A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function
Yi-wen Fan, Shao-wei Jiang, Jia-meng Chen, Hui-qi Wang, Dan Liu, Shu-ming Pan, Cheng-jin Gao
Department of Emergency Medicine, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200092, China
KEY WORDS: Sepsis; Infection source; Acute kidney injury; Lung injury; Renal function
INTRODUCTION
Sepsis has been described as the most common disease among critically ill patients.[1]Furthermore,severe sepsis is recognized as one of the leading causes of acute kidney injury (AKI);[2]this is an important clinical problem as AKI significantly increases the risk of mortality among patients with sepsis.[3]While research has attempted to determine the severity of this condition in order to treat patients appropriately, little is known about how the anatomical site of infection related with clinical outcomes in sepsis-associated (SA)-AKI patients.[4,5]
The Sepsis 3.0 criteria define sepsis as a lifethreatening organ dysfunction caused by dysregulation in a host's response to infection.[6]In other words, the primary driver of mortality in sepsis is the systemic inf lammatory response, and this response is triggered by uncontrolled infection arising from a specific anatomical source, which may influence the progression and clinical outcome of sepsis.[7]
Previous studies[8-10]have investigated the independent role of the anatomical site of infection with regard to mortality caused by sepsis, but with varying findings.SA-AKI patients have a high risk of mortality; previous studies[5,7,11,12]have shown that the most common source of infection is the lung, followed by the gastrointestinal and urinary tracts. Studies[13,14]also indicate that severe lung infection can induce acute lung injury (ALI) or acute respiratory distress syndrome (ARDS); these conditions are both directly associated with AKI.
As previous studies[15,16]were unable to reach a def initive conclusion, we hypothesized that the variations in the outcome in SA-AKI patients with sepsis can be explained, at least partly, by the anatomical source of infection. The study aims to explore the relationship between the anatomical source of infection and the outcome of SA-AKI patients.
METHODS
Study design, setting, and patient population
This was a retrospective study carried out in a single center and was supported by Xinhua Hospital, Shanghai Jiaotong University and the study complies with the Declaration of Helsinki. The requirement for ethical approval was waived due to the retrospective nature of this study.
Between January 2013 and January 2018, we identified 113 patients who were admitted to our Emergency Center with the diagnosis of sepsis and SAAKI. We reviewed epidemiological literature related to infections among patients receiving intensive care and identified the four most common sites of infection,[17-20]accounting for 90% of infections in patients with sepsis.[17]Evidence showed that the lungs were the primary source of such infections. Consequently, we divided the 113 patients into two groups: those with pulmonary infections and those without.
Our inclusion criteria were as follows: age >18 years; patients presenting in the first 48 hours of septic shock and developing AKI (Kidney Disease Improving Global Outcomes [KDIGO] stage 2 or 3, as defined by KDIGO criteria[21]); patients with a definite source of infection, as judged by medical records; and provision of informed consent (either by the patient or a person with appropriate responsibility for the patient). Patients were excluded if they had a diagnosis of AKI that was not associated with sepsis, if they had received treatment for AKI prior to admission, or if they had pre-existing diseases that required maintenance treatments, including renal replacement treatment (RRT) or previous RRT.
Data collection
All data were collated by trained research nurses and clinical doctors using a standardized and pilottested data form. Data were collated from admission until either hospital discharge or death. For those who were discharged, we collated data relating to the clinical outcome 90 days after admission. Our dataset was structured into three main components: admission,hospitalization, and outcome.
A range of data was collated from the time of admission, including the sources of infection, sex, age,urea creatinine, Acute Physiology and Chronic Health Evaluation (APACHE) II, Sepsis-Related Organ Failure Assessment (SOFA) score, laboratory blood analyses over the first 24 hours, and the presence of basic diseases, including atrial fibrillation (AF), deep-vein thrombosis (DVT), chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), and chronic kidney disease (CKD). With regard to the hospitalization period, we noted whether there was any evidence of shock and whether RRT was used, and we recorded PaO2and PaO2/FiO2data. Finally, we collected a range of outcome data. Our primary endpoint was death within 90 days of admission; the number of patients who died during hospitalization or after discharge was recorded. In addition, data related to renal recovery in 90 days after admission (as the secondary endpoint), as evidenced by the serum levels of creatinine and urea at discharge or at the last assessment, APACHE II scores, and SOFA scores, were collected. All data retrieved from medical records were checked by two or more researchers to ensure quality and consistency.
Key def initions
Sepsis
Sepsis was defined by the Sepsis 3.0 criteria,[6]which is based on the SOFA or quick SOFA assessment data.
AKI
AKI was defined and classified according to the KDIGO criteria during the first 24 hours after the diagnosis of sepsis, as described previously.[21]If a patient was diagnosed with AKI after admission, his/her serum creatinine level upon admission was used as the baseline value.
Renal recovery at 90 days post-admission
In the 90-day evaluation of renal function, we introduced the concept of acute kidney disease (AKD),which refers to disease progression in patients 7-90 days after the onset of AKI.
