Coconut oil nanoemulsion attenuates methotrexate-induced hepatotoxicity and nephrotoxicity in Ehrlich ascites carcinoma-bearing mice
Shaza A.Alyamani,Mayson H.Alkhatib✉,Faiza Abdu
1Department of Biochemistry,Faculty of Science,King Abdulaziz University,Jeddah,Saudi Arabia
2Department of Biological Sciences,Faculty of Science,King Abdulaziz University,Jeddah,Saudi Arabia
ABSTRACT
KEYWORDS: Chemotherapeutic agents; Oxidative stress; Serum analysis; Complete blood count; Nanocarrier
1.Introduction
Methotrexate,a chemotherapeutic drug,is used to treat different kinds of cancers,such as breast cancer,leukemia,lung cancer,lymphoma,and osteosarcoma.The most well-known negative effects of methotrexate include hepatotoxicity and renal toxicity[1-3].Methotrexate acts as an antimetabolite of the antifolate type since it inhibits the dihydrofolate reductase,which plays a major role in the tetrahydrofolate synthesis and affects the de novo synthesis of the thymidine and the biosynthesis of purine and pyrimidine base[4].Therefore,methotrexate restrains the synthesis of DNA,RNA,thymidylates,and proteins[5,6].
The principle mechanism of the effect of methotrexate on the liver is unknown.However,research studies had demonstrated that methotrexate therapy might cause hepatic folate deficiency and alterations in the liver histology[7,8].In addition,methotrexate treatment resulted in raising the liver oxidative stress since it enhanced the amount of lipid peroxidation and lowered the antioxidants[9,10].In addition,methotrexate caused kidney toxicity by either precipitation or affecting directly the renal tubules[11].
Nanoemulsion is a colloidal system that consists of water,oil,surfactant and/or cosurfactant and has many advantages including simplicity,the spontaneity of preparation,and the ability to solubilize a wide range of drugs,improve drug bioavailability and enhance the drug’s efficacy[12].The coconut oil stands out for its high nutritional and pharmaceutical value.An in vivo study revealed that coconut oil had the potential to ameliorate the negative side effects of methotrexate on oxidative stress and pro-inflammation[13].
The present study aimed to evaluate the impact of combination treatment of methotrexate with a coconut oil nanoemulsion on toxicity induced by methotrexate on the liver and kidney in vivo.
2.Materials and methods
2.1.Chemicals
A 100% pure methotrexate was commercially purchased from Al-Foad Pharmacy (Cairo,Egypt).Span 20 and Tween 80 were obtained from Sigma Aldrich (USA).Coconut oil was acquired from Abazeer for Natural Oils (Jeddah,Saudi Arabia).The Biodiagnostics and Research Reagents Co.(Cairo,Egypt) provided all of the colorimetric assays for measurement of biochemical parameters of liver and kidney functions,as well as the assays of the malondialdehyde (MDA,CAT NO.MD 25 29),catalase (CAT,CAT NO.CA 25 17),superoxide dismutase (SOD,CAT.NO.SD 25 21),and glutathione reductase (GR,CAT.NO.GR 25 23).
2.2.Preparation of coconut oil nanoemulsion
Coconut oil nanoemulsion was produced by mixing different weight percentages of 1.86 coconut oil,3.72 Tween 80,1.40 Span 20 and 93.02 distilled water.The resulting emulsion mixture was heated at a high temperature (~ 70 ℃) with constant mixing until a clear and transparent nanoemulsion was produced.The desired amount of methotrexate was dissolved in coconut oil nanoemulsion.
2.3.Experimental animals
A total of forty female Swiss albino mice weighing 25-30 g were placed in eight cages with five mice in each cage.The mice were first acclimatized for 7 d at the experiment site under optimal environmental conditions [12-hour light-dark cycles,temperature(25 ± 2) ℃ with moderate humidity (60 ± 5)%].
2.4.Experimental procedure
The forty mice were inoculated intraperitoneally (i.p.) with 2.5× 106Ehrlich ascites carcinoma (EAC)/mouse for 48 h and were separated into four groups.The first group included the untreated EAC-bearing mice (n=10) and served as the positive control.The other three groups of EAC-bearing mice were divided as follows: the second group received methotrexate (20 mg of methotrexate/kg of mouse/0.2 mL of distilled water)[2]; the third group was treated with 0.2 mL of coconut oil nanoemulsion/kg alone to testify the toxicity of the blank nanoemulsion; the fourth group was administered with methotrexate and coconut oil nanoemulsion (20 mg/kg of methotrexate solubilized in 0.2 mL/kg of coconut oil nanoemulsion)[2].
