Antioxidant and anti-inflammatory activities of Orostachys japonicus
Eun-Sun Hwang, Nhuan Do Thi
SKcohroeaol of Wellness Industry Convergence, Food & Nutrition, Hankyong National University, 327 Chungang-ro, Anseong-si, Gyeonggi-do 17579,
ABSTRACT
KEYWORDS:Orostachys japonicus; Antioxidant; RAW 264.7;Anti-inflammation; Nitrix oxide
1.Introduction
Orostachys japonicus (O.japonicus) A.Berger, a member of the perennial Crassulaceae family, is widely distributed in East Asian countries such as Korea, China, and Japan[1].The plant is usually used for medicinal purposes[2]and O.japonicus extracts have been used as a folk remedy for cancer treatment and as adjuvants or alternatives to chemotherapy[3].Many studies have reported vasoconstriction, respiratory stimulation, intestinal intensification,antidiabetic, antiobesity, antioxidant, and anticancer effects of O.japonicus[4-9].O.japonicus extract was recently shown to inhibit the proliferation of human colorectal cancer (HT-29 and SW480 cell lines) and proliferative myeloid leukemia cells (HL-60 cell line)by inducing cell cycle inhibition and apoptosis[10,11].Apoptosis induction through p53 protein expression has also been reported[12].The extracts were also demonstrated to induce growth inhibition and apoptosis of various cancer cells such as gastric, liver, cervical,lung, and prostate cancer cells[9,12,13].
O.japonicus contains sterol-based substances (campesterol and β-sitosterol), triterpenoid components (glutinol, glutinone,epi-friedianol, and friedelin), aromatic acids (gallic acid, 3,4-dihydroxybenzoic acid, and 4-hydroxybenzoic acid) and flavonoids(such as kaempferol and quercetin)[14].Among the O.japonicus extracts obtained by different solvents, the highest flavonoid content was observed in the 70% ethanol extract, followed by the 70% methanol, chloroform and 50% methanol, and water extracts.In addition, the extraction degree of the bioactive substance was dependent on the solvent used, and the higher the concentration of O.japonicus extract, the higher the antioxidant activity[15].
Reactive oxygen species (ROS) containing oxygen atoms are highly reactive and commonly produced in living organisms[16].It is oxygen with strong oxidizing power to attack biological tissues and damage cells.Molecules contain oxygen ions and hydrogen peroxide and are highly reactive due to unpaired electrons.Free radicals are known to occur in the normal metabolic process of oxygen and are known to play an important role in cellular signaling and homeostasis[17].Free radicals elevated by oxidative stress, such as ultraviolet light,environmental stress, diseases, etc., make them act as harmful substances for the body[16].Oxidative stress causes chronic fatigue,hyperlipidemia, arteriosclerosis, heart disease, peripheral vascular disease, allergic dermatitis, cancer, aging, and kidney disease, and exacerbates existing diseases[16].Fruits and vegetables contain physiologically active substances, including vitamins, minerals,antioxidant nutrients, polyphenols, flavonoids, etc., which are known to prevent aging and disease[18].
It is known that, when ROS is over-produced, the damage to cells and tissues is deeply related to the inflammatory reaction because of the destructive tendency of ROS[19].Inflammation is a biological defense mechanism that attempts to localize its effects by removing harmful factors and restoring the damaged area to normal when the biological tissue is stimulated from the outside[20].The purpose of inflammation is to suppress cell damage at an early stage, remove destroyed tissue and necrotic cells in the wound,and regenerate tissue at the same time[21].Substances that cause inflammatory reactions include pathogens, damaged cells, irritants,and danger signals.Inflammation itself is not a disease, but rather a defense system necessary for life[20].However, it is known that if the inflammatory response is poorly controlled, leading to chronic inflammation, it causes disease and promotes aging[22].Recently,many studies have been conducted to prevent inflammation of cells with foods rich in antioxidants[18,19].Studies have been conducted to prevent diseases through various bioactive substances, but few studies have been conducted on O.japonicus.