According to the Acute Dialysis Quality Initiative(ADQI)[22]for the diagnosis and staging of AKD, phase 0 corresponds to a creatinine level lower than 1.5 times that of the baseline with or without sustained renal injury,repair, and regeneration. In phase 3, the creatinine value is increased to 353.6 μmol/L, or RRT is initiated.
In this study, we conducted a review for the patients in 90 days. If the renal function of the patient corresponding to AKD 0 criteria, it was considered as“full renal recovery”; if that corresponding to AKD 3 criteria was considered as “non-recovery”.
For patients whose baseline of creatinine was unknown, we considered 1.5 times the high-normal value(144 μmol/L) as the cut-off point. For example, when the serum creatinine level was lower than 144 μmol/L, the kidney function was considered as “full recovery” (AKD 0 phase). When serum creatinine level was higher than 144 μmol/L and less than 353.6 μmol/L, it was considered as “partial recovery”. However, when the creatinine levels of patients were more than 353.6 μmol/L or still relied on RRT, it was considered as “non-recovery”. The renal recovery judgement for dead patient was determined by the last serum creatinine level before death.
Statistical analysis
In total, 113 patients were recruited and divided into two groups according to the site of infection. We then compared the baseline data between the two groups,including sex, age, underlying diseases, therapies received during hospitalization, and indications for discharge. Outcomes such as 90-day mortality and renal recovery were also compared. Binary logistic regression was used to identify independent factors, which could influence outcomes. Cumulative mortality rates and survival curves were calculated according to the Kaplan-Meier method. Complete and partial recovery of kidney function were analyzed using particular standards (as described in 2.3.3), and analysis of variance (ANOVA)was used to compare all other secondary endpoints.To take a more personalized and accurate approach to describe the recovery of renal function in our patients,especially for those with CKD, serum creatinine and urea nitrogen values at the time of initial diagnosis with AKI were compared with those at discharge or death using paired samples; the t-test was used to analyze the recovery of renal function. Student's t-test was used to compare independent variables, while the χ2test, or Fisher's exact test, was used to compare categorical variables. Wilcoxon's rank sum test was used to analyze ranked data, while paired sample t-tests were used to analyze the recovery of renal function.Cumulative survival rate was calculated using a life table. Data were expressed as mean ± standard deviation(SD). P values for all outcomes were two-sided, and values < 0.05 were considered to indicate statistical significance.
All statistical analyses were performed using the SPSS software, version 24 (IBM Corp. Released 2016, IBM SPSS Statistics for Windows, Version 24.0.Armonk, NY: IBM Corp.).
RESULTS
Baseline data
Of the 113 patients included in our analysis, 52(46%) experienced sepsis induced by pulmonary infection, 25 (22.1%) by gastrointestinal infections,22 (19.5%) by urinary tract infections, 4 (3.5%) by blood infections, and 10 (8.8%) from other sources of infections. The most common source of infection in SA-AKI patients was the lungs, followed by the gastrointestinal tract and urinary tract.
According to the site of infection, the 113 patients were assigned into one of two groups: a pulmonary group (PG, n=52) and an “other” sources group (OG,n=61); the OG group was further subdivided into four components (genitourinary, gastrointestinal, blood, and others). The differences between these two groups were then compared.
The patient population had several underlying diseases, including atrial f ibrillation (AF, n=12, 10.6%),DVT (n=6, 5.3%), CHF (n=32, 28.3%), cardiovascular disease, including acute coronary syndrome (n=4,3.5%) and coronary atherosclerotic heart disease (n=21,18.6%), hypertension (high blood pressure, n=71,62.8%), diabetes mellitus (n=42, 37.2%), and CKD(n=18, 15.9%).
The t test comparisons between the OG and PG groups with respect to underlying diseases are shown in Figure 1; these data showed that the morbidity associated with CKD and CHF was significantly different when compared between the groups (P=0.015 for CKD and P=0.027 for CHF).
We also collected other baseline data, such as age,sex, routine blood test results, SOFA and APACHE II scores on admission and discharge, ICU treatments (e.g.,RRT), mortality rate, times of death, and renal recovery 90 days after ICU admission (Table 1).
