火龙果采后病原菌的分离鉴定及抑制效应研究
胡翠平 黄慧燕 周桂
摘要:【目的】分離鉴定广西采后火龙果果实上的病原真菌,明确病原菌菌株对季铵化壳聚糖(HTCC)的敏感特性,为火龙果果实的采后病害防治提供科学依据。【方法】采用常规组织分离法从采后贮藏自然发病的火龙果果实上分离病原菌,经致病性鉴定后,依据菌株形态学特性和rDNA-ITS序列分析,确定病原真菌的分类地位。采用菌丝生长速率法和PI染色法,观察HTCC对病原真菌菌丝生长及菌丝细胞膜的影响。【结果】从采后贮藏自然发病的白心火龙果果实上分离到1株丝状真菌菌株E;致病性测定结果表明,菌株E为火龙果采后病害病原真菌;经形态学特性和rDNA-ITS序列分析,将菌株E鉴定为新月弯孢菌(Curvularia lunata)。HTCC对菌株E的作用结果显示,HTCC对菌株E的菌丝生长具有较强抑制作用,其抑制作用与HTCC浓度呈正相关,0.25、0.50、0.75和1.00 mg/mL HTCC对菌株E的抑菌率分别为20.84%、50.05%、72.91%和83.23%;HTCC处理能破坏菌株E的菌丝细胞膜通透性,降低细胞膜的完整性。【结论】新月弯孢菌能引起广西采后火龙果发生真菌病害,依据HTCC对该菌的抑制效果,火龙果采后流通过程中可使用HTCC来减轻病害发生。
关键词: 火龙果;采后病害;真菌;rDNA-ITS序列;季铵化壳聚糖;细胞膜
中图分类号: S436.679 文献标志码: A 文章编号:2095-1191(2020)07-1560-08
Abstract:【Objective】Isolation and identification of pathogenic fungi from postharvest pitaya in Guangxi and identification of the sensitive characteristics of the purified strains to quaternized chitosan(HTCC), so as to provide theoretical basis for the identification and control of postharvest fungal diseases of pitaya. 【Method】The fungi on the fruit of pitaya were isolated and purified from pitaya fruits which were naturally diseased after post harvest storage by common tissue isolation method. After pathogenicity identification, the taxonomic status of the fungi was determined according to the morphological characteristics and rDNA-ITS sequence of the strains. At the same time, the effects of HTCC on mycelial growth and cell membrane of pathogenic fungi were observed by the methods of mycelial growth rate and PI staining. 【Result】A filamentous fungus E was isolated from white pitaya fruits which were naturally diseased after post harvest storage. The results of pathogenicity test showed that strain E was pitaya post harvest disease pathogenic fungus, which was identified as Curvularia lunata by morphological characteristics analysis and rDNA-ITS sequence. The results showed that HTCC had a strong inhibitory effect on the mycelial growth of fungus E in a dose-dependent manner, and the inhibition rates of 0.25,0.50,0.75 and 1.00 mg/mL HTCC were 20.84%,50.05%,72.91% and 83.23%; HTTC treatment could destroy the membrane permeability and reduce the integrity of the membrane of fungus E. 【Conclusion】C. lunata can cause post harvest pitaya fungus disease in Guangxi. According to the inhibition effect of HTCC on the disease, HTCC can be used to reduce the occurrence of the disease in the process of post harvest circulation.