The methotrexate dose was selected according to the study of Abdel-Daim et al.[2]who reported that the in vivo lethal dose of methotrexate was 20 mg/kg administered intraperitoneally once a week.
2.5.Ethical statement
The mice were preserved in accordance with King Abdulaziz University’s policy and the International Ethical Guidelines on the Care and Use of Laboratory Animals[14].The ethical approval was obtained from the Research Ethics Committee in the Faculty of Medicine at King Abdulaziz University (DF-692-165-1441).
2.6.Blood and tissue sample collection
After one week of treatment,the mice were fasted overnight,anesthetized with diethyl ether and sacrificed via cervical dislocation as mentioned by Alkhatib et al[15].Blood samples were collected from the retro-orbital venous plexus after they had been anesthetized and were taken in a sterile EDTA or plain test tube for biochemical analysis.The blood was centrifuged at 3 000 rpm for 15 min to get the serum sample.The sample was then refrigerated at -80 ℃ prior to biochemical analysis as described by Almotwaa et al[16].The liver and kidney of mice were excised,washed with phosphate-buffered saline and subjected to histopathological examination.The portions of the liver and kidney were used to prepare 20% tissue homogenate for the determination of the oxidative status.
2.7.Assessment of liver and kidney function
2.7.1.Serum analysis
For testing the liver function,alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),total protein (TP),albumin (ALB),total (TBIL) and direct bilirubin(DBIL) were assessed.For testing the kidney function,creatinine(CREAT) and blood urea nitrogen (BUN) were examined.All of the parameters were measured according to the protocols of their respective kits.
2.7.2.Evaluation of the oxidative stress
The CAT activity was determined using the method described by Aebi[17].The concentration of MDA was measured based on a previously reported method[18].The concentration of GR was estimated as described in Carlberg et al.[19],while SOD activity was determined using the method of Flohé et al[20].
2.7.3.Histological study
Sections of excised liver and kidney were prepared for histological study as described by Alkreathy et al[21].The liver and kidney were fixed in 10% buffered formalin,and sections were cut less than 3 µm by a microtome.Tissue sections were then stained with haematoxylin and eosin (H & E) dyes and examined through a phase-contrast inverted light microscope (1X17 Olympus,Japan) for visualizing the histopathological changes.
2.8.Complete blood count analysis
The blood was collected by a retro-orbital plexus method followed by the determination of the white blood cells (WBCs),hemoglobin(Hb),red blood cells (RBCs) and platelets using an automated hematology analyzer (Sysmex Corporation,Kobe,Japan).
2.9.Statistical analysis
All data were expressed as mean ± standard deviation (SD).Statistical analysis was performed using MegaStat Excel (10.3).All of the variations between the samples were examined using Tukey’s range tests.The values were considered statistically significant when P < 0.05.
3.Results
3.1.Evaluation of the liver and kidney functions
3.1.1.Serum analysis
The intraperitoneal injection of methotrexate significantly increased the concentrations of liver parameters including TP,AST,ALT,ALP,TBIL and DBIL compared to the untreated EAC group (P <0.01).In contrast,these parameters were significantly decreased in the combination treatment group compared to the methotrexate group.The serum liver parameters of both EAC and coconut oil nanoemulsion groups were statistically comparable.Additionally,the amounts of ALB in all of the experimental groups did not differ.In terms of the kidney function,methotrexate-injected mice exhibited a significant increase in serum concentrations of CREAT and BUN when compared to EAC.Interestingly,there were significant decreases in the serum levels of CREAT and BUN in the group treated with methotrexate and coconut oil nanoemulsion relative to the methotrexate group.On the other hand,the serum CREAT and BUN for the coconut oil nanoemulsion alone group were markedly decreased when compared to EAC group (Table 1).