Table 1 shows that the overall mortality rate was 38.9%, which was slightly lower than that reported in other studies[1,13,14]involving SA-AKI patients, but still higher than the mortality rate for sepsis or AKI patients,respectively.[1,23-26]To make our study more rigorous, we only included patients who met the criteria of KDIGO 2 (n=50) or KDIGO 3 (n=63). Until either discharge or death, 52 patients (46%, n=113) experienced full recovery of renal function. Compared with the overall mortality rate of 38.9%, this may demonstrate that the overall situation of our patients seemed better than that reported in other study.[27]
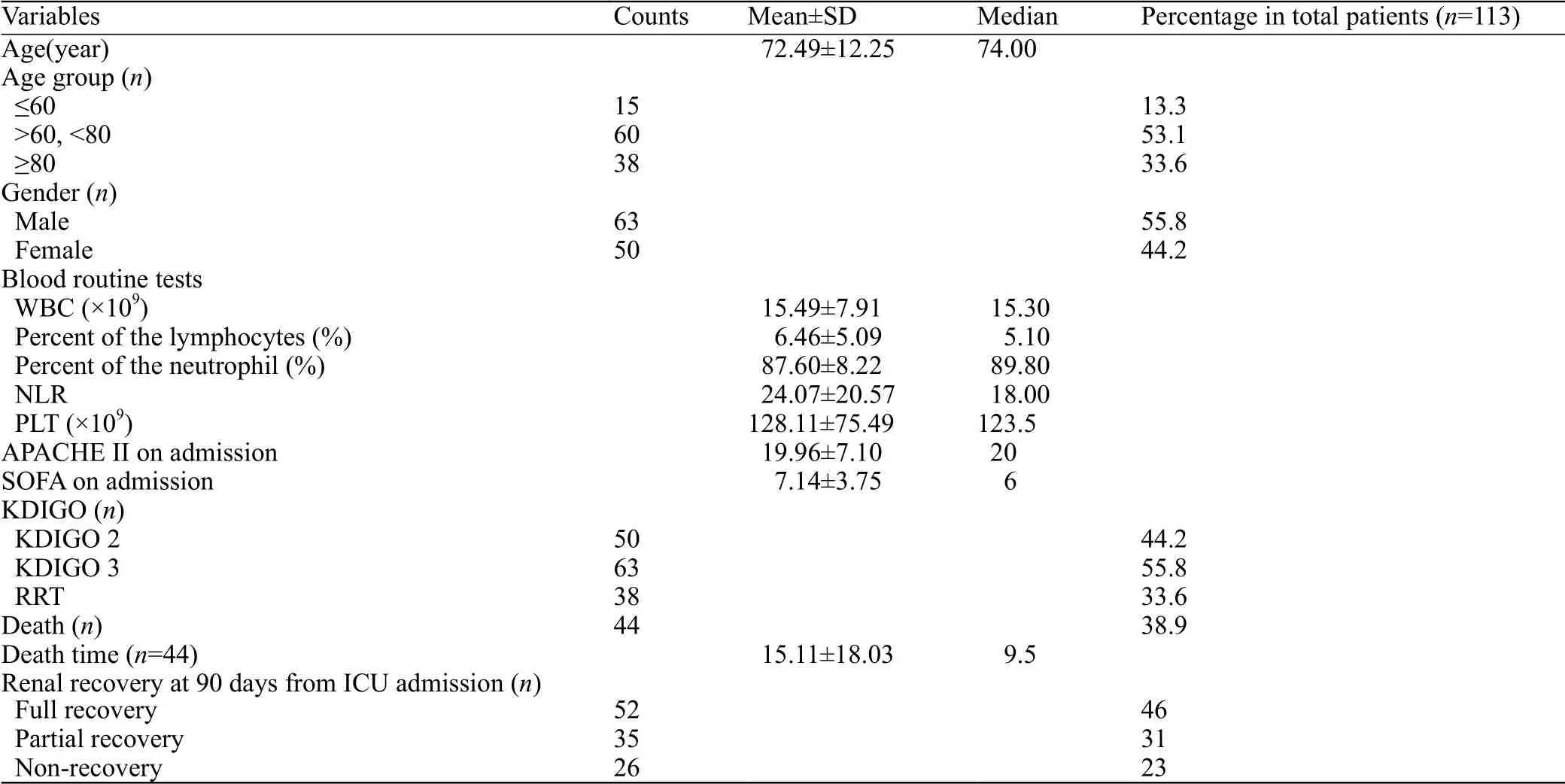
Table 1. Baseline data
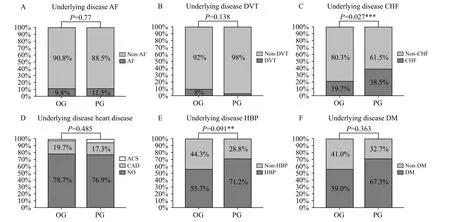
Figure 1. Comparisons between the OG and PG groups with respect to underlying diseases. OG: other sources infection group; PG: pulmonary infection group; AF: atrial fibrillation; DVT: deep-vein thrombosis; CHF: chronic heart failure; ACS: acute coronary syndrome; CAD: coronary atherosclerotic heart disease; HBP: high blood pressure; DM: diabetes mellitus; CKD: chronic kidney disease; **: using χ2 test, means P<0.1; ***:using χ2 test, means P<0.05.
Mortality rate
The PG group was associated with significantly worse outcomes than the OG group, including the 90-day mortality rate (P<0.001) and recovery of renal function(P=0.015). ANOVA was used to compare mortality rates across the four groups (pulmonary group, genitourinary group, gastrointestinal group, and others). Analysis showed that outcomes differed significantly according to the site of infection (Figure 2).
We also recorded the time of death of each deceased patient. Figure 3 shows survival curves for the OG and PG groups. It was clearly evident that patients in the PG group were associated with higher mortality in the early stages (until approximately day 30); thereafter, curves derived from the OG and PG groups were similar (Figure 3).