Key words: pitaya; post harvest disease; fungus; rDNA-ITS sequence; quaternized chitosan; cell membrane
Foundation item: Guangxi Natural Science Foundation(2014GXNSFAA118076); Project of Guangxi Scientific Research and Technology Development(14125008-1-2); Key Project of Science and Technology Research in Guangxi Universities(ZD2014041)
0 引言
【研究意义】火龙果(Hylocereus spp.)别称红龙果、青龙果、仙密果等,是一种仙人掌科量天尺属植物,原产于中美洲地区,20世纪90年代初引入我国台湾试种后陆续在广东、海南、福建和广西等地推广,是一种被广泛关注的热带亚热带果树(许伟东等,2002;王壮等,2014;张振华等,2019)。火龙果果实营养丰富,功用独特,除富含糖、维生素、蛋白质、氨基酸、脂肪酸及矿质元素等营养成分外,还含有多种功能性物质,如类黄酮、白蛋白、膳食纤维、色素、甾醇和苷类化合物,具有预防疾病、增强免疫力、调节激素及解毒等功效,是一种具有营养和药用价值的绿色保健水果(纵伟等,2007;蔡永强等,2008;王彬等,2009;徐慧等,2010)。由于火龙果潜在的利用价值,我国火龙果的种植规模不断扩大。广西地处亚热带季风气候区,具有适宜火龙果生长的温度、光照、降水和土壤等条件,火龙果产业发展迅猛,目前广西已成为我国火龙果种植面积最大的区域(黄艳芳等,2017)。但火龙果采后极易腐烂变质,一方面是由于火龙果呼吸代谢旺盛,自然衰老;另一方面则因微生物侵染,火龙果采后病害发生较严重,其中真菌病害最普遍(林珊宇等,2018)。因此,开展火龙果采后病害及其防治研究,对广西火龙果产业的可持续发展具有重要意义。【前人研究进展】火龙果常见病害主要有炭疽病、溃疡病、黑斑病、根霉病和果腐病等。Taba等(2007)研究发现仙人掌平脐蠕孢(Bipolaris cactivora)能引起火龙果腐烂。Hawa等(2009)首次报道新月弯孢菌(Curvularia lunata)可引起马来西亚红肉火龙果病害。崔志婧等(2011)研究结果表明尖孢镰刀菌(Fusarium oxysporum)和单隔镰刀菌(F. dimerum)是引起上海市进口火龙果软腐病的病原真菌。Guo等(2013)研究发现平头炭疽菌(Colletotrichum truncatum)可引起火龙果炭疽病,是我国首次报道该菌株引起的火龙果炭疽病。郭力维等(2014)首次发现引起火龙果采后果腐病的桃吉尔霉(Gilbertella persicaria)。胡美姣等(2015)鉴定出2种火龙果采后病害病原,即平头炭疽菌和单隔镰刀菌。姚昇华等(2015)研究结果表明仙人掌平脐蠕孢是引起上海市进口越南红心火龙果黑腐病的病原。Oeurn等(2015)从泰国洛伊火龙果果实上分离到仙人掌平脐蠕孢、胶孢炭疽菌(C. gloeosporiodes)和匍枝根霉(Rhizopus stolonifer)3种真菌,致病性试验确定3种真菌均能使采后火龙果发生病害。王会会等(2016)认为新暗色柱节孢是火龙果溃疡病致病菌。李国林等(2018)用电子鼻检测到桃吉尔霉侵染火龙果果实后气味的变化,表明桃吉尔霉可劣化火龙果品质。林珊宇等(2018)首次发现引起火龙果软腐病的木贼镰刀菌(F. equiseti)。目前对火龙果采后病害防治的方法有低温贮藏、热处理、辐射保鲜、化学药剂处理及气体熏蒸处理等(崔志婧等,2011;李敏等,2012;朱迎迎等,2014;Ngoc et al.,2017),物理防治成本较高,化学防治则使病原菌产生抗药性,药剂残留还会危害人体健康(杜彩莲等,2018)。随着人们对食品安全的重视,开发新型的绿色生物农药代替传统杀菌剂已成为当前研究热点之一。壳聚糖作为迄今为止在自然界中发现的唯一阳离子碱性多糖,不仅无毒安全、可生物降解,还具有广谱的抗菌性能,同时能促进植物宿主防御反应,对多种植物病害具有防治作用(Bautista-Ba?os et al.,2006;Wang et al.,2014;Kaur et al.,2018)。然而,壳聚糖溶解性较差,只溶于酸性溶液,限制了其应用范围。季铵化改性可有效提高壳聚糖水溶性。研究表明,季铵化壳聚糖(Quaternized chitosan,HTCC)比壳聚糖具有更好的抑菌作用,可用于控制一些植物病原菌(Guo et al.,2007;Badawy,2010;Badawy and Rabea,2014;Liu et al.,2018)。壳聚糖及其衍生物因其优异的性能,在果蔬贮藏保鲜和植物病害防治方面應用前景广阔。【本研究切入点】广西火龙果种植面积大,但采后火龙果真菌病害较普遍,严重制约其产业发展。目前,有关火龙果采后病害防治研究相对较少。【拟解决的关键问题】采用常规组织分离法从采后贮藏自然发病的火龙果果实上分离病原菌,经致病性测定后,结合菌株形态学特性和rDNA-ITS序列分析,确定病原真菌分类地位,并进一步测定HTCC对火龙果病原真菌的抑制效应,以期为火龙果果实的采后病害防治提供理论与实践依据。
