procedures, while, of course ensuring patient and provider safety is the need of the day. Canadian Association of Gastroenterology has recently laid a framework for resumption of nonurgent endoscopic procedures[177]. Stepwise increment and expansion of endoscopic services with a minimum of 2 wk’ time in between is recommended. Factors related to local risk of transmission, infection dynamics and success of mitigation measures in the community must considered before this[177]. Adequate availability of healthcare resources including personal protective equipment (PPE), staff and equipment, as well as the ability to implement recommended social distancing must be taken into consideration as well[177]. Lastly, triaging which is a powerful tool for efficient healthcare delivery should be vigilantly implemented at every level. Evidence based approach to triaging endoscopic procedures are summarized in Table 12.
When a procedure needs to be performed, proper personal protection equipment must be worn by the providers. Recommendations for PPEs during GI procedures are summarized in Table 13[171].
CONCLUSION
The disease caused by the novel coronavirus is more of a systemic condition than an isolated respiratory illness. GI and hepatic manifestations of COVID-19 are frequently reported and anorexia, nausea, vomiting, diarrhea are few of the commonly seen GI symptoms in COVID-19. Hepatic function derangement is also common, mostly mild, and transient and pancreatic injury is also reported. These symptoms can be a result of a direct infection or an indirect consequence of associated systemic inflammation or hypoxia from respiratory failure. Side effects of drugs currently being used to treat COVID-19 could also be a possible explanation for these manifestations. Fecal oral transmission in the setting of these symptoms poses a considerable risk of exposure for gastroenterologists who deal with these patients. The limited availability of PPE only makes matters worse. Effective and safe delivery of care while conserving these critical supplies is of utmost importance. For this purpose, various gastroenterology societies have also put forward their recommendations. With no end to the pandemic in sight, it is important for HCWs and gastroenterologists to be aware of and adherent to these guidelines.
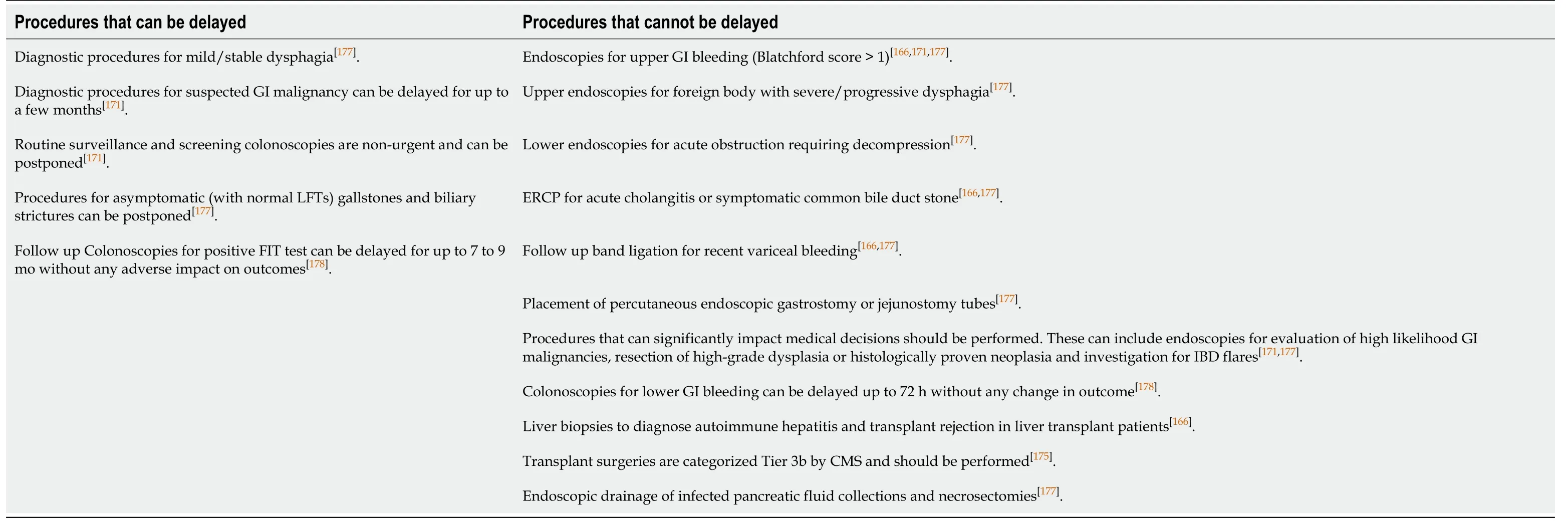
Table 12 Procedures that can be delayed during the ongoing pandemic

Table 13 Recommended personal protective equipment during gastrointestinal procedures[171]
 World Journal of Meta-Analysis2020年5期
World Journal of Meta-Analysis2020年5期
- World Journal of Meta-Analysis的其它文章
- Effects of antithrombotic agents on post-operative bleeding after endoscopic resection of gastrointestinal neoplasms and polyps: A systematic review and meta-analysis
- Comparing the incidence of major cardiovascular events and severe microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta-analysis
- Implications of COVID-19 for inflammatory bowel disease: Opportunities and challenges amidst the pandemic
- Current trend in the diagnosis and management of malignant pheochromocytoma: Clinical and prognostic factors
