Rotula aquatica Lour.inhibits growth and biofilm formation of clinically isolated uropathogenic Escherichia coli
A.Vysakh,Sebastian Jose Midhun,Ninan Jisha,Kuriakose Jayesh,V.Vijeesh,Mathew Jyothis,MS Latha
School of Biosciences,Mahatma Gandhi University,Priyadarshini Hills,Kottayam,Kerala,India
ABSTRACT
KEYWORDS: Rotula aquatica; Escherichia coli; Urinary tract infections; Anti-bacterial activity
1.Introduction
Escherichia coli (E.coli) is one of the most common pathogens that are related to nosocomial as well as community-associated infections[1,2].E.coli,the common flora of human intestines,is the major source of urinary tract infections (UTIs) and responsible for up to 85% of both complicated and uncomplicated UTIs[3].All over the world,40% of women and 12% of men experience UTI once in their lifetime.The current treatment therapies for infections due to uropathogenic E.coli mainly are based on antibiotics.Antibiotic resistance creates significant problems in the treatment and management of UTI in recent times[4].The emerging of drug-resistant strains is mainly due to the excessive and continuous use of antibiotics.Sometimes,it is difficult to find accessible effective antimicrobial agents for infections due to these multidrug-resistant bacteria[5].
According to the National Institute of Health,bacterial biofilm formation was involved in more than 60% of all bacterial infections[6].The organisms,which produce biofilms,have an innate capacity to resist antibiotics,disinfectants,and germicides.The inherent antibiotic resistance is mainly due to some specific defense mechanisms conferred by the biofilm environment.The mechanisms involved in the drug resistance include overexpression of stress-responsive genes,exopolysaccharide (EPS) based inactivation of anti-microbial agents,oxygen gradients within the biofilm matrix,and differentiation of a subpopulation of biofilm cells into resistant dormant cells[7].
Multidrug resistance and biofilm formation help the bacteria to overcome the current treatment strategies.So,there is an urgent need to develop safer antibacterial agents devoid of side effects and drug resistance from natural sources.Rotula aquatica (R.aquatica) Lour.(Family: Boraginaceae) is widely used as an important traditional medicine for treating stones (kidney/bladder),ulcer,and uterine diseases[8-10].The plant also has significant hypoglycemic,antidiabetic,and hypolipidemic activities.Scientific researches on the plant focus on its anti-mitotic[11],anti-bacterial[12],urolithiasis[13],anthelminthic[14],anti-diarrheal[15],analgesic,anti-inflammatory[16,17],anti-pyretic,psychoactive[18]and antioxidant[19]properties.The current study aimed to evaluate the antibacterial and anti-biofilm activity of ethyl acetate fraction of R.aquatica Lour.against clinically isolated uropathogenic E.coli.
2.Materials and methods
2.1.Chemicals
Ingredients of Mueller-Hinton agar,Nutrient agar used for bacterial studies and Acridine Orange/Ethidium Bromide stain used for fluorescent microscopical studies were from Himedia (Himedia,India).Propidium iodide was purchased from EMD Biosciences(Temecula,USA).All the solvents used in the study were purchased from Spectrochem Pvt.Ltd (Mumbai,India).
2.2.Plant materials
The roots of R.aquatica Lour.were collected from Kottayam district (9.7°N,76.78°E),Kerala,India.The authentication of plant material was done by Dr.Jomy Augustine (Taxonomist,St.Thomas College,Palai).A voucher specimen (SBSBRL.22) of the plant material was maintained in the institute.
2.3.Preparation of fractions
The liquid-liquid partition method was used to prepare various fractions of R.aquatica roots.Methanolic extract[17]was re-dissolved in 10% aqueous methanol (methanol: water,9:1 v/v) and fractionated with n-hexane (hexane),n-butanol,chloroform,and ethyl acetate,respectively.The residue left in separating funnel was re-fractionated twice by following the same procedure.Various fractions obtained were used for further analysis[20].Previous study reports from our laboratory showed that ethyl acetate fraction of R.aquatica (EFRA)possesses higher bioactivity than the other three fractions (data not shown here)[21].Thus,EFRA was chosen for the present study.