1 材料与方法
1. 1 试验材料
病原菌菌株与培养基:病原菌菌株于采后贮藏自然发病的白心火龙果果实上分离获得;PDA培养基购自北京索莱宝科技有限公司。主要试剂及仪器:DL2000 Plus DNA Ladder和2×Taq PCR Master Mix购自Biomiga公司;HTCC购自酷尔化学科技(北京)有限公司;碘化丙锭溶液(1 mg/mL)、活性氧检测试剂盒和ddH2O购自北京索莱宝科技有限公司;通用引物ITS1和ITS4由深圳华大基因科技有限公司合成;PCR仪购自Biometra公司;电泳仪和电泳槽购自Bio-Rad公司;荧光显微镜购自Olympus公司。
1. 2 试验方法
1. 2. 1 病原菌分离纯化 选取火龙果病果,参照常规组织分离法分离病原真菌(张成玲等,2019),取病健交界处组织块,用70%酒精和2%次氯酸钠溶液进行表面消毒后放入PDA培养基中,28 ℃恒温培养,待菌落长出后用无菌竹签挑取边缘菌丝植入另一PDA培养基内,28 ℃继续培养;经3次继代培养可得纯化菌种,将其接种于PDA斜面培养基,并置于4 ℃冰箱保存备用。
1. 2. 2 病原菌致病性能鉴定 参照许玲等(2003)的有伤接种方法进行菌株致病性鉴定。用70%酒精对火龙果果实进行表面消毒,再用无菌蒸馏水冲洗后晾干。用灭菌竹签在火龙果果实表面刺破5个孔,使孔眼集中在直径约5 mm的范围内,用5 mm无菌打孔器取纯化后的真菌块,将其反贴于伤口处,室温培养,以PDA培养基作为空白对照,定期观察火龙果接种处是否有病害症状出现。
1. 2. 3 病原菌形态学观察 用无菌竹签挑取纯化后病原真菌菌丝接种于PDA培养基上,28 ℃恒温培养,观察菌落形态、菌丝疏密性、颜色等特征。荧光显微镜下观察菌株菌丝、产孢结构和孢子形态等特征。
1. 2. 4 病原菌rDNA-ITS鉴定 用简易微波炉法提取DNA(潘力等,2010)。利用真菌通用引物ITS1(5'-TCCGTAGGTGAACCTGCGG-3')和ITS4(5'-TCCTCCGCTTATTGATATGC-3')进行PCR扩增。PCR反应体系20 μL:2×Taq PCR Master Mix 10 μL,10 μmol/L ITS1和ITS4引物各1 μL,DNA模板1 μL,ddH2O 7 μL。扩增程序:94 ℃预变性10 min;94 ℃ 30 s,55 ℃ 45 s,72 ℃ 1 min,进行35个循环;72 ℃延伸10 min。扩增产物经1%琼脂糖凝胶电泳检测后送至北京擎科新业生物技术有限公司进行纯化、测序。测序结果在NCBI上进行BLAST比对,选取同源性较高及形态相近的菌株序列,用MEGA 7.0中的邻接法(Neighbor-joining,NJ)构建其系统发育进化树,使用Bootstraps进行自检,1000次重复。
1. 2. 5 HTCC对病原真菌菌丝生长的抑制作用 参考Jia等(2016)的方法进行测定,配制含不同浓度HTCC的PDA培养基,121 ℃高压灭菌15 min后倒平板,以不含HTCC的PDA培养基作为空白对照。PDA培养基凝固后,在28 ℃培养7 d的菌株边缘,用无菌打孔器打下直径5 mm的菌饼,反贴于PDA培养基中央,Parafilm封口膜密封,28 ℃培养5 d后,用游标卡尺测量菌落直径。
1. 2. 6 HTCC对病原真菌菌丝细胞膜完整性的影响
参考Ouyang等(2018)的方法对病原菌菌丝进行碘化丙锭(Propidium iodide,PI)染色。无菌打孔器取直径5 mm的菌饼反贴于HTCC浓度为0.5 mg/mL的PDA培养基上,无菌水作对照,28 ℃培养3 d后,于菌落边缘斜插无菌盖玻片,继续培养2 d,取出盖玻片,置于洁净载玻片上,滴加50 μg/mL的PI染色液避光染色10 min后,蒸馏水洗去多余染色液,荧光显微镜下观察荧光强度。
1. 3 统计分析
试验数据利用Excel 2007和SPSS 23.0进行整理、绘图和统计分析。
2 结果与分析
2. 1 病原菌分离及致病性测定结果
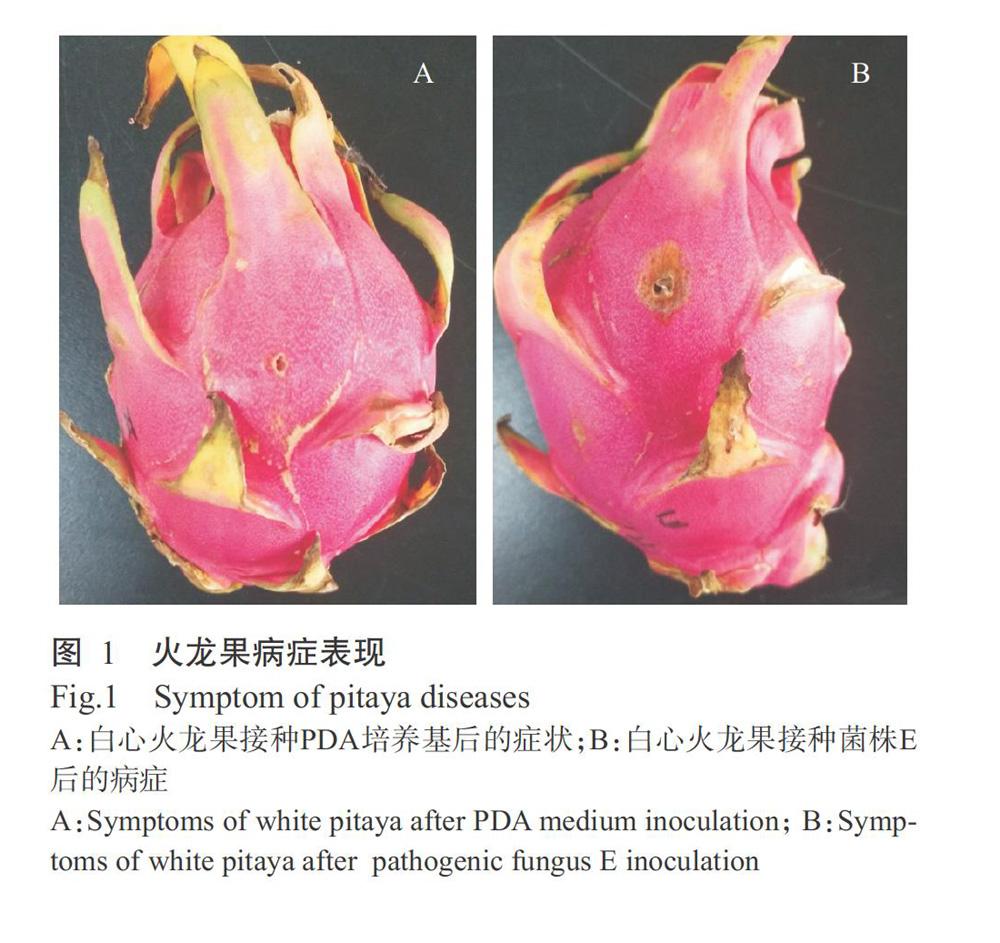
采用常规组织分离法从采后贮藏自然发病的白心火龙果果实上分离到1株丝状真菌,将其命名为E。采用有伤接种方法将菌株E回接至健康白心火龙果果实,结果显示,空白对照伤口无明显病害症状出现(图1-A),而回接菌株E的伤口出现略凹陷的病斑(图1-B)。由此确定菌株E为火龙果采后病害病原真菌。
2. 2 病原菌形态特征观察结果
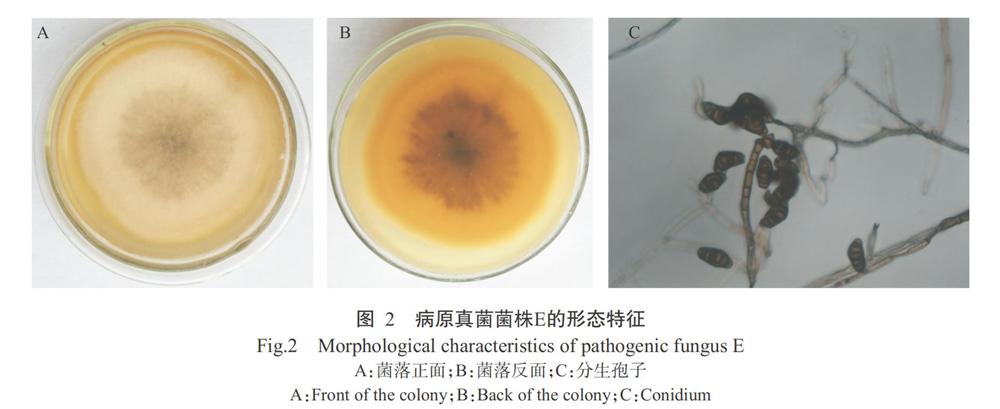