On comparing the outcomes using the t-test and χ2test between the two groups (Table 2), a significant difference in 90-day mortality rate (P<0.001), as well as renal recovery (P=0.015), was found between the two groups.
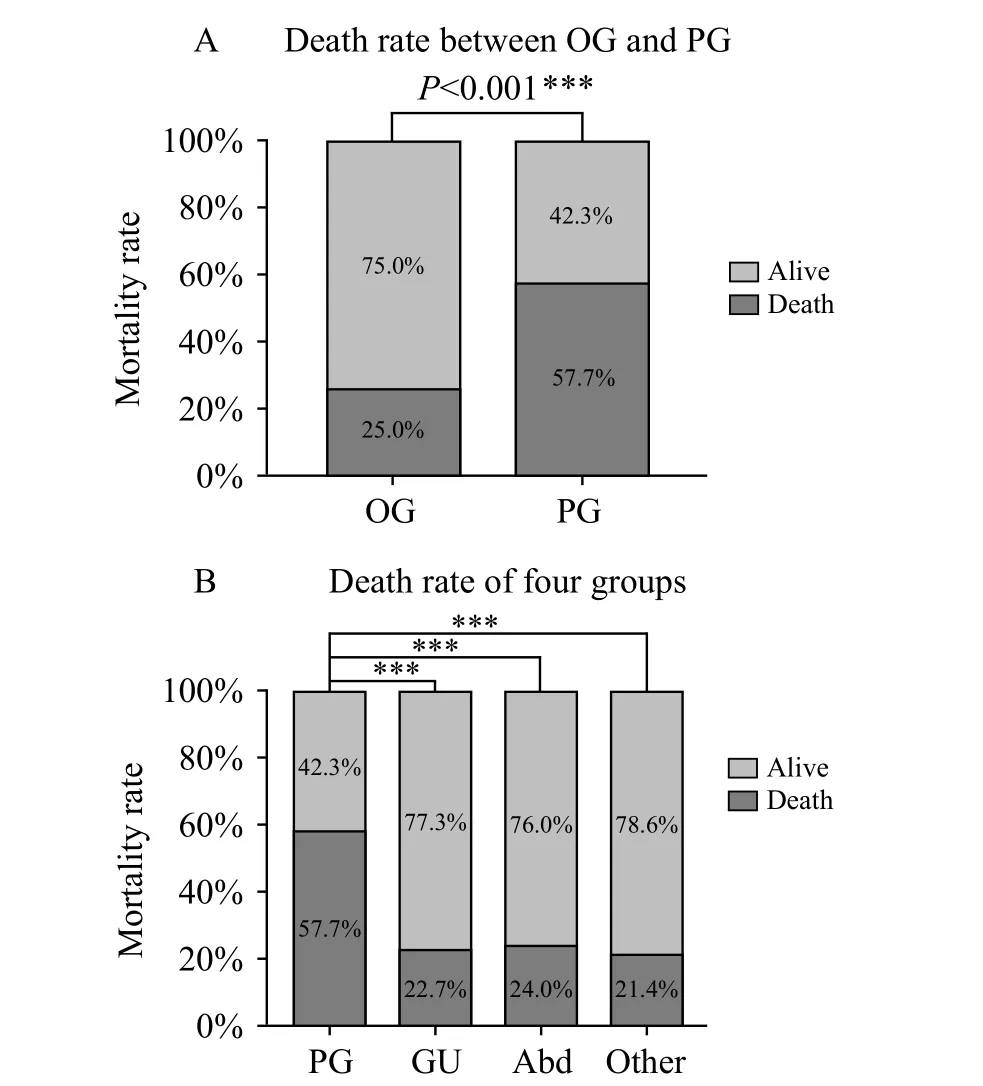
Figure 2. Comparison of the mortality rate between the OG and PG groups and across the four groups. PG: pulmonary infection group;GU: genitourinary infection group; Abd: abdominal infection; Other:other sources of infection including blooding, etc. ***: using χ2 test,means P<0.05 (A); ***: one way ANOVA analysis post hoc, using LSD method, means P<0.05 (B).
Lung function and renal recovery
We found that PaO2and PaO2/FiO2during hospitalization differed significantly when compared between the two groups; values for these parameters were lower in the PG group owing to severe pneumonia occurring in the PG group (Figure 4).
In terms of renal recovery, we first divided the group into three using the standards previously described in the methods section. Then, we used the Wilcoxon's rank sum test to analyze differences between the OG and PG groups (P<0.001) (Figure 5).
Serum levels of creatinine and urea nitrogen at the time of AKI diagnosis and at the time of discharge or death were recorded. On comparison of the two groups of patients with regard to renal recovery (paired sample t-test), the OG and PG groups differed greatly with respect to serum creatinine (P=0.001 and P=0.139,respectively) and urea nitrogen (P=0.001 and P=0.2,respectively) (Figure 6).