2.4.Microorganism
The clinically isolated E.coli strain BRL-17 (E.coli BRL-17)[22](Accession no: MF185683) was used for the present study.
2.5.Evaluation of antimicrobial activity of EFRA
Antimicrobial activity of EFRA was carried out by agar well diffusion assay[23].The zone of diameter was measured and recorded[24].
2.6.Determination of minimum inhibitory concentration(MIC) and minimum bactericidal concentration (MBC)
For MIC determination,microbroth dilution method in 96 multiwell microtiter plates with slight modifications was used[25].The different concentrations (0.156 to 5 mg/mL) of EFRA was prepared in 96 multi-well microtiter plate,and 50 µL of standardized bacterial suspension (2×105CFU/mL) was added to all wells.The positive(Medium+ E.coli) and negative controls (Medium without E.coli) were also prepared.After incubation for 24 h at 37 ℃,30 µL resazurin (0.015%) was added to all wells.The plate was again incubated for 2 to 4 h for the observation of color change[26].Resazurin (purple color) reduced in the presence of living bacteria from purple to pink or to colorless.The lowest concentration at which the color change observed was taken as MIC value.The minimum bactericidal concentration (MBC) for EFRA was determined by spread plating method.The bacterial culture from wells with concentrations higher than the MIC value was subjected to spread plating and plates were incubated at 37 ℃ for 24 h.The concentration at which the bacteria were completely killed was taken as MBC.
2.7.Tolerance level of E.coli BRL-17
The tolerance level of E.coli BRL-17 against EFRA was determined according to the method of May et al.[27].The tolerance level was calculated by using the formula: Tolerance=MBC/MIC.
2.8.Killing kinetic assay
EFRA at MBC (5 mg/mL) concentration was used for the killing kinetic assay.The inoculum density used for killing kinetic assay was 2×105CFU/mL.For killing kinetic assay,two controls were prepared.The Mueller Hinton broth (MHB) inoculated with the test organism was used as first control,and the other was MHB with EFRA at the test concentration (5 mg/mL) without the bacterial cells.The test was prepared by adding bacterial cells to MHB.The EFRA was added to the test only after 6 h of incubation.The bottles were incubated at 37 ℃ on an orbital shaker at 120 rpm.A total of 100 µL of aliquots were removed from each bottle at specific time intervals(0,1,2,3,4,5,6,7,8,9,10,11,and 12 h) for the determination of CFU/mL by plate count technique.The percentage of survived bacterial cells was calculated according to the method described by Neethu et al.[28].
2.9.Acridine orange (AO)-ethidium bromide (EB) staining
The bacterial cells (2×105CFU/mL) were treated with EFRA at MBC (5 mg/mL) for 6 h at 37 ℃.After incubation,the cells were placed on a glass slide and dried at 50 ℃,fixed with absolute methanol for 2 min and air-dried.The slides were stained with 50µL of a fluorescent dye (10 mg AO and 10 mg EB in 10 mL of PBS).After staining and PBS wash,the slides were observed under OLYMPUS Bx43F fluorescence microscope (Olympus Corporation,Japan)[28].
2.10.Propidium iodide (PI) uptake assay
The standardized inoculum concentration of E.coli cells was treated with EFRA (2.5 mg/mL) and incubated at 37 ℃.After EFRA exposure,the cells were washed with PBS buffer.Then the cells were incubated with PI (1.3 µg/mL) at 37 ℃ for 20 min in the dark.The PI fluorescence was measured at an excitation of 544 nm and an emission of 620 nm[29].
