Role of penehyclidine in acute organophosphorus pesticide poisoning
Shi-yuan Yu, Yan-xia Gao, Joseph Walline, Xin Lu, Li-na Zhao, Yuan-xu Huang, Jiang Tao, An-yong Yu, Na Ta, Ren-ju Xiao, Yi Li
1 Department of Emergency Medicine, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
2 Department of Emergency Medicine, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
3 Accident and Emergency Medicine Academic Unit, Prince of Wales Hospital, the Chinese University of Hong Kong, Hong Kong, China
4 Department of Emergency Medicine, the Fourth Affiliated Hospital of Jishou University, Huaihua, China
5 Department of Emergency Medicine, the First Affiliated Hospital of Zunyi Medical University, Zunyi, China
6 Department of Emergency Medicine, Chifeng Municipal Hospital, Chifeng, China
7 Department of Emergency Medicine, Xingyi People's Hospital, Xingyi, China
KEY WORDS: Penehyclidine; Organophosphorus pesticide poisoning; Meta-analysis
INTRODUCTION
Acute severe organophosphorus pesticide (OP)poisoning, which is defined as cholinesterase activity less than 30% in a patient presenting with an acute onset M- and N-cholinergic receptor activation syndrome,is a common type of accidental or suicidal pesticide poisoning in developing countries,[1]especially in rural China.[2,3]Although the mortality rate for OP poisonings in China has dropped over the past several years,OP poisoning still accounts for more than half of all intoxication cases in emergency departments throughout China and leads to more than 80% of total poisoning deaths.[2,4,5]
Anticholinergic agents are the cornerstone of therapy for relieving symptoms in OP patients, especially pulmonary edema. Atropine is the most commonly used anticholinergic antidote for treating cholinergic syndrome, especially in OP patients.[2,3,6]However, its M-receptor blockade ability sometimes leads to adverse effects on intoxicated patients, particularly concerning vital signs (e.g., elevated heart rate and blood pressure).In the initial phase of treating an OP poisoning patient in the emergency department, the proper initial dose of atropine is hard to judge and may lead to severe complications.[7-9]The dose of atropine administration does vary a lot between different medical centers and managing the effect of atropine is challenging.[10]The adverse effects of atropine are another cause of poor prognosis or even death in OP patients.[6,11]
Penehyclidine is a relatively new anticholinergic agent developed by the Chinese Academy of Military Medical Sciences targeting mainly M1 and M3 cholinergic receptors.[12]After intramuscular injection of 1 mg of penehyclidine hydrochloride in healthy adults, penehyclidine hydrochloride can be detected in the bloodstream in two minutes. The peak plasma concentration (13.20 μg/L) occurs in about 30 minutes,and the elimination half-life is 10.35 hours. Due to penehyclidine's high-selectivity for M-receptors, it relieves the symptoms associated with M-receptor activation in OP patients and also reduces some of the adverse effects of atropine caused by its M-receptor blockade mechanism, such as fever and bradycardia.However, the time to atropinization is possibly prolonged due to the selectivity of penehyclidine as compared to the general cholinergic receptor-blocking ability of atropine,and penehyclidine needs longer period of time to reach functional volume in the blood as compared to atropine(almost 1 hour vs. 4-8 minutes).[13,14]
Clinical observations showed that atropine combined with penehyclidine is a potentially effective therapy for OP poisoning patients, reducing the rate of adverse drug reactions (ADR) of atropine and improving overall prognosis.[3,10,15,16]Nevertheless, despite the affordable price for people in developing countries and wide usage of penehyclidine, it has not yet been clearly recommended in any guideline or consensus worldwide.A published meta-analysis of clinical trials between 2000 and 2010 concluded that the administration of penehyclidine compared to atropine for severe OP poisoning patients could increase the cure rate and reduce adverse effects significantly and recommended that atropine combined with penehyclidine may also be beneficial for OP poisoning patients.[17]However, the quality of included articles was relatively low and there have since been few published guidelines advocating for atropine combined with penehyclidine as a standard treatment for OP patients. We aimed to synthesize the most recent publications and evaluate the effects of penehyclidine alone or combined with atropine toward OP poisoning patients.
METHODS
Search strategy
Our research team searched the following databases:Pubmed, Cochrane library, EMBASE, Chinese National Knowledge Infrastructure (CNKI), Chinese Biomedical literature (CBM) and Wanfang. English search terms included organophosphate poisoning (MeSH term),organophosphorus, organophosphorus poisoning, OP poisoning, OP, atropine, and penehyclidine. Chinese search terms were also included, consisting of words with the same meaning as the aforementioned English terms, including (in pinyin romanization): “a tuo pin”,“wu yi kui mi”, “you ji lin”, “chang tuo ning” and“zhong du”. Afterwards, two members of our research team cross-checked all reference lists independently for agreement.