To rule out the effects of different RRT upon renal function recovery during admission, we also performed the χ2test to compare RRT across the two groups. This test failed to reveal any significant difference between the two groups(P=0.645); consequently, there was no apparent effect of RRT, thus excluding its effect upon the recovery of renal function. It is evident that patients in the PG group not only had worse outcome (mortality rate and time of death), but also were associated with poorer recovery of renal function.
Binary logistic regression model and cox regression model
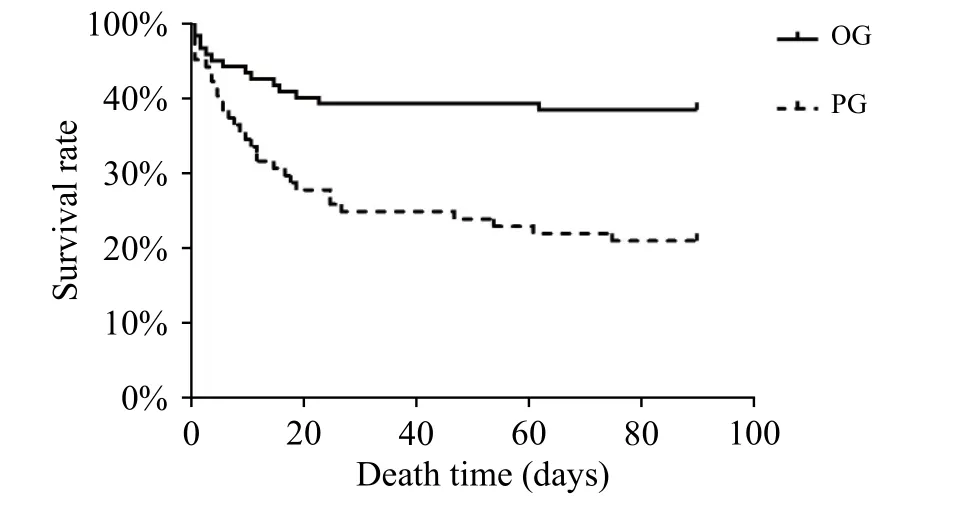
Figure 3. Survival curves for the OG and PG groups.

Table 2. The outcome results between the OG and the PG
Additional analysis identified further statistical differences between the two groups. White blood cell count (WBC) was significantly higher in the OG group than that in the PG group. The neutrophil to lymphocyte ratio was also significantly higher in the OG group than that in the PG group. Unexpectedly, there were reduced levels of shock in the PG group compared to the OG group (Table 3).
Then, we used the indices showing statistical differences between the two groups in a binary logistic regression model using SPSS (using 0.05 for entry and 0.10 for removal). The model achieved 71.7% accuracy in terms of classification. This model identified only one significant variable: the site of infection (P=0.026, odds ratio=3.08 [95% confidence interval: 1.146-8.287]).This provided strong evidence that a pulmonary source of infection could exert a significant influence on the outcome of SA-AKI patients.
To further explore the relationship between the site of infection and mortality, we used the Cox regression analysis by SPSS (using 0.05 for entry and 0.10 for removal), and the results showed a strong association between pulmonary source of infection and mortality(P=0.043, odds ratio=1.352, 95% confidence interval:0.625-2.926), after adjustments for SOFA admission(P=0.095, odds ratio=1.113), APACHE II admission(P=0.801, odds ratio=1.007), shock (P=0.034,odds ratio=2.42), lymphocyte count (P=0.029,odds ratio=1.08), and platelet count(P=0.717, odds ratio=0.999).
DISCUSSION
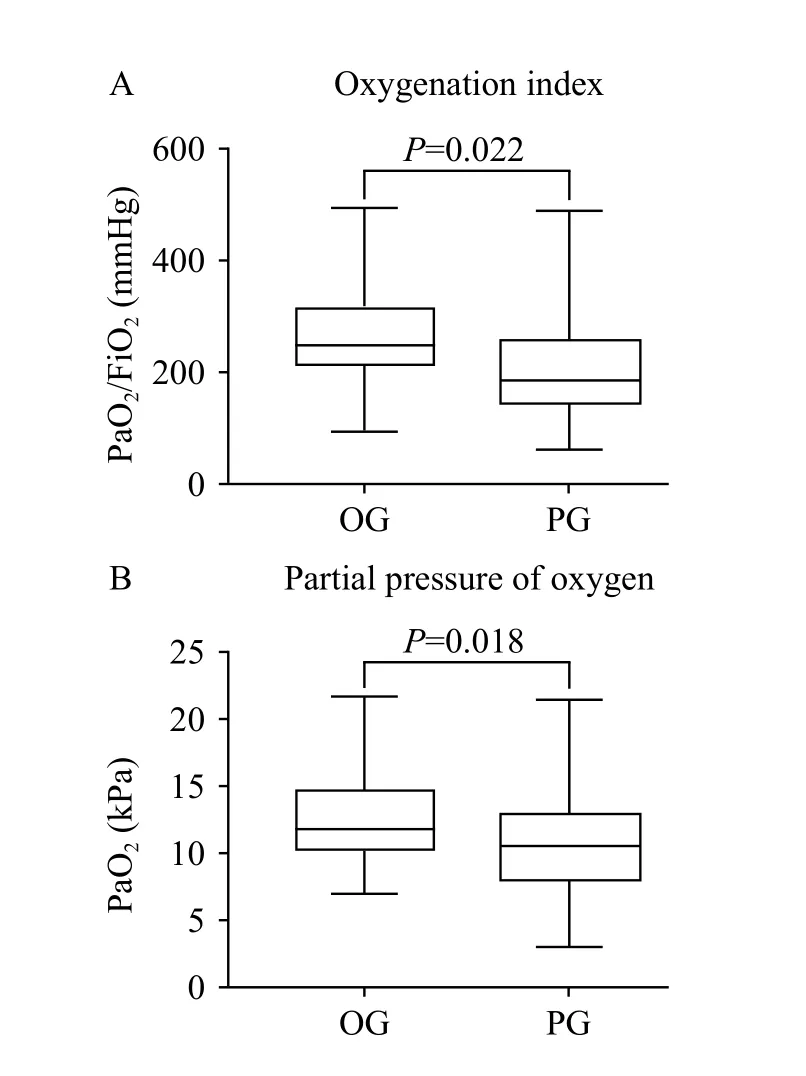
Figure 4. Comparison of PaO2 and PaO2/FiO2 between OG and PG groups.