菌株E在PDA培养基上生长旺盛,菌落均匀、近似圆形,初期为白色,后菌落中心逐渐变为灰黑色,菌丝绒状致密(图2-A和图2-B)。在光学显微镜下观察,该菌株菌丝透明,分枝,有隔膜;分生孢子梗丛生或散生;分生孢子呈褐色,梭形或近椭圆形,直立或弯曲,有隔膜,无缢缩(图2-C)。
2. 3 rDNA-ITS同源性比对及系统发育分析结果
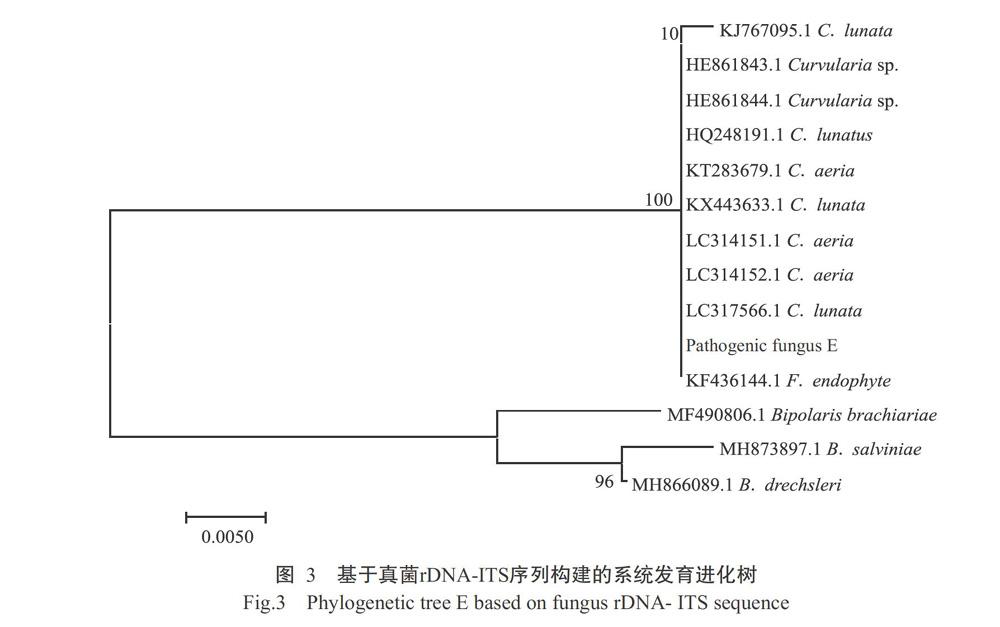
测序结果表明,菌株E的rDNA-ITS序列大小为556 bp,将其上传至NCBI进行BLAST比对,结果(图3)显示,菌株E的rDNA-ITS与C. lunata(KJ767095.1/KX443633.1/LC317566.1)、Curvularia sp.(HE86184 3.1/HE861844.1)、Cochliobolus lunatus(HQ248191.1)、C. aeria(KT283679.1/LC314151.1/LC314152.1)和Fungal endophyte(KF436144.1)的同源性均在99.0%以上。基于真菌rDNA-ITS序列構建的系统发育进化树也显示,病原真菌菌株E与C. lunata、Curvularia sp.、C. lunatus、C. aeria和F. endophyte在同一分支。结合菌株E的形态学特征并参考前人的相关研究(Avasthi et al.,2015),将其鉴定为新月弯孢菌(C. lunata)。
2. 4 HTCC对菌株E菌丝生长的抑制作用
如图4所示,HTCC能抑制菌株E的菌丝生长,其抑制作用与HTCC浓度呈正相关,0.25、0.50、0.75和1.00 mg/mL HTCC对菌株E的抑菌率分别为20.84%、50.05%、72.91%和83.23%,均与CK(0.0 mg/mL)差异显著(P<0.05)。
2. 5 HTCC对菌株E菌丝细胞膜完整性的影响
PI本身不荧光,但与细胞内DNA结合后,在绿色激发光下能发出红色荧光,当完整的细胞膜受损后,PI进入细胞内并与核酸结合发出荧光,其荧光强度能反映出细胞膜完整性。如图5所示,未经处理的菌丝,暗场下基本无红色荧光产生,而经0.50 mg/mL HTCC处理过的菌丝,则显示出较强烈的红色荧光。表明HTCC处理后病原真菌菌株E的菌丝细胞膜完整性受损,使PI进入胞内与核酸结合,故荧光强度增强。
3 讨论
查阅文献发现,火龙果采后常见的病原真菌有仙人掌平脐蠕孢(Taba et al.,2007;姚昇华等,2015;Oeurn et al.,2015)、尖孢镰刀菌(崔志婧等,2011),单隔镰刀菌(崔志婧等,2011;胡美姣等,2015)、桃吉尔霉(郭力维等,2014;李国林等,2018)、平头炭疽菌(胡美姣等,2015)、胶孢炭疽菌(Oeurn et al.,2015)和匍枝根霉(Oeurn et al.,2015)等。本研究从发病的白心火龙果果实上分离得到1株病原真菌,经形态学特性、rDNA-ITS序列分析,将其鉴定为新月弯孢菌,是首次发现新月弯孢菌引起我国火龙果采后病害。此外,Hawa等(2009)也发现新月弯孢菌是马来西亚红肉火龙果的病原真菌。
在我国,由新月弯孢菌引起的玉米弯孢叶斑病(Curvularia leaf spot of maize)是一种严重的玉米病害,对我国玉米生产造成巨大影响(Liu et al.,2015;常佳迎等,2019)。新月弯孢菌具有广泛的寄主范围,包括膜稃草(Monteiro et al.,2003)、小麦(Iram and Ahmad,2006)、结缕草(Roberts and Tredway,2008)、黑麦草(Tian et al.,2008)、草莓(Verma and Gupta,2010)、菠菜(Pandey et al.,2011)、高粱(Panchal and Dhale,2011)、莲花(Cui and Sun,2012)、水稻(Liu et al.,2014;杨小林等,2019)和番茄(Iftikhar et al.,2016)等。与大多数病原真菌相似,新月弯孢菌侵染植物的过程包括附着、萌发、感染结构形成、宿主渗透和宿主组织定殖,已证实多种毒力因子参与其感染过程,如黑色素、毒素、细胞壁降解酶、角质酶和激素(Gao et al.,2014b)。Liu等(2016a)通过研究新月弯孢菌中大量的角质酶基因,认为其产生的角质酶能降解不同寄主植物不同类型的角质层,可能是新月弯孢菌拥有广泛寄主的原因之一。致病基因是疾病发展的必要条件,在了解新月弯孢菌的相关毒力因子后,科学家开始致力于研究一些毒力相关基因,如角质酶基因ClCUT7参与发病过程,在发病早期,其转录水平逐渐升高,ClCUT7基因缺失时,其致病性降低(Liu et al.,2016a);丝裂原活化蛋白激酶(MAPK)基因Clk1参与菌丝生长、细胞壁降解酶生物合成的正调控,Clk1功能域缺乏,影响分生孢子的产生,但有形成厚垣孢子状结构的倾向,Clk1突变体的致病性降低(Gao et al.,2013);Ras基因的同源基因Clg2p对新月弯孢菌附着孢的形成和致病性至关重要,Clg2p通过其RA结构域与clf相互作用,在clf上游调控附着孢的形成和致病性(Liu et al.,2016b);Clt-1基因与毒素的产生和致病性密切相关(Gao et al.,2014a);Brn1基因参与1,8-二羟基萘黑色素的合成(Liu et al.,2011)。