The purpose of this study was to find out which solvent conditions are most suitable for extracting bioactive substances from O.japonicus as effectively as possible and to evaluate the inhibitory activity of oxidative and inflammatory reactions in 70% ethanolic or water extract of O.japonicus.
2.Materials and methods
2.1.Chemicals
Folin-Ciocalteu's phenol reagent, 2,2'-diphenyl-1-picrylhydrazyl radical (DPPH), 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), gallic acid, and catechin were purchased from Sigma Chemical (Sigma-Aldrich Co., St.Louis, MO, USA).All chemicals used were of analytical grade.
2.2.Sample preparation
O.japonicus in this experiment was purchased directly from cultivated farms in Anseong, Gyeonggido, Southe Korea in 2018.O.japonicus was harvested just before flowering in late August and the leaves of plants were used for the experiment.There was no apparent damage to the plant and no signs of insect infestation.They were washed with water, naturally dried, removed from the water,and then put in an aluminum lunch box for freeze-drying and stored at -80 ℃ for 24 h.The samples were put in a freeze-drier (Ilshin,Seoul, Korea) and dried, then finely ground with a food grinder(Hanil, Korea) to make a fine powder.After mixing well by adding 125 mL of 70% ethanol or water to 5 g of the powder sample, the active ingredient was extracted for 2 h at 60 ℃ or 85 ℃, respectively,and the mixture was filtered under vacuum using Whatman #2 filter paper.
The extracted filtrate was then evaporated using a rotary evaporator(EYELA, Tokyo, Japan) under reduced pressure at 40 ℃.The extracts were dried in a freeze-drier (Ilshin, Seoul, Korea), finely ground with a cooled mortar and pestle to a particle size smaller than 1.0 mm, and kept in air-tight plastic bags at -20 ℃ until analysis.
2.3.Total polyphenol, flavonoid and anthocyanin content analysis
The total polyphenol content was measured by the method of Sembiring et al.[23]using Folin-Ciocalteu's phenol reagent.The total polyphenol content in the sample was calculated by the standard curve using gallic acid and expressed as the gallic acid equivalents(GAE) per gram dry weight (GAE mg/g).
The total flavonoid content in the sample was measured by the method of Sembiring et al.[23], calculated by the standard curve using catechin, and expressed as catechin equivalents (CE) per gram of dry weight (CE mg/g).
The total anthocyanin content in the sample was measured by the method of Yang et al.[24], calculated by the standard curve using cyanidin-3-glucoside (C3G) and was expressed as the C3G per 100 g of dry weight (C3G mg/100 g).
2.4.Determination of antioxidant activity
The antioxidant activities of O.japonicus were examined through several antioxidant assays.The activity of scavenging DPPH and ABTS radical by water and 70% ethanol extracts of O.japonicus at concentrations of 12.5-400 µg/mL was measured by the method of Thi and Hwang[25], and the superoxide anion-scavenging activity was determined by the method of Jayalakshmi and Devika[26].
2.5.Cell viability assay
The effect of O.japonicus extract on the growth of RAW 264.7 mouse macrophages was determined by MTT assay.RAW 264.7 cells, distributed at the Korea Cell Line Bank (Seoul, Korea),were cultured in Dulbecco's Modified Eagle's Medium (DMEM)supplemented with 10% fetal calf serum and penicillin/streptomycin in a humidified incubator containing 5% CO2at 37 ℃.Cells were seeded in a 96-well plate at a concentration of 2×104/well, cultured with or without 50 µmol/L malvidin for the first 30 min, again with 1 µg/mL lipopolysaccharide (LPS) and further cultured for 24 h.The cells cultured for 24 h without adding LPS under the same conditions were used as a control.After culturing for 24 h, the existing medium was removed and fresh DMEM medium in which 0.5% MTT reagent was dissolved was added to the cells.The culture continued for another 3 h.To terminate the MTT reaction, 10% sodium lauryl sulphate dissolved in 0.1 mol/L HCl was added to the cells.The blue formazan dye formed in the cells was dissolved with a 10% sodium lauryl sulphate solution containing 0.1 mol/L HCl to measure absorbance at 550 nm with a microplate reader (Infinite M200 Pro, Tecan Group Ltd.San Jose, CA).The blue formazan dye is formed in proportion to the number of living cells and the number of living cells can be predicted through the intensity of color.