Inclusion and exclusion criteria
Articles were included if they met all of the following criteria: (i) randomized controlled trial (RCT);(ii) study population included accidental or suicidal acute OP patients; (iii) study included comparisons at least between atropine combined with penehyclidine group,a penehyclidine group and an atropine group; (iv) one or more of the following indexes were reported in the article: cure rate, mortality rate, time to atropinization,time to 60% normal acetylcholinesterase (AchE) level,rate of intermediate syndrome (IMS), and rate of ADR.
Articles were excluded for the following reasons: (i)language other than Chinese or English; (ii) study not carried out on human beings; (iii) data was unavailable or unextractable; (iv) important outcomes (cure rate or mortality rate) were not reported; (v) study was performed primarily on special populations (e.g., patients with HIV or tuberculosis); (vi) study consisted of patients who were not initially treated with penehyclidine or penehyclidine combined with atropine; (vii) study was a review article or case report.
Quality assessment
Two reviewers independently evaluated the included studies under the guide of the Cochrane Collaboration's quality assessment tool. First, they excluded duplicate articles and then performed title and abstract screening.Second, they screened the full-text articles for inclusion or exclusion. If there was a controversial decision, a third reviewer from our team was consulted as a tie-breaker. The following seven aspects were evaluated, including allocation concealment, blinding of participants and personnel, blinding of outcome assessment, random sequence generation,incomplete outcome data, selective reporting and other sources of bias. The results were stratified as low risk of bias,high risk of bias and unclear risk of bias.
Statistics
Two researchers independently extracted and evaluated the data of baseline characteristics using Excel 2016 (Microsoft Corporation, Redmond, USA). The main outcomes were cure rate (defined as all clinical syndromes attenuated and vital signs returned to be normal), mortality rate, time to atropinization (which was defined as first the occurrence of any of the following findings: dry skin, dilated pupils, reduced levels of abnormal breath sounds, and a heart rate beyond 120 bpm), time to 60% normal AchE level, rate of IMS, rate of ADR and duration of hospitalization. The estimated risk ratio (RR), standardized mean difference (SMD) and 95% confidence intervals (95% CI) were pooled using Review Manager 5.3 (Cochrane Collaboration, UK).A P-value less than 0.05 was defined as statistically significant. Heterogeneity level was evaluated using I2.An I2> 50% suggested high heterogeneity among studies and a randomized model was used to pool the data, while a fixed model was used if I2was no more than 50%.Funnel plots were used to demonstrate reporting bias.
RESULTS
Studies included
Our study found 3,918 articles in the first screening stage, 154 of these articles came from PubMed, 98 from EMBASE, 0 from the Cochrane library, 1,878 from CNKI,1,005 from CBM and 783 from Wanfang. After secondary abstraction and full-text screening by our reviewers, 16 articles (all from the CNKI database) were identified,recruiting 1,334 patients in total.[2,4,10,11,13,14,18-27]The screening procedure is illustrated in Figure 1. All included studies were RCTs published in Chinese. Details of the articles included are shown in Table 1.
Baseline characteristics
Sixteen RCTs enrolled 1,334 OP patients in total.There were 388 patients in the penehyclidine+atropine group, 378 patients in the penehyclidine-only group and 568 patients in the atropine-only group. The mean age was 35.1±6.2 in the penehyclidine+atropine group,38.8±2.3 in the penehyclidine-only group and 34.2±11.0 in the atropine-only group (P=0.556). The ingestion amount was 90.7±26.1 mL in the penehyclidine+atropine group, 62.9±28.1 mL in the penehyclidine-only group and 76.0±30.3 mL in the atropine-only group(P=0.521). The AchE level was 159.3±41.4 U/L in the penehyclidine+atropine group,158.2±118.7 U/L in the penehyclidine-only group and 168.2±103.1 U/L in the atropine-only group (P=0.212). All enrolled studies gave their patients a standardized amount of oxime.
Evaluation of bias
The methodological quality of included studies was assessed under the guidelines of the Cochrane Collaboration's tool. All 16 studies included mentioned a randomization procedure and reported the process of random sequence generation, however, no included studies reported details regarding allocation concealment.There was also a high risk of bias concerning both participants and personnel as well as outcome assessment.The reasons for missing data between groups are similar in the recruited studies. There was a low risk of bias regarding selective reporting. Thirteen studies met criteria of low risk of other bias while one of the studies failed to report baseline characteristics. Therefore, all studies were judged to be of poor methodological quality (Figure 2).

Figure 1. Flow diagram.
Cure rate
Penehyclidine + atropine vs. atropine alone
Seven studies included eligible data on cure rates between penehyclidine+atropine and atropine-alone groups, including 594 individuals. A fixed model was used due to low heterogeneity in these studies(I2=49%). Pooled statistics showed that the cure rate was significantly higher in the penehyclidine+atropine group than in the atropine-alone group (0.97 vs. 0.86, RR 1.13,95% CI 1.07-1.19, P<0.00001).