壳聚糖是甲壳素脱乙酰化产物,是自然界最丰富的生物聚合物之一,具有无毒安全、环境兼容性好、可生物降解、可再生、生物活性和来源广泛等优点。壳聚糖不仅可抑制多种植物病原菌的生长,还可诱导植物产生防御反应,对多种植物病害具有防治作用。壳聚糖处理能提高番茄植株对尖孢镰刀菌引起的根腐病和冠腐病的抗性(Benhamou and Thériaul,1992)。Meng等(2010)報道350 kD壳聚糖能抑制梨黑斑病菌和轮纹病菌的菌丝生长和孢子萌发,并使梨果实过氧化物酶活性显著提高。壳聚糖可通过诱导花生种子产生更多的酚类化合物来限制黄曲霉生长和黄曲霉毒素B1产生(Fajardo et al.,1995)。Liu等(2007)研究表明,壳聚糖能防治番茄果实采后病害,能显著提高番茄果实中多酚氧化酶、过氧化物酶活性和酚类物质的含量。
壳聚糖线型分子链上含有氨基、羟基和乙酰基等功能基团,使其表现出独特的物理、化学和生物特性,如抗菌性、生物相容性及生物可降解性等(Ali et al.,2010;Deng et al.,2010)。然而,壳聚糖溶解性较差,只溶于酸性溶液,限制了其应用范围。对壳聚糖进行物理、化学和生物改性,如酶催化(Sun and Payne,2000)、共混(Dunia et al.,2008; Abdelghany et al.,2013 )、交联(Wang et al.,2013)、衍生化(Luan et al.,2018)及酸解(Zhang et al.,2018)等,可增强其的溶解性、抗菌性、抗病毒、抗肿瘤、吸附性和螯合性等,因而在食品、生物医药和环境等领域具有广泛的应用前景。
4 结论
从采后自然发病的白心火龙果果实上分离得到1株病原真菌菌株E,经形态学特征和分子生物学分析,确定其为新月弯孢菌。HTCC对该病原真菌的菌丝生长具有较强的抑制作用,并呈剂量依赖性,火龙果采后流通过程中可使用HTCC来减轻病害发生。
参考文献:
常佳迎,刘树森,马红霞,石洁,郭宁,张海剑. 2019. 黄淮海地区夏玉米弯孢叶斑病菌遗传多样性分析[J]. 中国农业科学,52(5):822-836. [Chang J Y,Liu S S,Ma H X,Shi J,Guo N,Zhang H J. 2019. Genetic diversity analysis of Curvularia lunata in summer maize in Huang-Huai-Hai Region[J]. Scientia Agricultura Sinica,52(5):822-836.]
蔡永強,向青云,陈家龙,彭玉基,王彬. 2008. 火龙果的营养成分分析[J]. 经济林研究,26(4):53-56. [Cai Y Q,Xiang Q Y,Chen J L,Peng Y J,Wang B. 2008. Analysis of nutritional components in pitaya fruit[J]. Nonwood Forest Research,26(4):53-56.]
杜彩莲,张灿,袁芳,李佳颖,孙廷飞,乐扬,Shaukat Ali. 2018. 温度、pH和培养基对长毛拟青霉菌株SP053生长和繁殖的影响[J]. 南方农业学报,49(12):2447-2453. [Du C L,Zhang C,Yuan F,Li J Y,Sun T F,Yue Y,Shaukat Ali. 2018. Effects of temperature,pH and culture media on growth and sporulation of Paecilomyces penicillatus strain SP053[J]. Journal of Southern Agriculture,49(12):2447-2453.]
崔志婧,王奕文,于岳,许玲. 2011. 上海市进口火龙果软腐病病害分析[J]. 微生物学通报,38(10):1499-1506. [Cui Z J,Wang Y W,Yu Y,Xu L. 2011. Pathogens analysis of soft rot disease of imported pitaya in Shanghai[J]. Microbiology China,38 (10):1499-1506.]
郭力维,吴毅歆,何汉兴,毛自朝,何鹏博,何月秋. 2014. 云南省火龙果采后果腐病研究(英文)[J]. 果树学报,31(1):111-114. [Guo L W,Wu Y X,He H X,Mao Z C,He P B,He Y Q. 2014. A new fruit rot disease in Hylocereus costaricensis in Yunnan Province of China[J]. Journal of Fruit Science,31(1):111-114.]