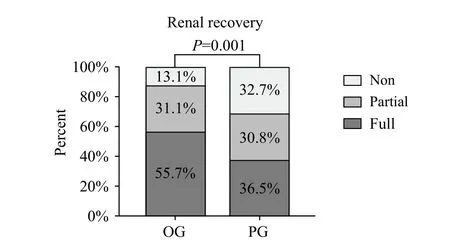
Figure 5. Comparison of renal recovery between OG and PG groups.Non: non-recovery patients; partial: partial renal recovery patients;full: full renal recovery patients. The P value was the result of Wilcoxon rank sum test, and we assigned “full” as a value of 1,“partial”a value of 2, and “non” a value of 3.
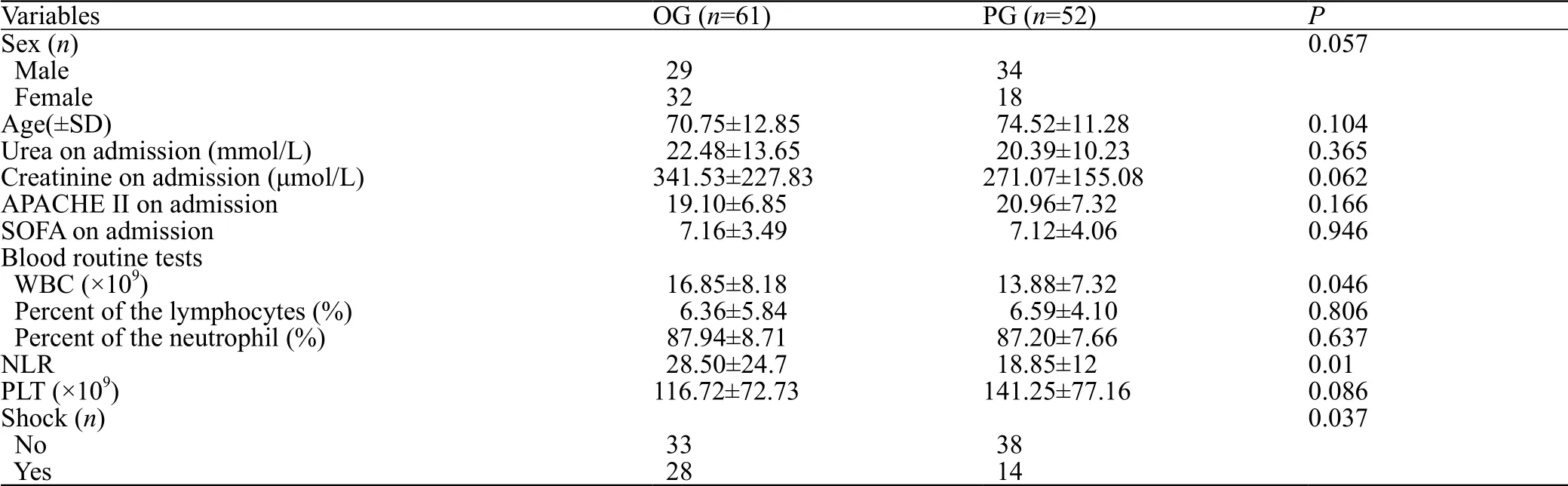
Table 3. The comparison of baseline for OG and PG
Our study showed that the most common source of infection was the lung (52/113 cases, 46%), followed by gastrointestinal (GI) (25/113 cases, 22.1%), and urinary(22/113, 19.5%) sources. Our analysis showed that patients with SA-AKI had a significantly worse outcome(30/52 cases, P<0.001) and poorer kidney recovery(P=0.015) with pulmonary sources of infection than those infected by another source. Data also showed that patients who were not infected by a pulmonary source more likely experienced shock (28/61 cases, P=0.037).