2.11.Scanning electron microscopy (SEM)
The cultured bacterial cells treated with EFRA at MBC (5 mg/mL)were taken as the test sample,and the untreated cells were taken as control.After incubation (24 h at 37 ℃),the cells were placed on a glass slide.For SEM analysis,the samples were prepared by the method described by Vysakh et al.and Midhun et al.[22,23].The glutaraldehyde solution (2.5%,v/v) was used for the sample fixation.It was then dehydrated using ethanol at increasing concentrations for 2 min in each.The slides were sputter-coated with gold and samples were examined under the JEOL 6390 SEM JSM instrument (Jeol USA Inc,USA).
2.12.Inhibition of biofilm formation
2.12.1 MTT assay
The effect of EFRA on biofilm formation of E.coli BRL-17 was studied by using the modified microdilution method[30].Briefly,different concentrations (0.156 to 5 mg/mL) of EFRA and tested strains were prepared as described in the MIC assay.The 100 µL of E.coli BRL-17 was inoculated in each well of 96-well plates.After incubation (37 ℃ for 24 h),the supernatants were discarded and washed three times with PBS.The quantification of biofilm formation was carried out by using the MTT assay.
2.12.2.Crystal violet assay
The effect of EFRA on biofilm formation was evaluated in 96-well plates.Briefly,300 µL of inoculated fresh trypticase soy broth was aliquoted into each well of the microplate.The different concentrations of (75%,50%,and 25% of MBC) EFRA were added to the cultured bacterial cell in the microplate.Wells containing medium and those without fractions were used as controls.After incubation (37 ℃ for 48 h),the supernatant was removed,and each well was washed thoroughly with sterile distilled water and airdried.The biofilm was stained with a 0.1% aqueous solution of crystal violet for 15 min at room temperature.After incubation,the excess stain was removed by washing.Finally,250 µL of 95% ethanol was added to each well for solubilizing dye bound to the cells.After 15 min of incubation,the absorbance was measured using the microplate reader at a wavelength of 570 nm[31].The index of specific biofilm formation (SBF) was calculated based on the method described by Sánchez et al.[32].Biofilm determination was made using the formula SBF = (AB×CW)/G,where SBF is the specific biofilm formation,AB is the OD570nm of the attached and stained bacteria,CW is the OD570nm of the stained control wells containing only bacteria-free medium,and G is the OD630nm of cell growth in broth.SBF index > 2.00 is considered as strong biofilm producers; SBF index between 1 and 2 as intermediate and SBF index < 1.00 as weak.
2.13.Statistical analysis
The GraphPad Prism©version 5.03 for Windows (GraphPad Software,San Diego,CA,USA) was used for the statistical analysis.Data of specific biofilm formation was subjected to one way ANOVA and Tukey’s test,P<0.05 was considered significant.The data were expressed as mean ± standard deviation (n=3).The data of PI uptake assay and biofilm inhibition were analyzed by Kruskal-Wallice nonparametric test and performed Dunn's multiple comparison posthoc tests.A P-value < 0.05 was considered significant.
3.Results
3.1.Antibacterial effect of EFRA
The antibacterial effect of EFRA was evaluated by measuring the zone of inhibition against E.coli BRL-17.The EFRA showed a zone of clearance of 16 mm in diameter on MHA agar plates (Figure 1).
3.2.MIC and MBC
The result of Resazurin assay showed that bacterial growth was arrested at 2.5 mg/mL.The MBC value of EFRA against E.coli BRL-17 was 5 mg/mL.
3.3.Tolerance determination
MIC and MBC values were used for the calculation of the tolerance level of the bacterial pathogen.The tolerance level of E.coli BRL-17 against EFRA was found to be 2,which indicated the bactericidal nature of the EFRA.
3.4.Time kill assay
The bactericidal activity of EFRA was progressively increased with increase in time (P<0.05).The whole bacteria were killed by EFRA within 6 h of exposure time (Figure 2).Up to 6 h from the initial inoculum,the bacterial growth was 8.62 log CFU/mL.The EFRA exhibited a significant killing of ≥5 log reduction in the bacterial growth within 5 h of exposure.In killing kinetic assay,it was found that the survival of E.coli BRL-17 was in the range of 89.91%,71.44%,60.31%,51.53%,34.02% at 1,2,3,4 and 5 h,respectively,after treatment with EFRA.