胡美姣,高兆银,李敏,朱迎迎,陈亮. 2015. 火龙果2种采后病害病原菌的鉴定及生物学特性研究[C]//中国热带作物学会. 中国热带作物学会第九次全国会员代表大会暨2015年学术年会论文摘要集. [Hu M J,Gao Z Y,Li M,Zhu Y Y,Chen L. 2015. Identification and biological characteristics of two pathogens of postharvest diseases of pitaya[C]//China Tropical Crop Society. Proceedings of the 9th National Meeting and 2015 Academic Annual Meeting of China Tropical Crop Society.]
黄艳芳,秦媛媛,阮晓静,兰宗宝. 2017. 广西火龙果产业发展现状及可持续发展对策研究[J]. 热带农业科学,37(6):97-99. [Huang Y F,Qin Y Y,Ruan X J,Lan Z B. 2017. Present situation and countermeasures of sustainable development of pitaya industry in Guangxi[J]. Chinese Journal of Tropical Agriculture,37(6):97-99.]
李国林,孟繁博,郑秀艳,黄道梅,陈曦,林茂. 2018. 红肉火龙果贮藏期间气味监测及桃吉尔霉对气味的影响[J]. 食品安全质量检测学报,9(18):74-78. [Li G L,Meng F B,Zheng X Y,Huang D M,Chen X,Lin M. 2018. Odor monitoring during storage of red pitaya and effect of Gilbertella persicariaon odor[J]. Journal of food safety and quality inspection,9(18):74-78.]
李敏,胡美姣,高兆银,张正科,薛丁榕,杨冬平,杨波. 2012. 海南火龙果采后病害调查及防治技术研究[J]. 中国热带农业,(6):42-44. [Li M,Hu M J,Gao Z Y,Zhang Z K,Xue D R,Yang D P,Yang B. 2012. Investigation and control of postharvest diseases of pitaya in Hainan[J]. China Tropical Agriculture,(6):42-44.]
林珊宇,贤小勇,韦小妹,韦继光,朱桂宁. 2018. 广西火龙果采后病害主要病原菌分离与鉴定[J]. 中国南方果树,47(2):6-12. [Lin S Y,Xian X Y,Wei X M,Wei J G,Zhu G N. 2018. Isolation and identification of pathogens for the postharvest diseases in pitaya fruits[J]. South China Fruits,47(2):6-12.]
潘力,崔翠,王斌. 2010. 一种用于PCR扩增的丝状真菌DNA快速提取方法[J]. 微生物学通报,37(3):450-453. [Pan L,Cui C,Wang B. 2010. Rapid extraction of filamentous fungal DNA for PCR amplification[J]. Microbiology China,37(3):450-453.]
王彬,蔡永强,郑伟. 2009. 火龙果果实氨基酸含量及组成分析[J]. 中国农学通报,25(8):210-214. [Wang B,Cai Y Q,Zheng W. 2009. Analysis on the amino acid content and the composition in the pitaya fruit[J]. Chinese Agricultural Science Bulletin,25(8):210-214.]
王会会,符碧海,戴俊,徐倩,王萌,陶挺燕,谢昌平,朱朝华. 2016. 火龙果溃疡病菌的鉴定及室内药剂筛选[J]. 中国南方果树,45(1):8-12. [Wang H H,Fu B H,Dai J,Xu Q,Wang M,Tao T Y,Xie C P,Zhu C H. 2016. Identification of dragon fruit canker pathogen and indoor scree-ning of fungicides[J]. South China Fruits,45(1):8-12.]
王壯,王立娟,蔡永强,周俊良,马玉华. 2014. 火龙果营养成分及功能性物质研究进展[J]. 中国南方果树,43(5):25-29. [Wang Z,Wang L J,Cai Y Q,Zhou J L,Ma Y H. 2014. Present research of the nutritional components and functional substances of pitaya fruit[J]. South China Fruits,43(5):25-29.]
徐慧,王秋玲,韦刚,莫建光. 2010. 火龙果的保健功效及其研究进展[J]. 广西科学院学报,26(3):383-385. [Xu H,Wang Q L,Wei G,Mo J G. 2010. The health benefits and research progress of pitaya[J]. Journal of Guangxi Academy of Sciences,26(3):383-385.]
许玲,李学文,滕康宁. 2003. 果蔬采后致病真菌的检测及其控制[J]. 食品科学,24(7):155-158. [Xu L,Li X W,Teng K N. 2003. Detection and control of postharvest pathogenic fungi in fruits and vegetables[J]. Food Science,24 (7):155-158.]
许伟东,廖剑锹,刘加建,关金顺. 2002. 火龙果引种初报[J]. 中国南方果树,31(1):33-34. [Xu W D,Liao J Q,Liu J J,Guan J S. 2002. Preliminary report on the introduction of pitaya[J]. South China Fruits,31(1):33-34.]
杨小林,韩烨,殷得所,张瑞洋,张佑宏,张舒. 2019. 湖北稻区穗腐病病原ITS鉴定及其生物学特性分析[J]. 河南农业科学,48(4):82-87. [Yang X L,Han Y,Yin D S,Zhang R Y,Zhang Y H,Zhang S. 2019. ITS identification and biological characterization of the pathogens of rice spikelet rot disease in Hubei rice region[J]. Journal of Henan Agricultural Sciences,48(4):82-87.]
姚昇华,范诗睿,邢云莱,何嘉琳. 2015. 越南‘红心火龙果黑腐病病原真菌鉴定及环境因素影响分析[J]. 植物生理学报,51(9):1419-1424. [Yao S H,Fan S R,Xing Y L,He J L. 2015. Pathogen identification and environmental effects analysis of black rot disease of imported Vietna-mese ‘Red pitaya[J]. Plant Physiology Journal,51(9):1419-1424.]
纵伟,刘艳芳,白新鹏. 2007. 火龙果的营养保健成分及加工[J]. 中国食物与营养,(10):46-48. [Zong W,Liu Y F,Bai X P. 2007. Nourishment components and processing of pitaya[J]. Food and Nutrition in China,(10):46-48.]