3.1.2.Oxidative stress assessment
As exhibited in Figure 1,CAT and SOD activities were significantly reduced while GR activity was increased in both liver and kidney tissues of the methotrexate group compared to the EAC group.The combination group reversed the changes induced by methotrexate.Moreover,MDA level in the liver was enhanced,whereas it was decreased in the kidney of the methotrexate group.In contrast,the combination treatment with methotrexate and coconut oil nanoemulsion decreased the MDA level in both liver and kidney tissues.
3.2.Complete blood count analysis
According to Table 2,the WBC counts and platelet amounts were elevated in the methotrexate group compared to the EAC group.However,there were no significant changes in the RBC and Hb amount between methotrexate and EAC groups.The combination treatment caused a reduction in the WBC count,whereas the Hb amount was increased compared to the methotrexate group.There were no significant changes in the RBC and platelet amounts between methotrexate and combination groups.The WBC,Hb,and platelet amounts in the coconut oil nanoemulsion alone group were higher than those of the EAC group with no changes in the RBC counts.

Table 1.Effect of drug formulations on liver and kidney functions of mice.

Table 2.Effect of drug formulations on the hematological parameters of the experimental mice.

Figure 1.The antioxidant status (A: CAT; B: SOD; C: GR; D: MDA) of the liver and kidney tissues for the experimental groups.The superscripts (a,b,c)display the statistical significant variations between the tested groups as follows: a) EAC and MTX groups,b) MTX-COPRA and MTX,c) EAC and COPRANEMUL,respectively.CAT: catalase; SOD: superoxide dismutase; GR: glutathione reductase; MDA: malondialdehyde.

Figure 2.Photomicrographs of the hepatic tissues.(A) The EAC group shows the central vein (CV) surrounded by hepatocytes (H),excessive dilated blood sinusoids (S),and the presence of excess Kupffer cells (K); (B) the MTX group shows disruption in radial arrangement around the CV with hepatocytes,more dilated blood sinusoids,and the presence of Kupffer cells; (C) the COPRA-NEMUL group shows narrow blood sinusoid spaces,and the presence of few Kupffer cells; (D) the MTX-COPRA group exhibits fewer dilated blood sinusoids,and the presence of few Kupffer cells.H&E × 400.
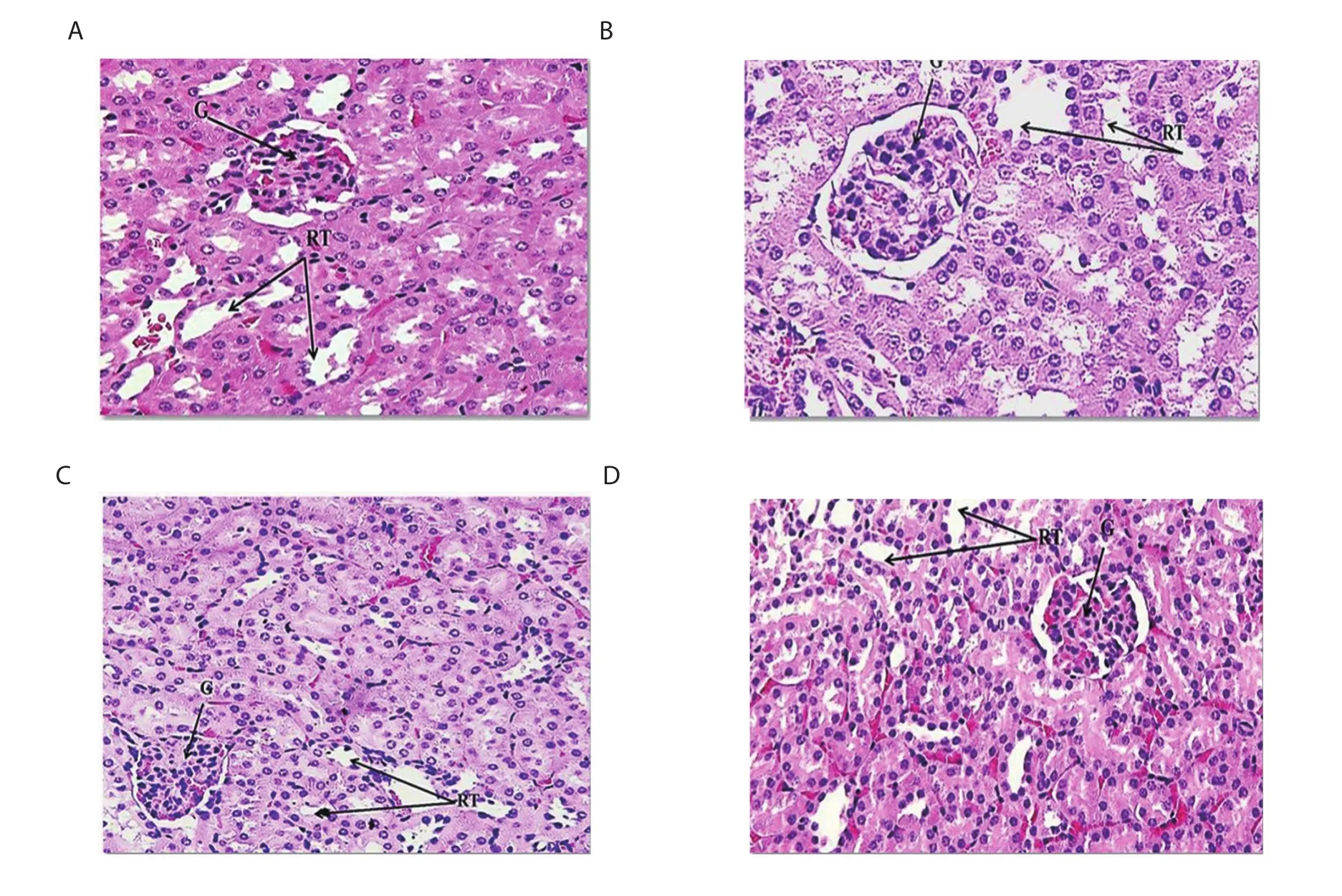