Penehyclidine alone vs. atropine alone
Eight studies included eligible data on cure rates between penehyclidine alone and atropine alone groups,including 569 individuals. A fixed model was used due to low heterogeneity in these studies (I2=48%). Pooled statistics showed that the cure rate was significantly higher in the penehyclidine group than in the atropine group (0.93 vs. 0.80, RR=1.16, 95% CI 1.08-1.24, P<0.00001).
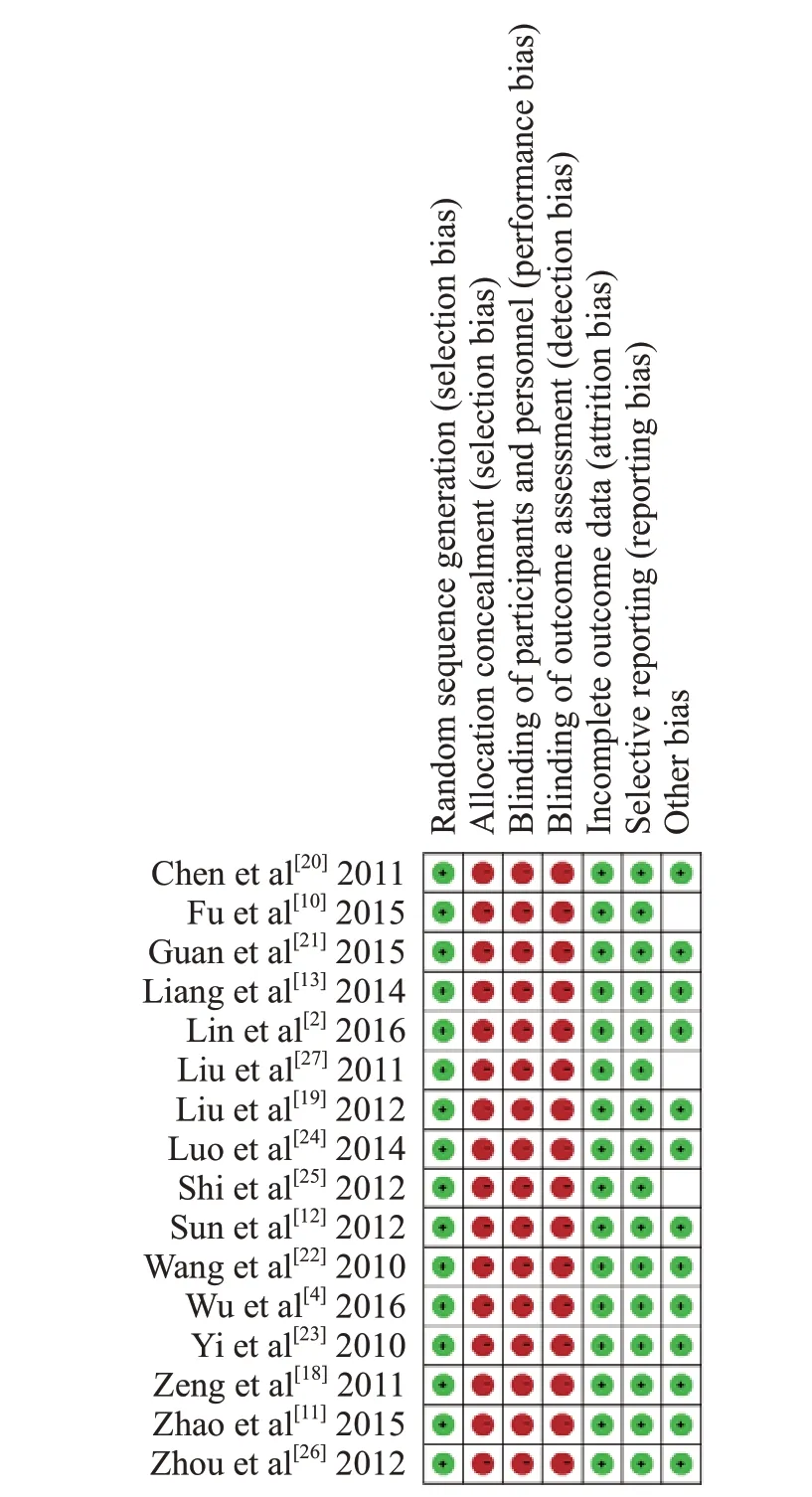
Figure 2. Risk of bias summary.
Penehyclidine+atropine vs. penehyclidine alone
Three studies included eligible data on cure rates between penehyclidine+atropine and penehyclidinealone groups, including 267 individuals. A fixed model was used due to low heterogeneity in these studies(I2=0%). Pooled statistics showed that cure rate was significantly higher in the penehyclidine+atropine group than in the penehyclidine-alone group (0.93 vs. 0.80,RR=1.08, 95% CI 1.01-1.15, P=0.02). Forest plots of the comparative cure rates are shown in Figure 3.