朱迎迎,陈亮,祝庆刚,张正科,李敏,高兆银,胡美姣. 2014. 火龙果采后病害与防控技术研究进展[J]. 中国热带农业,(4):55-58. [Zhu Y Y,Chen L,Zhu Q G,Zhang Z K,Li M,Gao Z Y,Hu M J. 2014. Present research of postharvest diseases and control technology of pitaya[J]. China Tropical Agriculture,(4):55-58.]
张成玲,孙厚俊,赵永强,杨冬静,徐振,马居奎,谢逸萍. 2019. 甘薯根腐病病原分子鉴定及防治药剂筛选[J]. 江西农业学报,31(8):46-51. [Zhang C L,Sun H J,Zhao Y Q,Yang D J,Xu Z,Ma J K,Xie Y P. 2019. Molecular detection of sweetpotato root rot disease and screening of fungicides[J]. Acta Agriculturae Jiangxi,31(8):46-51.]
張振华,林江,王文雅,许畅,杨开样,何世伟. 2019. 火龙果溃疡病原菌拮抗菌株的筛选与生物防治效果初探[J]. 河南农业科学,48(8):88-94.[Zhang Z H,Lin J,Wang W Y,Xu C,Yang K Y,He S W. 2019. Screening of antagonistic strains of pitaya ulcer pathogen and primary study on biocontrol effects[J]. Journal of Henan Agricultural Sciences,48(8):88-94.]
Abdelghany S M,Schmid D,Deacon J,Jaworski J,Fay F. 2013. Enhanced antitumor activity of the photosensitizer meso-Tetra(N-methyl-4-pyridyl) porphine tetra tosylate through encapsulation in antibody-targeted chitosan/alginate nanoparticle[J]. Biomacromolecules,14(2):302-310.
Ali S W,Joshi M,Rajendran S. 2010. Modulation of size,shape and surface charge of chitosan nanoparticles with reference to antimicrobial activity[J]. Journal of Computational and Theoretical Nanoscience,3(4):452-460.
Avasthi S,Gautam,A. K,Bhadauria R. 2015. Occurrence of leaf spot diseases on(L.) Burm.f. caused by species from Madhya Pradesh,India[J]. Biodiversitas Journal of Biological Diversity,16(1):79-83.
Badawy M E I. 2010. Structure and antimicrobial activity relationship of quaternary N-alkyl chitosan derivatives against some plant pathogens[J]. Journal of Applied Polymer Science,117(2):960-969.
Badawy M E I,Rabea E I. 2014. Synthesis and antifungal property of N-(aryl) and quaternary N-(aryl) chitosan derivatives against Botrytis cinerea[J]. Cellulose,21(4):3121-3137.
Bautista-Ba?os S,Hernandez-Lauzardo A N,Velazquez-Del Valle M G,Hernandez-Lopez M,Ait Barka E,Bosquez-Molina E,Wilson C L. 2006. Chitosan as a potential na-tural compound to control pre and postharvest diseases of horticultural commodities[J]. Crop Protection,25(2):108-118.
Benhamou N,Thériault G. 1992. Treatment with chitosan enhances resistance of tomato plants to the crown and root rot pathogen Fusarium oxysporum f. sp. radicislycopersici[J]. Physiological and Molecular Plant Pathology,41(1):33-52.
Cui R Q,Sun X T. 2012. First report of Curvularia lunata causing leaf spot on lotus in China[J]. Plant Disease,96(7):1068-1068.
Deng H B,Zhou X,Wang X Y,Zhang C Y,Ding B,Zhang Q H,Du Y M. 2010. Layer-by-layer structured polysaccharides film-coated cellulose nanofibrous mats for cell culture[J]. Carbohydrate Polymers,80(2):474-479.
Dunia M,García C,José L,Gomez R,Manuel S S. 2008. Blending polysaccharides with biodegradable polymers. I. Properties of chitosan/polycaprolactone blends[J]. Journal of Biomedical Materials Research. Part B:Applied Biomaterials,85B (2):303-313.
Fajardo J E,Waniska R D,Cuero R G,Pettit R E. 1995. Phenolic compounds in peanut seeds:Enhanced elicitation by chitosan and effects on growth and aflatoxin B1 production by Aspergillus flavus[J]. Food Biotechnology,9(1-2):59-78.
Gao J X,Liu T,Chen J. 2014a. Insertional mutagenesis and cloning of the gene required for the biosynthesis of the non-host-specific toxin in Cochliobolus lunatus that causes maize leaf spot[J]. Phytopathology,104(4):332-339.
Gao S G,Li Y Q,Gao J X,Suo Y J,Fu K H,Li Y Y,Chen J. 2014b. Genome sequence and virulence variation-related transcriptome profiles of Curvularia lunata,an important maize pathogenic fungus[J]. BMC Genomics,15(1):627.
Gao S G,Zhou F H,Liu T,Li Y Y,Chen J. 2013. A MAP kinase gene,Clk1,is required for conidiation and pathogenicity in the phytopathogenic fungus Curvularia lunata[J]. Journal of Basic Microbiology,53(3):214-223.
Guo L W,Wu Y X,Ho H H,Su Y Y,Mao Z C,He P F,He Y Q. 2013. First report of dragon fruit(Hylocereus undatus) anthracnose caused by Colletotrichum truncatum in China[J]. Journal of Phytopathology,162(4):272-275.
Guo Z Y,Xing R,Liu S,Zhong Z M,Ji X,Wang L,Li P C. 2007. Antifungal properties of Schiff bases of chitosan,N-substituted chitosan and quaternized chitosan[J]. Carbohydrate Research,342(10):1329-1332.
Hawa M M,Salleh B,Latiffah Z. 2009. First report of Curvularia lunata on red-fleshed dragon fruit(Hylocereus poly-rhizus) in Malaysia[J]. Plant Disease,93(9):971-971.
Iftikhar S,Shahid A A,Nawaz K,Ali S W. 2016. First report of Curvularia lunata causing fruit rot of tomato(Lycopersicum esculentum) in Pakistan[J]. Plant Disease,100(5):1013.
Iram S,Ahmad I. 2006. Aggressiveness analysis of foliar fungi to leaves of wheat and rice crops[J]. Archives of Phytopathology and Plant Protection,39(6):429-437.