3.5.AO/EB staining
The results AO/EB staining observed under fluorescence microscopy showed that the EFRA was capable of killing all living bacterial cells in 6 h of treatment (Figure 3).The green colour of living cells in the control group (Figure 3A) was turned to red colour(dead cells) in the EFRA treatment group (Figure 3B).
3.6.PI uptake assay
The result of PI uptake assay showed that the percentage of PI uptake increased significantly (P<0.05) with increase in time (Figure 4).This could be due to the formation of cell wall pores in the bacteria by the action of EFRA,which enhances the uptake of PI and intercalates it into the DNA.

Figure 1.Antimicrobial sensitivity testing of ethyl acetate fraction of Rotula aquatica Lour.(EFRA).A: EFRA,B: Standard drug (Gentamicin 10 mcg/mL).

Figure 2.Time kill assay of EFRA against Escherichia coli (E.coli) BRL-17.The bacterial growth from initial (0 h) to injection point (6 h) were similar in both groups.The significant difference between the two groups starts from the injection point.The EFRA was added to the test only after 6 h of incubation (Injection point).EFRA can kill the bacterial cells within 6 h of exposure time.P<0.05 was considered significant.

Figure 3.Effect of EFRA against E.coli BRL-17 by acridine orangeethidium bromide staining.A: Untreated cells,B: Treated cells.The green colour of living cells in the control group (A) turned red (B) (dead cells) in the EFRA treatment group.Magnification: 200×.

Figure 4.Propidium iodide (PI) uptake assay.Different letters mean statistical significant difference (P<0.05).

Figure 5.SEM analysis of E.coli BRL-17 treated with EFRA.A: Untreated cells,B: Treated cells.The EFRA treated group (B) showed signs of cell wall damage.The slime production in the control group (A) also disappeared in EFRA treatment (B).
3.7.SEM analysis
The SEM images of E.coli BRL-17 cells treated and untreated with EFRA at MBC concentration (5 mg/mL) were shown in Figure 5.The EFRA treated group showed signs of cell wall damage.The cells were shrunken and wrinkled.The changes like the formation of holes in the cell wall and disorganization of cell structure were observed.The slime production in the control group was also disappeared in EFRA treatment.
3.8.Inhibition of biofilm formation
In MTT assay,the percentage of biofilm inhibition increased significantly (P<0.05) with increase in the concentration of EFRA.At MBC concentration,around 99.06% of biofilm formation was inhibited by EFRA (Figure 6A).In crystal violet assay,the result showed that the higher biofilm reduction was observed in a higher concentration of EFRA (75% of MBC).The concentrations corresponding to 75% (SBF=0.73; weak biofilm formation) and 50% (SBF=1.34; intermediate biofilm formation) of MBC showed significant reduction (P<0.05) in SBF of E.coli BRL-17 (Figure 6B).
4.Discussion
E.coli is the dominant organism responsible for extra-intestinal infections,enteric disease,and systemic infections in humans and animals.The predominant cause of UTIs is uropathogenic E.coli,one of the members of the extra-intestinal pathogenic E.coli[33].It is the leading cause of 80%-90% community-acquired and 30%-50% nosocomial acquired UTIs[34].These strains are having a variety of virulence factors that permit them to establish infection[35].For the current study,we used clinically isolated uropathogenic strain E.coli BRL-17 with strong biofilm-forming ability and multidrugresistance.