Figure 3.Photomicrographs of the kidney tissues.(A) The EAC group exhibits normal histopathological structure in the glomeruli (G) and renal tubules (RT); (B)the MTX group shows variable histological changes in glomeruli (G) and some parts of the renal urinary tubules (RT); (C) the COPRA-NEMUL group shows no histopathological changes in glomeruli (G) and renal tubules (RT); (D) the MTX-COPRA group shows significant improvement in glomerular damage,and moderately organized tubular and glomerular structures with mild inflammation.H&E × 400.
3.3.Histological study
3.3.1.Liver histology
The morphological changes of the resected liver for the tested mice are displayed in Figure 2.The hepatic tissues of the untreated EAC-bearing mice and methotrexate-injected EAC mice revealed several microscopic changes such as the presence of prominent Kupffer cells and leukocyte infiltration around the central vein and excessively dilated blood sinusoids (Figure 2A & B).In contrast,the leukocyte infiltration around the central vein was significantly reduced,and fewer dilated blood sinusoids were seen in the liver structure of coconut oil nanoemulsion treated groups (Figure 2C & D).
3.3.2.Kidney histology
The kidney sections of all tested groups are displayed in Figure 3.The tissues of EAC and coconut oil nanoemulsion alone groups exhibited no histopathological changes in glomeruli and renal tubules(Figure 3A & C).In contrast,the kidney tissue of the methotrexate group revealed histological changes in glomeruli and some parts of the renal urinary tubules (Figure 3B).Interestingly,the kidney tissue of the methotrexate group treated with coconut oil nanoemulsion displayed an improvement in glomerular damage and moderately organized tubular and glomerular structures with mild inflammation(Figure 3D).
4.Discussion
The current study assessed the protective role of methotrexate combined with coconut oil nanoemulsion in diminishing the associated toxicity of methotrexate in the liver and kidney.Methotrexate is an antifolate belonging to the antimetabolite class of antineoplastic agents that treat many cancer types.The toxicity of methotrexate stems from its oxidative reactions in the liver due to the transformation of methotrexate to its main product 7-hydroxymethotrexate that gets stored in the liver[6,22].The long term treatment of methotrexate may cause precipitation of polyglutamate and reduction of folate levels which might cause liver toxicity,including fibrosis and cirrhosis[7,23].