Mortality rate
Penehyclidine+atropine vs. atropine alone
Five studies included eligible data on mortality rates between penehyclidine+atropine and atropinealone groups, including 399 individuals. A fixed model was used because of low heterogeneity in these studies(I2=0%). Pooled statistics showed that the mortality rate was significantly lower in the penehyclidine+atropine group than in the atropine-alone group (0.015 vs. 0.11,RR=0.17, 95% CI 0.06-0.49, P=0.0009).
Penehyclidine alone vs. atropine alone
Seven studies included eligible data on mortality rates between penehyclidine-alone and atropine-alone groups, including 429 individuals. A fixed model was used due to low heterogeneity in these studies (I2=0%).Pooled statistics showed that the mortality rate was significantly lower in the penehyclidine-alone group than in the atropine-alone group (0.06 vs. 0.16, RR=0.35, 95%CI 0.19-0.65, P=0.001).
Penehyclidine+atropine vs. penehyclidine alone
Two studies included eligible data on mortality rates between penehyclidine+atropine and penehyclidinealone groups, including 151 individuals. A fixed model was used because of low heterogeneity in these studies(I2=0%). Pooled statistics showed that the mortality rate was comparable between the penehyclidin+atropine group and the penehyclidine-alone group (0.013 vs. 0.08,RR= 0.23, 95% CI 0.04-1.28, P=0.09). Forest plots of the comparative mortality rates are shown in Figure 4.
Time to atropinization
Penehyclidine+atropine vs. atropine alone
Seven studies included eligible data on time to atropinization between penehyclidine+atropine and atropine-alone groups, including 546 individuals. A randomized model was used due to high heterogeneity in these studies (I2=96%). Pooled statistics showed that the time to atropinization was significantly shorter in the penehyclidine+atropine group than in atropine-alone group (SMD=-1.44, 95% CI -2.37- -0.51, P<0.00001).
Penehyclidine alone vs. atropine alone
Five studies included eligible data on time to atropinization between penehyclidine-alone and atropinealone groups, including 344 individuals. A randomized model was used because of high heterogeneity in these studies (I2=97%). Pooled statistics showed that the time to atropinization was significantly longer in the penehyclidine-alone group than in the atropine-alone group (SMD=1.00, 95% CI 0.75-1.26, P<0.00001).
Penehyclidine+atropine vs. penehyclidine alone
Four studies included eligible data on time to atropinization between penehyclidine+atropine and penehyclidine-alone groups, including 284 individuals.A randomized model was used because of high heterogeneity in these studies (I2=97%). Pooled statistics showed that the time to atropinization was significantly shorter in the penehyclidine+atropine group than in the penehyclidine-alone group (SMD=-1.53, 95% CI -1.83--1.24, P<0.00001).
Time to 60% normal AchE level
Penehyclidine+atropine vs. atropine alone
Six studies included eligible data on time to 60%normal AchE level between penehyclidine+atropine and atropine-alone groups, including 543 individuals. A randomized model was used due to high heterogeneity in these studies (I2=90%). Pooled statistics showed that the time to 60% normal AchE level was significantly shorter in the penehyclidine+atropine group than in the atropinealone group (SMD=-1.69, 95% CI -1.89- -1.48, P<0.00001).
Penehyclidine alone vs. atropine alone
Five studies included eligible data on time to 60%normal AchE level between penehyclidine-alone and atropine-alone groups, including 411 individuals. A fixed model was used due to low heterogeneity in these studies(I2=31%). Pooled statistics showed that the time to 60% normal AchE level was significantly shorter in the penehyclidine-alone group than in atropine-alone group(SMD=-1.27, 95%CI -1.53- -1.01, P<0.00001).
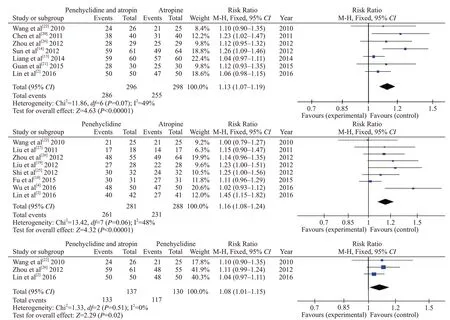
Figure 3. Cured rate.
Penehyclidine+atropine vs. penehyclidine alone
Three studies included eligible data on time to 60%normal AchE level between penehyclidine+atropine and penehyclidine-alone groups, including 284 individuals. A randomized model was used because of high heterogeneity in these studies (I2=94%). Pooled statistics showed that the time to 60% normal AchE level was comparable between the penehyclidine+atropine and the penehyclidine-alone groups(SMD=-0.77, 95%CI -1.83-0.30, P=0.16).