The anti-microbial and anti-biofilm studies are carried out by different researches around the world.The studies conducted by Mohammadi et al.proved the antibacterial activities of Carum copticum extracts against six pathogenic bacteria[36].Antibacterial activity of Euphorbia hebecarpa alcoholic extract and antimicrobial property of Quercus brantii fruits were studied by Mohsenipour et al.and Sadeghian et al.,respectively[37,38].These studies revealed the importance of plants in the treatment of bacterial infections.The antibacterial effect of the ethyl acetate fraction of R.aquatica against E.coli BRL-17 was evaluated by agar well diffusion method.The EFRA showed potent antimicrobial activity against E.coli BRL-17.The MIC of the EFRA was evaluated by Resazurin method.The redox-sensitive dye,Resazurin,was used to check cell viability.In the presence of live cells,the nonfluorescent blue Resazurin reduced to fluorescent red Resorufin,and dead cells do not reduce the Resazurin[39].This fluorescence and visible change in colour were used for the identification of MIC of EFRA.The EFRA at 2.5 mg/mL can reduce the visible growth of the bacteria,and the EFRA at 5 mg/mL has the capability to kill the bacteria completely.
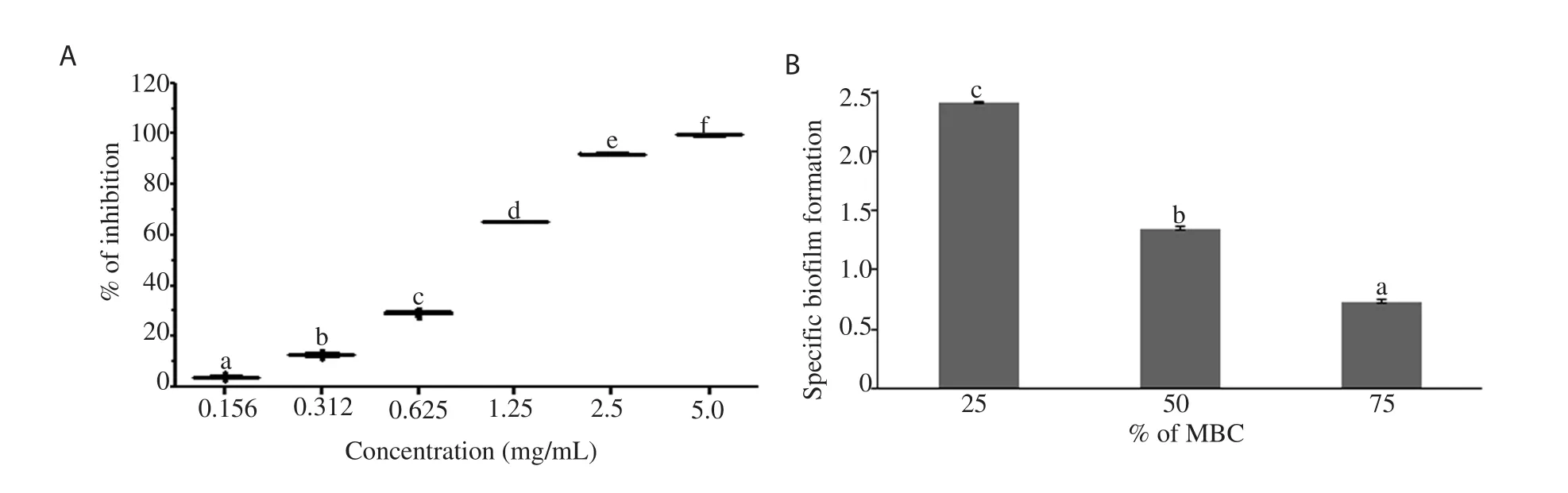
Figure 6.Effect of EFRA on biofilm inhibition against E.coli BRL-17.A: MTT assay,B: crystal violet assay.Different letters mean statistically significant difference (P<0.05).
The MBC/MIC ratio showed a more significant impact in the identification of the bacteriostatic or bactericidal nature of the antimicrobial agent.The antimicrobial agent is bacteriostatic when the MBC/MIC ratio is greater than or equal to 16,but when its value is found less than or equal to 4,it represents the bactericidal nature[39].It is well known that bactericidal agents promote microbial killing,whereas the bacteriostatic agents only promote the inhibition of bacterial growth.The MBC/MIC ratio obtained from the study was 2 and the EFRA was found capable of exerting a bactericidal effect against E.coli BRL-17.Mostly,the MBC of an antibacterial agent was 1 or 2 fold higher than the MIC value[40].