2.6.Quantification of nitric oxide (NO) production
RAW 264.7 cells were seeded in a 96-well plate at a density of 5 ×105cells/well and incubated for 2 h to allow cells to adhere to the plate bottom.Subsequently, 2 mg of LPS was dissolved per mL of DMEM medium and 100 µL was added to each well containing cells.After 1 h of incubation, different concentrations of O.japonicus(25-400 µg/mL) were added to the cells and cultured for 12 h, only DMEM medium containing no cells was collected.Then, 50 µL of Griess reagent was mixed in 50 µL of the collected medium and reacted for 15 min at room temperature.Then, the absorbance was measured at 540 nm using a microplate reader.The content of nitrite contained in the sample was calculated based on the standard curve of NaNO2.
2.7.Determination of tumor necrosis factor (TNF-α) and interleukin 6 (IL-6)
LPS at 1-2 µL/mL was treated into the RAW 264.7 cells and incubated with different concentrations of O.japonicus extract.TNF-α and IL-6 levels were then determined by a microplate reader.The cells were grown with 0-400 µg/mL of O.japonicus and captured antibody solution (100 µL), which was incubated overnight at 4 ℃and blocked at the bottom of 96-well plate using the assay diluent(100 µL).After incubation for 2 h with shaking (100 rpm) at ambient temperature, the detection antibody solution (50 mL) was replaced with the medium, and incubation continued for 1 h with shaking(100 rpm) at 25 ℃.Subsequently, the detected antibody solution was exchanged for 50 µL of avidin-horse radish peroxidase solution and incubated at 25 ℃ for 30 min.The 50 µL of TMB substrate solution was added after removing the avidin-horse radish peroxidase solution and incubation proceeded in the dark with shaking at 100 rpm for 30 min.Finally, absorbance was measured at 450 nm and 570 nm after adding 50 µL of the stop solution.The amount of TNF-α and IL-6 in the sample was calculated by the standard curve.
2.8.Statistical analysis
All results are presented as mean±SD.The statistical analysis of the results was performed using SPSS software package version 17.0(Statistical Package for the Social Sciences, Spss Inc., Chicago, IL,USA).One-way analysis of variance was performed and P<0.05 was considered statistically significant.
3.Results
3.1.Total polyphenol, flavonoid, and anthocyanin
The contents of the bioactive substances contained in the O.japonicus extract by water and 70% ethanol were measured and the results are shown in Table 1.The total polyphenols of 70% ethanol extracts of O.japonicus were 3.76 times higher than that of water extract.The total flavonoid content in 70% ethanol extract was 3.38 times higher than that of water extract.These results indicated that 70% ethanol was more effective than water when extracting polyphenols and flavonoids from O.japonicus.
In contrast, the total anthocyanin content showed a higher value in the water extract than the 70% ethanol extract, about 1.60 times higher.
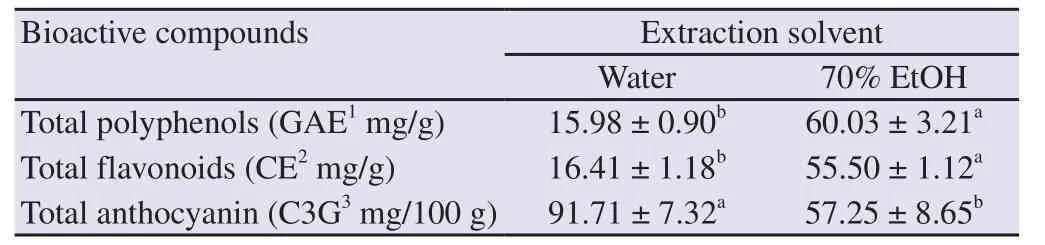
Table 1.Total polyphenol, flavonoid, and anthocyanin contents of Orostachys japonicus extracted by different solvents.