Rate of IMS
Penehyclidine+atropine vs. atropine alone
Three studies included eligible data on the rate of IMS between penehyclidine+atropine and atropinealone groups, including 275 individuals. A fixed model was used due to low heterogeneity in these studies(I2=0%). Pooled statistics showed that the rate of IMS was significantly lower in the penehyclidine+atropine group than in the atropine-alone group (0.058 vs. 0.20,RR=0.31, 95% CI 0.15-0.64, P=0.002).
Penehyclidine alone vs. atropine alone
Four studies included eligible data on the rate of IMS between penehyclidine-alone and atropine-alone groups,including 305 individuals. A fixed model was used because of low heterogeneity in these studies (I2=0%).Pooled statistics showed that the rate of IMS was significantly lower in the penehyclidine-alone group than in the atropine-alone group (0.082 vs. 0.22, RR=0.39,95% CI 0.21-0.70, P=0.002).
Penehyclidine+atropine vs. penehyclidine alone
Two studies included eligible data on the rate of IMS between penehyclidine+atropine and penehyclidinealone groups, including 184 individuals. A fixed model was used due to low heterogeneity in these studies(I2=0%). Pooled statistics showed that the rate of IMS was comparable between the penehyclidine+atropine and the penehyclidine-alone groups (0.072 vs. 0.11, RR=0.70,95% CI 0.28-1.78, P=0.45).
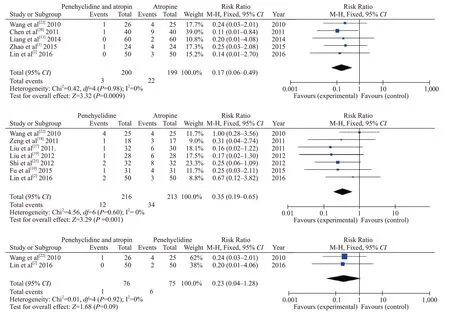
Figure 4. Mortality rate.
Rate of total ADR
Penehyclidine+atropine vs. atropine alone
Four studies included eligible data on the rate of total ADR between penehyclidine+atropine and atropine-alone groups, including 323 individuals. A fixed model was used because of low heterogeneity in these studies (I2=12%).Pooled statistics showed that the rate of total ADR was significantly lower in the penehyclidine+atropine group than in the atropine-alone group (0.044 vs. 0.24, RR=0.19, 95%CI 0.09-0.41, P<0.0001).
Statistics on the rates of total ADR were not extractable for the penehyclidine-alone vs. atropinealone groups nor for the penehyclidine+atropine vs.penehyclidine-alone groups.
Duration of hospitalization
Penehyclidine+atropine vs. atropine alone
Seven studies included eligible data on duration of hospitalization between penehyclidine+atropine and atropine-alone groups, including 556 individuals.A randomized model was used because of high heterogeneity in these studies (I2=77%). Pooled statistics showed that the duration of hospitalization was significantly shorter in the penehyclidine+atropine group than in the atropine-alone group (SMD=-1.35, 95% CI-1.74- -0.95, P<0.00001).
Penehyclidine alone vs. atropine alone
Three studies included eligible data on duration of hospitalization between penehyclidine-alone and atropine-alone groups, including 247 individuals.A randomized model was used because of high heterogeneity in these studies (I2=64%). Pooled statistics showed that the duration of hospitalization was comparable between the penehyclidine-alone and atropine-alone groups (SMD=-0.42, 95% CI -0.85-0.01,P=0.06).
Penehyclidine+atropine vs. penehyclidine alone
Two studies included eligible data on duration of hospitalization between penehyclidine+atropine and penehyclidine-alone groups, including 181 individuals.
A randomized model was used due to high heterogeneity in these studies (I2=97%). Pooled statistics showed that the duration of hospitalization was comparable between the penehyclidine+atropine and penehyclidine-alone groups (SMD=-1.40, 95% CI -3.64-0.84, P=0.22). Main outcomes are summarized in Table 2.
DISCUSSION
Accidental and suicidal OP poisoning is still a major cause of in-hospital death among all types of pesticide poisonings.[15]Developing countries account for a majority of these patients.[29]Conventional therapy for OP poisoning consists of two major treatments: reactivating the function of AchE and relieving symptoms (i.e.attenuating cholinergic receptor activity) until AchE function recovers. Unfortunately, despite OP poisoning alone being fully capable of causing high mortality rates,atropine in overdose or from its direct adverse effects is also a source of poor outcomes.[9,30]
Penehyclidine is a newly developed anticholinergic agent that selectively targets M1 and M3 receptors, reducing the adverse effects of atropine due to M-receptor blockade.The time to atropinization is prolonged, however, when using penehyclidine.[3,6,17]Thus, many medical centers combine the use of atropine and penehyclidine in order to maintain a balanced outcome of time to atropinization and cure rate.[11,14,29]A retrospective study of two cases of OP poisoning in pregnant women also showed beneficial effects of penehyclidine.[16]Although both fetuses died,the two women both reported no complications and recovered. Despite its reported utility, there is still a lack of high-quality evidence or standardized regimens of penehyclidine combined with atropine for OP poisoning patients.