A 3log reduction in the CFU/mL or a 99.9% killing for a specified time period can be related to the bactericidal effect of an antimicrobial agent.The National Committee for Clinical Laboratory Standards (M26-A)[41]with modifications based on Handweger and Tomasz’s recommendation proposed that a kill can be determined at a 6 h time treatment period in liquid media[42].Based on this recommendation,the killing kinetics of the bactericidal activity of EFRA against E.coli BRL-17 for 6 h was examined.The results obtained from the killing kinetic assay revealed the fact that EFRA can kill the bacterial cells within 6 h of exposure time.The bactericidal property exhibited by EFRA strongly conforms to the suggestion of Handweger and Tomasz that a bactericidal agent should eradicate the pathogen within 6 h of treatment[42].
The results from the killing kinetic assay of the EFRA against E.coli BRL-17 were also confirmed by performing AO/EB staining.During AO/EB staining,live cells radiate a green fluorescence,and dead cells radiate a red fluorescence[43].As shown in our results,a prompt killing effect was demonstrated by EFRA against E.coli BRL-17,which appeared as a green fluorescence from the viable cells at zero minute and turned into the red colour of dead cells after 6 h.
PI is a dye used to evaluate the cell membrane disruption in bacterial cells.PI can enter the bacterial cell membrane and intercalate with bases of DNA only when it has been permeabilized through an agent/drug.PI uptake was increased during the EFRA treatment,which confirmed the ability of EFRA to kill bacteria by creating cell membrane pores.This was also evidenced by SEM analysis.The cell wall pores are easily visible in the SEM image of EFRA treated bacterial cells.These results prove the ability of EFRA to kill the uropathogen in a short period of time.
Microorganisms with biofilm are highly resistant to antimicrobial agents,and they are safe from immune system attack.The biofilm formation was found associated with chronic and recurrent human infections,which increase the severity of the diseases in most of the cases[44].An effective barrier created with exopolymeric substance prevents the entry of antibiotics and antimicrobial peptides into the biofilm[45].In the current study,the EFRA showed biofilm inhibition activity at its MIC and MBC concentrations,and proved its role in preventing biofilm formation associated with diseases like cystitis.The results of specific biofilm formation assay also confirmed the ability of EFRA to prevent biofilm formation against E.coli BRL-17.
5.Conclusion
To prevent colonization and attachment of uropathogenic E.coli,a drug with potential anti-bacterial and anti-biofilm activity would play an important role.The EFRA is capable of preventing bacterial growth and also inhibiting the biofilm-forming capability of the pathogen.The current study suggests the potential use of EFRA as an anti-bacterial drug.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This study gained financial assistance in the form of JRF (to A.Vysakh),from CSIR,Government of India [09/499(0093)/2017-EMR-I].
Acknowledgments
The authors are grateful to the School of Biosciences,Mahatma Gandhi University,Kottayam,India,for providing excellent research facilities.
Authors’ contributions
AV performed experiments,data analysis,interpretation and manuscript writing.VV and SJM contributed in writing section and data processing.MJ and MSL performed critical revisions of the manuscript and prepared the final approved version for publication.NJ and KJ reviewed manuscript draft.All authors participated in experimental design.
 Asian Pacific Journal of Tropical Biomedicine2020年12期
Asian Pacific Journal of Tropical Biomedicine2020年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Ferulic acid alleviates lipopolysaccharide-induced depression-like behavior by inhibiting inflammation and apoptosis
- Role of higher levels of post-challenge antibodies in protective vaccination against Leishmania tropica infection of BALB/c mice
- Coconut oil nanoemulsion attenuates methotrexate-induced hepatotoxicity and nephrotoxicity in Ehrlich ascites carcinoma-bearing mice
- Potential bioactive phytochemicals,antioxidant properties and anticancer pathways of Nymphaea nouchali
- Teicoplanin is a potential inhibitor of SARS CoV-2 replication enzymes: A docking study