3.2.Antioxidant activity
The antioxidant activity of water and 70% ethanol extract of O.japonicus was measured through three assays at different concentrations (12.5 to 400 µg/mL), and the results are shown in Tables 2-4.As the concentration of the O.japonicus extract increased,the antioxidant activities also increased in three assays.In addition,the antioxidant activities of O.japonicus extracted with 70% ethanol were significantly higher compared with extracted with water at all concentrations in three assays (P<0.05).

Table 2.DPPH radical-scavenging activity (%) of Orostachys japonicus extracted by different solvents.
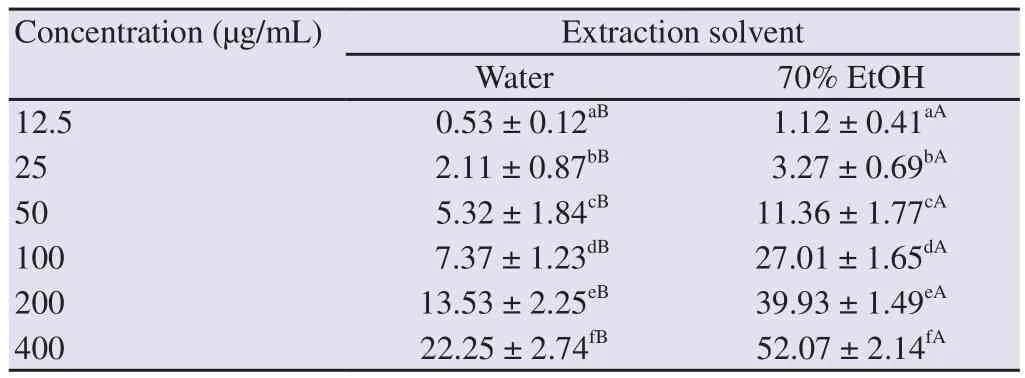
Table 3.ABTS radical-scavenging activity (%) of Orostachys japonicus extracted by different solvents.

Table 4.Superoxide radical-scavenging activity (%) of Orostachys japonicus extracted by different solvents.
3.3.Cell cytotoxicity
As a result of the previous experiment, since 70% ethanol extract had higher antioxidant activity than water extract, 70% ethanol extract was used for subsequent experiments.
In order to investigate cytotoxicity, RAW 264.7 cells were incubated in DMEM supplemented with various concentrations(0-400 µg/mL) of O.japonicus extract for 24 h.The viability of RAW 264.7 cells ranged between 97.3% and 92.8% within the 0-400 µg/mL (Figure 1).O.japonicus extract thus did not affect cell viability in this concentration range.Therefore, 70% ethanol extract of O.japonicus of up to 400 µg/mL was used in subsequent experiments.
3.4.NO production, TNF-α, and IL-6
NO levels in control cells and cells treated with LPS were 13.6 and 97.2 µmol/L, respectively.O.japonicus extract significantly inhibited LPS-induced NO production in a concentration-dependent manner(P<0.05) (Figure 2).