The mechanism of action of atropine combined with penehyclidine on severe OP poisoning patients is not yet clearly understood. While penehyclidine can relieve the effects of muscarinic activation by competing with accumulated acetylcholine against the M1 and M3 receptors, the faster time to reach 60% normal AchE level we found in this study is not well explained by thismechanism. Future pharmacological investigations into the mechanism(s) of penehyclidine are still needed to explain some findings of this study.

Table 2. Main outcomes
Other effects of penehyclidine on OP-damaged tissue have been observed.[31]Besides the direct cellular injury effect of OP on organs such as the liver and kidneys, auto-immune reaction is another underlying cause of tissue damage in OP patients. Rat models showed protective effects of penehyclidine on cerebral tissue after ischemia and reperfusion injury by reducing the activation of inflammation and down-regulating cell apoptosis after oxidative stress.[32-34]This kind of neural cell damage can also be caused by intoxication and therefore penehyclidine may help modulate cerebral function after OP poisoning, leading to a reduced IMS rate.[35-37]Similarly, some other in vitro studies and animal models also revealed an immune modulation function of penehyclidine, such as attenuating renal ischemia and reperfusion injury as well as enhancing respiratory function in COPD patients.[38,39]These immune modulation mechanisms of penehyclidine are likely also present in OP patients but future research is still needed to confirm.
Compared with the last meta-analysis from 2012,[17]our research integrates more clinical trials concerning acute OP poisoning patients and evaluated more indexes(such as time to atropinization and adverse drug effects).Our study analyzed the most up to date RCTs, focusing on the effects of atropine combined with penehyclidine on OP poisoning patients. Our pooled statistics revealed that compared to atropine alone, penehyclidine combined with atropine significantly increases the cure rate,reduces the mortality rate, time to atropinization, time to 60% normal AchE level, rate of IMS and total ADR.Also, compared to penehyclidine alone, the combined therapy significantly increases the cure rate and reduces mortality, time to atropinization and duration of hospitalization. Based on the pooled data from this study, it seems likely that combining penehyclidine with atropine leads to the best outcomes for OP poisoning patients. Given the pharmacokinetics of penehyclidine and its ease of use, potentially having the medication be given by pre-hospital staff in cases of likely OP poisoning is in intriguing avenue of further study.[40]
This study has some limitations. First, all the included RCTs were carried out in China. Although not surprising considering that Chinese patients make up a majority of OP poisoning cases worldwide, it would have been better to have more geographical diversity represented. Second, the methodological quality of the included studies was also relatively low, especially concerning the procedures for allocation concealment and blindness. Third, baseline characteristics such as the amount of OP ingested, baseline serum AchE levels, and dose of atropine and penehyclidine administrated were not reported in most studies. In particular, the dosing of atropine is known to very considerably due to a lack of standardization in treating OP poisoning patients.Fourth, some important outcomes such as the rate of ICU admission and intubation rates were lacking. Finally, it is possible that a more careful administration of atropine could lead to a significant improvement in mortality.[41,42]We are unsure as to why the time to atropinization was significantly reduced in the penehyclidine groups and further study is urgently needed. Additionally, the economic effects of adding penehyclidine (such as its effect on overall medication costs) are still unknown.
Despite of these limitations, our meta-analysis synthesized the most up to date RCTs comparing the outcomes of penehyclidine added to atropine for OP poisoning patients. Our study suggests that combining penehyclidine with atropine for OP poisoning patients until atropinization improves overall outcomes for OP poisoning patients by increasing the cure rate, reducing mortality, time to atropinization, time to AchE recovery,IMS rate and total ADR. Nevertheless, current results are based on studies of relatively poor methodological quality and small sample sizes. Despite the favorable outcomes these studies reported, a single-center RCT cannot provide enough additional evidence for the use of penehyclidine in OP poisoning patients. We believe that our updated meta-analysis provides the current best evidence for the use of penehyclidine in conjunction with atropine on OP poisoning patients, and a future multicenter, large scale RCT is still needed to best determine the effectiveness, economics and proper dosing protocol of penehyclidine combined with atropine therapy for OP poisoning patients.
CONCLUSION
Penehyclidine combined with atropine for OP poisoning patients is likely to improve mortality and overall clinical condition. Future high-quality multicenter RCTs are still needed to determine best administration procedures for these drugs.
Funding:None
Ethical approval:Not needed.
Conflicts of interests:The authors declare no competing interests.
Contributors:SYY, RJX and YXG searched the database,collected the data and performed the meta-analysis. XL, YXH,AYY and JT also searched the database and helped prepare the manuscript; YL, NT, LZ and JW reviewed and revised the manuscript.