According to our findings,methotrexate treatment raised the levels of AST,ALT,and ALP levels.Similarly,some research studies demonstrated that methotrexate caused alterations in the liver enzymes which resulted in liver diseases[24,25].The hepatotoxicity is a consequence of methotrexate binding to the dihydrofolic enzyme which in turn prevents the conversion of folic acid into its active form,5-methyltetrahydrofolate[24,25].Accordingly,the synthesis of nucleic acids and some amino acids and proteins is inhibited indirectly which may cause damage in the organelles and plasma membranes of hepatic parenchymal cells that intervene with their function and permit the enzymatic leakage[26].Other studies reported that the liver damage caused by methotrexate is owing to the aggregation of polyglutamine methotrexate in hepatocytes[7,8].
Many studies exhibited the renal toxicity of methotrexate was caused by its poor solubility and metabolite in the acidic environments of the urine which leads to the elevation of CREAT and BUN levels[11,27].In particular,the basic mechanism of methotrexate to initiate renal toxicity is its crystallization in the renal tubular lumen; in addition,methotrexate clearance is by means of renal glomerular filtration and active tubular excretion leads to renal tubular injury[2,27,28].
The present study revealed that the peritoneal injection of methotrexate and coconut oil nanoemulsion into the mice lowered the ALT,AST,ALP,CREAT and BUN levels which were raised in methotrexate treated mice.The inclusion of coconut oil in the nano-formulation may play an important role in reducing the hepatotoxicity and nephrotoxicity induced by methotrexate.Coconut oil has antioxidant activities,renoprotective and regenerative effects on the kidney[13,29]which may be attributed to its high nutritional value and the presence of phenolic acid[30].Moreover,the incorporation of the coconut oil and methotrexate into the nanoemulsion maximizes their solubility in the aqueous media and hence improves their bioavailability and permeation to the targeted cancer cells without affecting the other organs[31].
In addition to the serum analysis of the experimental mice,the oxidative stress of the liver and kidney tissues was evaluated in order to testify the functions of the organs.The administration of methotrexate and coconut oil nanoemulsion into the mice attenuated the oxidative stress in the liver and kidney organs caused by methotrexate treatment.Oxidative stress and the production of reactive oxygen species have been reported to play a central role in the toxicity of methotrexate[32].Elevating these oxidative stresses can alter the morphological and physiological structures in the liver and kidney tissues[1,10].
According to the histological examination,the hepatic tissues of EAC and methotrexate groups displayed pathological alterations and inflammation which might be caused by the toxic metabolites of methotrexate that could reduce the GR level and enhance the lipid peroxidation in the hepatocytes[23].In contrast,the hepatic tissue of the methotrexate group treated with coconut oil nanoemulsion revealed an improvement in hepatocytes with fewer dilated blood sinusoids,which indicates that the combination significantly decreases the toxicity of methotrexate in the liver.
In terms of the kidney,the tissue sections of the methotrexate group exhibited significant histological changes in glomeruli and some parts of the renal urinary tubules.In agreement with our findings,methotrexate caused oxidative stress and peroxidation in mice kidney tissues which might be one of the reasons for methotrexate-induced nephrotoxicity[33].By contrast,the combination of methotrexate and coconut oil nanoemulsion reduced the glomerular damage and showed moderately organized tubular and glomerular structures.
In conclusion,the results obtained in this study suggest that the coconut oil nanoemulsion as a delivery system markedly reduces the liver and kidney toxicity induced by methotrexate.It is recommended to make further investigations on the molecular mechanism of the coconut oil nanoemulsion in suppressing the toxicity of methotrexate.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ conbributions
MHA and SAA wrote the concept of the study and designed the experiments.SAA performed the experiments.MHA and SAA implemented the data analysis and interpretation.All authors drafted and revised the manuscript.The whole study was performed under the supervision of MHA and FA.
 Asian Pacific Journal of Tropical Biomedicine2020年12期
Asian Pacific Journal of Tropical Biomedicine2020年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Ferulic acid alleviates lipopolysaccharide-induced depression-like behavior by inhibiting inflammation and apoptosis
- Role of higher levels of post-challenge antibodies in protective vaccination against Leishmania tropica infection of BALB/c mice
- Rotula aquatica Lour.inhibits growth and biofilm formation of clinically isolated uropathogenic Escherichia coli
- Potential bioactive phytochemicals,antioxidant properties and anticancer pathways of Nymphaea nouchali
- Teicoplanin is a potential inhibitor of SARS CoV-2 replication enzymes: A docking study