Generally, pulmonary and gastrointestinal infections are two of the most common causes of sepsis,[28-30]and the mortality rate associated with these sources of infection can be over 60%, higher than that for other sources of infection-induced sepsis.[4,25,26]One of the most dangerous complications of septic shock is AKI,which occurs in approximately 50% of patients, with a mortality rate of about 60% at 3 months.[3,31]A previous study[3]showed that the mortality rate was notably higher in SA-AKI patients than that in patients with AKI or sepsis alone. The specific characteristics and pathogenesis of sepsis induced by pulmonary infection have been described previously, although there are few published studies[4,5]in this area. In the present study, the outcomes of SA-AKI patients with pulmonary infection were significantly worse (P<0.001).

Figure 6. Serum levels of creatinine and urea between OG and PG groups before and after admission. ***: the P value was the result of Student's t-test; ***means P<0.001.
We consider that our results might be related to the common occurrence of ALI/ARDS in severe lung infections,as described in a previous study.[7]Moreover, the close relationship and interaction between ALI/ARDS and AKI can lead to a worse outcome among patients.[13,14,26]The high mortality rate of SA-AKI patients who developed ARDS and lung infections in our study concurred with previously published data;[25,26]12 of the 17 patients experiencing this combination of factors died (12/17;70.6%, n=51). This rate was higher than that for SA-AKI patients, which was 41.1%, 53.9%, and 66.3% for the AKI subtypes I, II, and III, respectively, as classified by KDIGO.
It was evident that our patients with pulmonary infection showed hypoxemia and lower oxygenation index (212.92 mmHg in the PG group vs. 255.62 mmHg in the OG group, P=0.022). These conditions directly lead to the increasing possibility of the patient receiving mechanical ventilation; this may induce acute tubular necrosis (ATN) and worsen the outcome.[32]Worse still, the adverse effects of mechanical ventilation may be further complicated by reduced cardiac output induced by high intrathoracic pressure. However, such detrimental effects are not limited to the lung and can induce a systemic inflammatory response.[33]Several evidences now indicate that biotrauma induced by mechanical ventilation not only affects the lung but also leads to further systemic inf lammation and organ dysfunction via the release of inflammatory cytokines.[7,26,34-36]These factors, therefore, provide an explanation, at least in part, of why patients with a pulmonary source of infection have poorer outcomes.
Further, we found that in the OG group, SA-AKI patients were associated with a poorer renal recovery.ALI/ARDS often occurs with severe lung infections. A large body of evidence now indicates that AKI is closely related to ALI and that AKI is an extrapulmonary factor of ALI/ARDS. Previous studies[13,14]also documented the role of cytokines and inflammatory mediators in AKI/ARDS caused by AKI. We believe that once sepsis patients with pulmonary source of infection are complicated by kidney damage, they will have a poorer recovery of kidney function.
Our study has yielded a useful new concept, that is,among patients with SA-AKI, the initial anatomy of the sepsis has an important effect on patient outcome. We also found that the recovery of renal function in patients with pulmonary infection was significantly worse than that among patients with other sources of infection. This provides a new direction for clinical research and has special guiding significance for treatment and prevention.
Despite the novelty and significance of our findings for clinical translation, several limitations should be considered. First, this was a retrospective study of a relatively small number of patients from a single center.Second, given our small sample size, we focused on the outcomes of patients with pulmonary infection and ignored patients with other sources of infection. Further studies are needed to verify our results.
CONCLUSION
Our study demonstrates that patients with SA-AKI induced by pulmonary infection have significantly poorer outcomes and unique pathophysiological mechanisms and characteristics. Furthermore, lung infection influences the clinical outcome in an independent manner. This study also shows that lung injury may affect renal function through unknown mechanisms,which also affects the recovery of kidney function in SAAKI patients. Consequently, we must pay closer attention to these patients and take more effective measures to improve their outcome.
Funding:This work is supported by the National Natural Science Foundation of China (81873947) and Hospital Development center(SHDC120161).
Ethical approval:Not needed.
Conflicts of interest:The authors have no conflict of interest to declare.
Contributors:YWF proposed and wrote the paper. All authors read and approved the f inal version.
REFERENCESS
1 Siew ED, Deger SM. Recent advances in acute kidney injury epidemiology. Curr Opin Nephrol Hypertens. 2012;21(3):309-17.
2 Poukkanen M, Vaara ST, Pettila V, Kaukonen KM, Korhonen AM, Hovilehto S, et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units. Acta Anaesthesiol Scand. 2013;57(7):863-872.
3 Koyner JL. Assessment and diagnosis of renal dysfunction in the ICU. Chest. 2012;141(6):1584-94.
4 Fan PC, Chang CH, Tsai MH, Lin SM, Jenq CC, Hsu HH, et al.Predictive value of acute kidney injury in medical intensive care patients with sepsis originating from different infection sites. Am J Med Sci. 2012;344(2):83-9.