REFERENCESS
1 Perera PM, Jayamanna SF, Hettiarachchi R, Abeysinghe C,Karunatilake H, Dawson AH, et al. A phase II clinical trial to assess the safety of clonidine in acute organophosphorus pesticide poisoning. Trials. 2009;10: 73.
2 Lin TL. Atropine, penehyclidine hydrochloride combined with blood perfusion on severe organophosphorus poisoning patients.Chin J Mod Drug Appl. 2016;10(12):156-7.
3 Chen W, Huang ML, Wei SC. Rate of delirium between Penehyclidine and atropine therapy on acute organophosphorus poisoning. Chin J Mod Med. 2016;2 (11): 68-71.
4 Wu HK. Clinical observation of penehyclidine hydrochloride treating 42 cases of severe acute organophosphorus patients.Heilongjiang Med J. 2016;40(10):928-29.
5 Li M. Consensus on diagnosis and management of acute poisoning. Chinese Journal of Hygiene Rescue (Electronic Edition). 2016;12(6):333-47.
6 Ai L. Administration of combined anticholinergic agents on acute organophophours poisoning patients. Journal of Nursing.2015;2(5): 91-3.
7 Katz FS, Pecic S, Schneider L, Zhu Z, Hastings A, Luzac M,et al. New therapeutic approaches and novel alternatives for organophosphate toxicity. Toxicol Lett. 2018;291:1-10.
8 Brvar M, Chan MY, Dawson AH, Ribchester RR, Eddleston M. Magnesium sulfate and calcium channel blocking drugs as antidotes for acute organophosphorus insecticide poisoning - a systematic review and meta-analysis. Clin Toxicol (Phila). 2018;56(8):725-36.
9 Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium - theory, evidence and practice. Br J Clin Pharmacol. 2016;81(3):516-24.
10 Fu SZ, Yang LY, Tan Y, Dong H. Comparison between penehyclidine and atropine on rescuing severe acute organophosphorus poisoning patients. 11th National Conference on Disaster Medicine with Integrated Traditional Chinese Medicine and Western Medicine. P2.
11 Zhao JS. Clinical analysis of penehyclidine hydrochloride injection combined with atropine in the treatment of acute severe organophophorus pesticide poisoning. World Notes on Antibiotics. 2015;36 (4):191-2.
12 Guo Y, Wei M, Yan Z, Wang G. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury in rats. Molecular Medicine Journal. 2017;33(11):1486-90.
13 Liang CK, He SF, Li WS. Clinical analysis of Penehyclidine combined with atropine in the resuscitation procedure of acute severe organophophorus poisoning. Contemporary Medicine.2014;20(19):86-7.
14 Sun LS. Clinical outcomes and prognosis of penehyclidine hydrochloride combined with atropine on treating acute organophosphorus pesticide poisoning patients. Chian Modern Medicine. 2012;19(21):102-4.
15 Li CM. Study of management on severe acute organophosphorus pesticide poisoning. China Pharmacology. 2015;20 (17): 95-6.
16 Sun L, Li GQ, Yan PB, Liu Y, Li GF, Wei LQ. Clinical management of organophosphate poisoning in pregnancy. Am J Emerg Med. 2015;33(2): 305.e1-3.
17 Chen JY, Duan B, Liu JY. Outcomes of penehyclidine hydrochloride and atropine in treating acute organophosphorus pesticide poisoning: A meta-analysis. Clinical Misdiagnosis &Mistherapy. 2012;7 (5):71-4.
18 Zeng P, Lei LH, Wang WP. Clinical observation of Penehyclidine treating severe organophosphorus poisoning and analysis of 62 cases. Fujian Medical Journal. 2011;33 (4):126-7.
19 Liu YB, Huang YH, Chen XZ, Zeng MH. Comparison between penehyclidine and atropine of outcomes on resuscitation procedure of acute severe organophosphorus poisoning. Medical Innovation of China. 2012;9 (14): 46-7.
20 Chen BX. Outcomes of penehyclidine combined with atropine on treating severe acute organophosphorus poisoning. Guide of China Medicine. 2011;15(9):225-6.
21 Guan HY. Outcomes of penehyclidine combined with atropine in treating severe organophosphorus poisnoing. World Latest Medicine Information. 2015;15(53):62.
22 Wang LX. Outcomes of penehyclidine combined with atropine on treating severe organophosphorus pesticide poisoning. Chin J Mod Drug Appl. 2010;11(4):121-2.
23 Yi F, Lu HH, Wang Y, Peng K, Chen P, Tang B. Clinical observation of penehyclidine combined with atropine on treating severe organophosphorus pesticide poisoning. Journal of Lingnan Emergency Medicine. 2010;15(2):133-5.
24 Luo SH. Clinical outcomes of Penehyclidine combined with atropine on treating severe organophosphorus pesticide poisoning. Medicine. 2014;8(9):415-6.
25 Shi J. Application of penehyclidine hydrochloride in severe organophosphorus poisoning. Journal of Clinical Emergency Call (China). 2012;13(2):132-3.