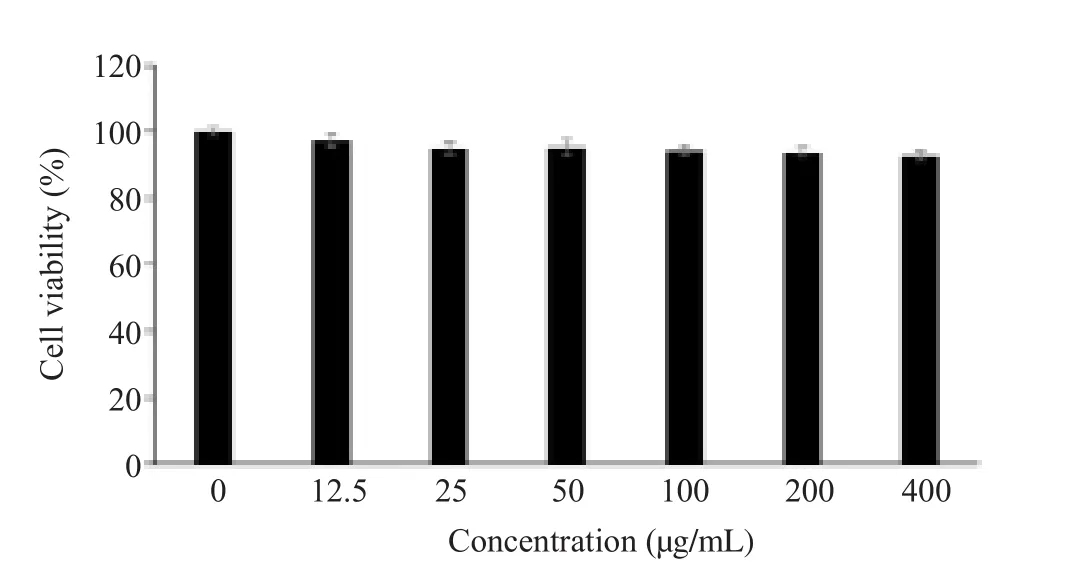
Figure 1.Effect of Orostachys japonicus extract on the viabilities of RAW 264.7 macrophage.
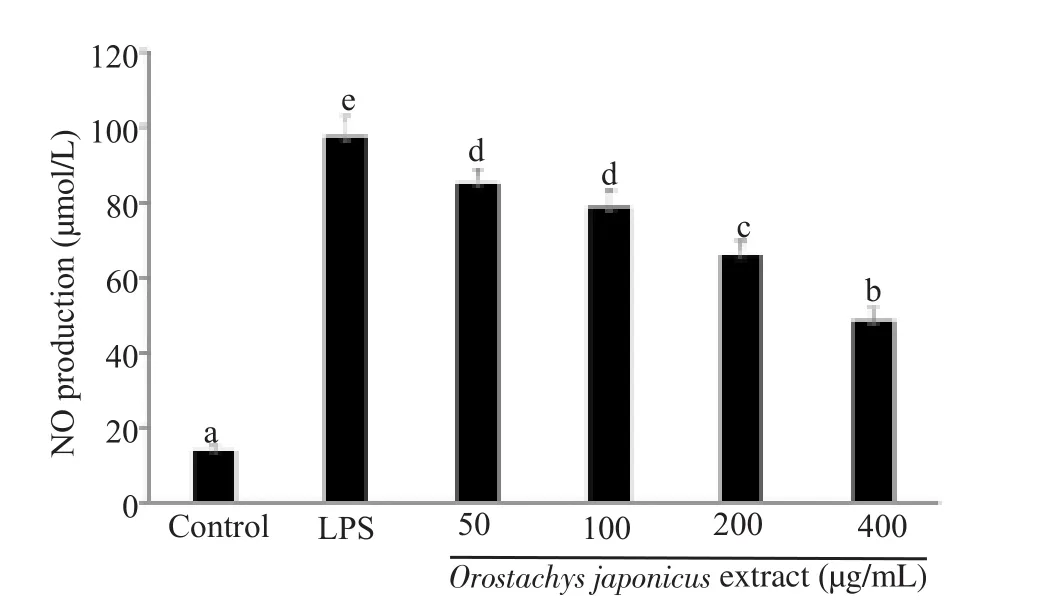
Figure 2.Effect of Orostachys japonicus extract on intracellular NO production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage.Values with different letters (a-e) indicate a significant difference (P<0.05).