5 Sood M, Mandelzweig K, Rigatto C, Tangri N, Komenda P,Martinka G, et al. Non-pulmonary infections but not specific pathogens are associated with increased risk of AKI in septic shock. Intensive Care Med. 2014;40(8):1080-8.
6 Singer M, Deutschman CS, Seymour CW, Shankar-Hari M,Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA.2016;315(8):801-10.
7 Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med.2000;342(18):1301-8.
8 Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesin A, Descorps-Declere A, et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med.2011;39(8):1886-95.
9 Chambers KA, Park AY, Banuelos RC, Darger BF, Akkanti BH,Macaluso A, et al. Outcomes of severe sepsis and septic shock patients after stratification by initial lactate value. World J Emerg Med. 2018;9(2):113-7.
10 Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180(9):861-6.
11 Yoshida J, Furugaki K, Oyama M. Perioperative antimicrobials in chest surgery patients positive for methicillin-resistant Staphylococcus aureus. Gen Thorac Cardiovasc Surg.2010;58(12):657-9.
12 Shorr AF, Bernard GR, Dhainaut JF, Russell JR, Macias WL,Nelson DR, et al. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006;10(3):R92.
13 Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, et al. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Renal Physiol. 2012;303(6):F864-F872.
14 Bhargava R, Janssen W, Altmann C, Andres-Hernando A,Okamura K, Vandivier RW, et al. Intratracheal IL-6 protects against lung inflammation in direct, but not indirect, causes of acute lung injury in mice. PLoS One. 2013;8(5):e61405.
15 Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A,Kumar A, et al. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204-13.
16 Volakli E, Spies C, Michalopoulos A, Groeneveld AB, Sakr Y,Vincent JL. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14(2):R32.
17 Nielsen SL, Roder B, Magnussen P, Engquist A, Frimodt-Moller N. Nosocomial pneumonia in an intensive care unit in a Danish university hospital: incidence, mortality and etiology. Scand J Infect Dis. 1992;24(1):65-70.
18 Arumugam SK, Mudali I, Strandvik G, El-Menyar A, Al-Hassani A, Al-Thani H. Risk factors for ventilator-associated pneumonia in trauma patients: A descriptive analysis. World J Emerg Med.2018;9(3):203-10.
19 Rello J, Jubert P, Valles J, Artigas A, Rue M, Niederman MS.Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 1996;23(5):973-98.
20 Stevens RM, Teres D, Skillman JJ, Feingold DS. Pneumonia in an intensive care unit. A 30-month experience. Arch Intern Med.1974;134(1):106-11.
21 Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR,Thakar CV, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-72.
22 Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery:consensus report of the Acute Disease Quality Initiative(ADQI) 16 workgroup. Nat Rev Nephrol, 2017;13(4):241-57.
23 Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576-82.
24 Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-9.
25 Awad AS, Okusa MD. Distant organ injury following acute kidney injury. Am J Physiol Renal Physiol.2007;293(1):F28-F29.
26 Darmon M, Clec'h C, Adrie C, Argaud L, Allaouchiche B,Azoulay E, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol.2014;9(8):1347-53.
27 Peng Q, Zhang L, Ai Y, Zhang L. Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin Med J (Engl). 2014;127(10):1820-26.
28 Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM,Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock:2008. Intensive Care Med. 2008;34(1):17-60.
29 Carrigan SD, Scott G, Tabrizian M. Toward resolving the challenges of sepsis diagnosis. Clin Chem. 2004;50(8):1301-14.
30 Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors,mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16(3):379-414.
31 Barbar SD, Binquet C, Monchi M, Bruyere R, Quenot JP.Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL-ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomized controlled trial. Trials. 2014;15:270.
32 Kuiper JW, Groeneveld AB, Slutsky AS, Plotz FB.Mechanical ventilation and acute renal failure. Crit Care Med.2005;33(6):1408-15.
33 Parra ER, Kairalla RA, Ribeiro de Carvalho CR, Eher E,Capelozzi VL. Inf lammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration.2007;74(2):159-69.
34 Liu YC, Qi YM, Zhang H, Walline J, Zhu HD. A survey of ventilation strategies during cardiopulmonary resuscitation.World J Emerg Med. 2019;10(4):222-7.
35 Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inf lammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54-61.
36 Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB,Wheeler A, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35(12):2755-61.
 World journal of emergency medicine2020年1期
World journal of emergency medicine2020年1期
- World journal of emergency medicine的其它文章
- Surgical closure of large splenorenal shunt may accelerate recovery from hepato-pulmonary syndrome in liver transplant patients
- Investigations for the assessment of adult patients presenting to the emergency department with supraventricular tachycardia
- Epidemiological characteristics and disease spectrum of emergency patients in two cities in China: Hong Kong and Shenzhen
- Role of penehyclidine in acute organophosphorus pesticide poisoning
- The first two cases of transcatheter mitral valve repair with ARTO system in Asia
- Admission delay is associated with worse surgical outcomes for elderly hip fracture patients: A retrospective observational study