26 Zhou JW, Xue Y, Zhang RG, Yao L, Tian ZY, Du GJ. Clinical observation of penehyclidine hydrochloride combined with atropine in the treatment of acute severe organophosphorus poisoning. Occupation and Health. 2012;28(15):1918-20.
27 Liu M, Liang XG. Clinical outcomes of penehyclidine hydrochloride in rescuing severe organophosphorus poisoning.Modern Journal of Integrated Traditional Chinese and Western Medicine. 2011;20 (30): 3816-7.
28 Xue F. Outcomes of Penehyclidine combined with atropine in treating acute severe organophosphorus pesticide poisoning patients. Chinese Folk Medicine. 2010;12(7): 180-9.
29 Mew EJ, Padmanathan P, Konradsen F, Eddleston M, Chang SS, Phillips MR, et al. The global burden of fatal self-poisoning with pesticides 2006-15: Systematic review. J Affect Disord.2017;219:93-104.
30 Moffatt A, Mohammed F, Eddleston M, Azher S, Eyer P,Buckley NA. Hypothermia and fever after organophosphorus poisoning in humans--a prospective case series. J Med Toxicol.2010;6(4):379-85.
31 Jayasinghe SS, Pathirana KD, Buckley NA. Effects of acute organophosphorus poisoning on function of peripheral nerves: a cohort study. PLoS One. 2012;7(11):e49405.
32 Yuan QH, Xiao F, Liu QS, Zheng F, Shen SW, He QW, et al. M 3 receptor is involved in the effect of penehyclidine hydrochloride reduced endothelial injury in LPS-stimulated human pulmonary microvascular endothelial cell. Pulm Pharmacol Ther.2018;48(2):144-50.
33 Yu C, Wang J. Neuroprotective effect of penehyclidine hydrochloride on focal cerebral ischemia-reperfusion injury.Neural Regen Res. 2013;8(7):622-32.
34 Shu Y, Yang Y, Zhang P. Neuroprotective effects of penehyclidine hydrochloride against cerebral ischemia/reperfusion injury in mice. Brain Res Bull. 2016;121:115-23.
35 Wang J, Ren Y, Zhu Y, Chen JW, Zhu MM, Xu YJ, et al. Effect of penehyclidine hydrochloride on the incidence of intra-operative awareness in Chinese patients undergoing breast cancer surgery during general anaesthesia. Anaesthesia. 2013;68(2):136-41.
36 Cao HJ, Sun YJ, Zhang TZ, Zhou J, Diao YG. Penehyclidine hydrochloride attenuates the cerebral injury in a rat model of cardiopulmonary bypass. Can J Physiol Pharmacol. 2013;91(7):521-7.
37 Pandit JJ, Picton P, Mashour GA. Penehcyclidine and awareness during anaesthesia: caution with zero numerators. Anaesthesia.2013;68(2):131-5.
38 Wang YP, Li G, Ma LL, Zheng Y, Zhang SD, Zhang HX, et al. Penehyclidine hydrochloride ameliorates renal ischemiareperfusion injury in rats. J Surg Res. 2014;186(1):390-7.
39 Xiao HT, Liao Z, Tong RS. Penehyclidine hydrochloride:a potential drug for treating COPD by attenuating Toll-like receptors. Drug Des Devel Ther. 2012;6:317-22.
40 Shadnia S, Zamani N, Hassanian-Moghaddam H, Shafaroodi H,Padandar M, Rezaeizadeh MH. Prognostic value of cortisol and thyroid function tests in poisoned patients admitted to toxicology ICU. World J Emerg Med. 2018;9(1):51-5.
41 Eddleston M, Dawson A, Karalliedde L, Dissanayake W,Hittarage A, Azher S, et al. Early management after selfpoisoning with an organophosphorus or carbamate pesticide - a treatment protocol for junior doctors. Crit Care. 2004;8(6):R391-7.
42 Wang W, Chen QF, Li QB, Wu YB, Chen K, Chen B, et al.Efficiency of anisodamine for organophosphorus-poisoned patients when atropinization cannot be achieved with high doses of atropine. Environ Toxicol Pharmacol. 2014;37(2):477-81.
 World journal of emergency medicine2020年1期
World journal of emergency medicine2020年1期
- World journal of emergency medicine的其它文章
- Surgical closure of large splenorenal shunt may accelerate recovery from hepato-pulmonary syndrome in liver transplant patients
- Epidemiological characteristics and disease spectrum of emergency patients in two cities in China: Hong Kong and Shenzhen
- Mobile technology: Usage and perspective of patients and caregivers presenting to a tertiary care emergency department
- Retrospective analysis of eFAST ultrasounds performed on trauma activations at an academic level-1 trauma center
- A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function
- Admission delay is associated with worse surgical outcomes for elderly hip fracture patients: A retrospective observational study