Jia R X,Duan Y F,Fang Q,Wang X Y,Huang J Y. 2016. Pyri-dine-grafted chitosan derivative as an antifungal agent[J]. Food Chemistry,196:381-387.
Kaur P,Duhan J S,Thakur R. 2018. Comparative pot studies of chitosan and chitosan-metal nanocomposites as nano-agrochemicals against fusarium wilt of chickpea(Cicer arietinum L.)[J]. Biocatalysis and Agricultural Biotechnology,14:466-471.
Liu J,Tian S P,Meng X H,Xu Y. 2007. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit[J]. Postharvest Biology and Technology,44(3):300-306.
Liu L M,Huang S W,Wang L,Hou E Q,Xiao D F. 2014. First report of leaf blight of rice caused by Cochliobolus lunatus in China[J]. Plant Disease,98(5):686-686.
Liu T,Hou J M,Wang Y Y,Jin Y Z,Borth W,Zhao F Z,Liu Z,Hu J,Zuo Y H. 2016a. Genome-wide identification,classification and expression analysis in fungal-plant interactions of cutinase gene family and functional analysis of a putative ClCUT7 in Curvularia lunata[J]. Molecular Genetics and Genomics,291(3):1105-1115.
Liu T,Wang Y Y,Ma B C,Hou J M,Jin Y Z,Zhang Y L,Ke X W,Tai L M,Zuo Y H,Dey K. 2016b. Clg2p interacts with Clf and ClUrase to regulate appressorium formation,pathogenicity and conidial morphology in Curvula-ria lunata[J]. Scientific Reports,6(1):24047.
Liu T,Xu S F,Liu L X,Zhou F H,Hou J M,Chen J. 2011. Cloning and characteristics of Brn1 gene in Curvularia lunata causing leaf spot in maize[J]. European Journal of Plant Pathology,131(2):211-219.
Liu T,Zhao F Z,Wang Y Y,Hou J M,Liu L Z,Shen Y Q,Liu Z,Zhang H T,Zuo Y H. 2015. Comparative analysis of phylogenetic relationships,morphologies,and pathogenicities among Curvularia lunata isolates from maize in China[J]. Genetics & Molecular Research,14(4):12537-12546.
Liu W X,Qin Y K,Liu S,Xing R,Yu H H,Chen X L,Li K C,Li P C. 2018. Synthesis,characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups[J]. International Journal of Biological Macromolecules,114:942-949.
Luan F,Wei L J,Zhang J J,Tan W Q,Chen Y,Dong F,Li Q,Guo Z Y. 2018. Preparation and characterization of qua-ternized chitosan derivatives and assessment of their antioxidant activity[J]. Molecules,23(3):516.
Meng X H,Yang L Y,Kennedy J F,Tian S P. 2010. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit[J]. Carbohydrate Polymers,81(1):70-75.
Monteiro F T,Vieira B S,Barreto R W. 2003. Curvularia lunata and Phyllachora sp.:Two fungal pathogens of the grassy weed Hymenachne amplexicaulis from Brazil[J]. Australasian Plant Pathology,32(4):449-453.
Ngoc N K,Phong N V,Tung N T,Hos N V,Woolf A B,Fullerform R A. 2017. Postharvest diseases and effect of hot water treatments on white fleshed dragon fruit [Hyloce-reus undatus(Haw.) Britton & Rose][J]. International Journal of Current Research in Biosciences and Plant Bio-logy,4(5):30-37.
Oeurn S,Jitjak W,Sanoamuang N. 2015. Fungi on dragon fruit in Loei Province,Thailand and the ability of Bipolaris cactivora to cause post-harvest fruit rot[J]. Asia-Pacific Journal of Science and Technology,20(4):405-418.
Ouyang Q L,Tao N G,Zhang M L. 2018. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicillium digitatum[J]. Frontiers in Microbiology. doi:10.3389/fmicb.2018.00239.
Panchal V H,Dhale D A. 2011. Isolation of seed-borne fungi of Sorghum(Sorghum vulgare pers.)[J]. Journal of Phytology,3(12):45-48.
Pandey R K,Gupta P K,Srivastava M,Singh S R,Gogo R. 2011. First report of brown leaf spot disease caused by Curvularia lunata infecting Indian spinach or poi(Basella rubra)[J]. Indian Phytopathology,64(2):207.
Roberts J A,Tredway L P. 2008. First report of Curvularia blight of zoysiagrass caused by Curvularia lunata in the United States[J]. Plant Disease,92(1):173.
Sun W Q,Payne G F. 2000. Tyrosinase-containing chitosan gels:A combined catalyst and sorbent for selective phenol removal[J]. Biotechnology and Bioengineering,51(1):79-86.
Taba S,Miyahira N,Nasu K,Takushi T,Moromizato Z. 2007. Fruit rot of strawberry pear(pitaya) caused by Bipolaris cactivora[J]. Journal of General Plant Pathology,73(5):374-376.
Tian P,Nan Z B,Li C J,Spangenberg G C. 2008. Effect of the endophyte Neotyphodium lolii on susceptibility and host physiological response of perennial ryegrass to fungal pathogens[J]. European Journal of Plant Pathology,122(4):593-602.
Verma V S,Gupta V K. 2010. First report of Curvularia lunata causing root rot of strawberry in India[J]. Plant Di-sease,94(4):477.
Wang L T,Wu H,Qin G Z,Meng X H. 2014. Chitosan disrupts Penicillium expansum and controls postharvest blue mold of jujube fruit[J]. Food Control,41:56-62.
Wang W B,Huang D J,Kang Y R,Wang A Q. 2013. One-step in situ fabrication of a granular semi-IPN hydrogel based on chitosan and gelatin for fast and efficient adsorption of Cu2+ ion[J]. Colloids and Surfaces B:Biointerfaces,106:51-59.
Zhang H K,Lu Y T,Wang Y H,Zhang X R,Wang T Y. 2018. D-Glucosamine production from chitosan hydrolyzation over a glucose-derived solid acid catalyst[J]. RSC Advances,8(10):5608-5613.
(責任编辑 麻小燕)