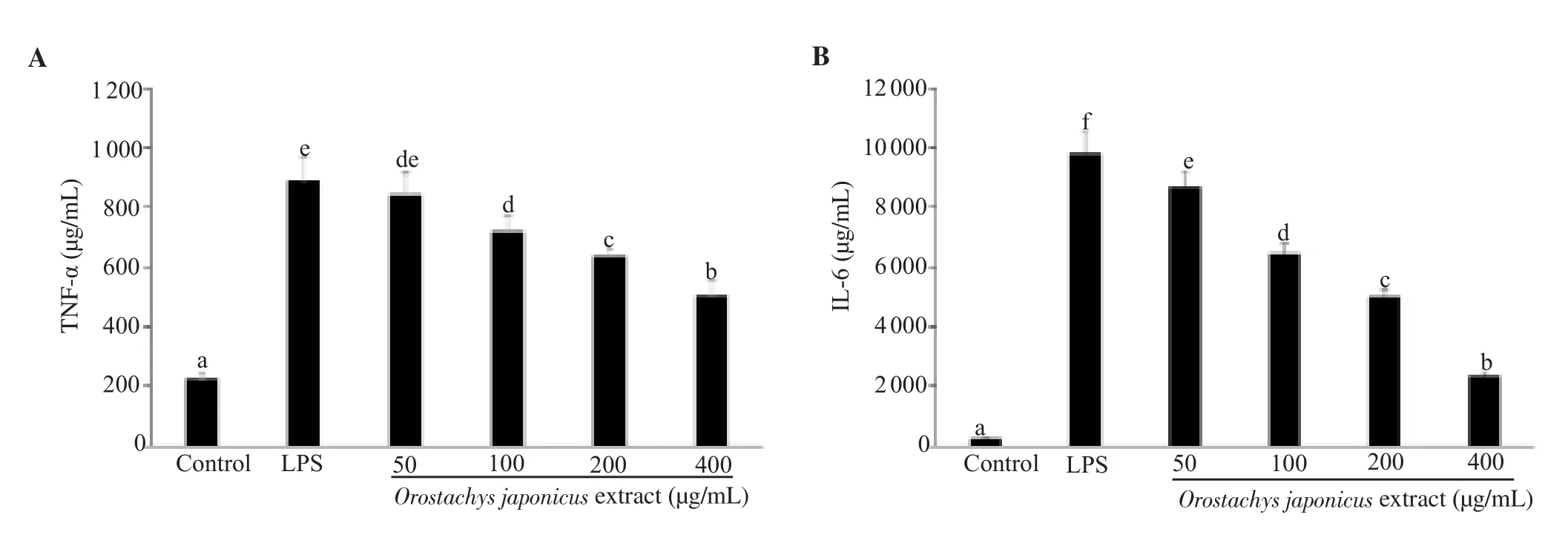
Figure 3.Effect of Orostachys japonicus extract on the level of (A) TNF-α and (B) IL-6 in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage.Values with different letters (a-f) indicate a significant difference (P<0.05).
IL-6 levels in control cells without LPS and cells treated with LPS were 251.8 and 9 823.1 µg/mL, respectively; while TNF-α levels were 224.1 and 890.2 µg/mL, respectively.O.japonicus significantly suppressed IL-6 and TNF-α production in a concentration-dependent manner (P<0.05) (Figure 3).
4.Discussion
The choice of solvent is important to extract useful components such as polyphenols, flavonoids in the plant matrix.Solvents used to extract phenolic compounds are often mixed with water in different proportions[27].Plant phenolic compounds are usually polar,and therefore extracted using polar solvents such as acetone and methanol[28].Using water with a solvent such as methanol, ethanol,and acetone enhances the extraction of phenolic compounds[28,29].Ethanol is a good solvent for polyphenol extraction[30].Several studies showed that ethanol and water combinations were more effective in extracting phenolic compounds than water alone and that ethanol extracts exhibited a higher antioxidant activity than aqueous extracts[31-33].This may be due to the higher performance of aqueous solvents in the extraction of highly polar bioactive compounds; whereas ethanol or ethanol/water solvent is more suitable for extracting bioactive compounds with broad ranges of polarity[32].Anthocyanin is a polar molecule with an aromatic ring conjugated with multiple hydroxyl, methoxy, and glycosyl groups.Hence, its extraction is more effective with water and ethanol mixture rather than only water.Several studies also reported high total anthocyanin content in 70% ethanol extracts of black currant and red cabbage[34,35].
According to previous studies, the antioxidant effect of O.japonicus has been reported.Lee et al.[2]previously determined DPPH radical scavenging activities in different parts of O.japonicus and showed 96% radical scavenging activity at 4 mg/mL in leaves,stems, and roots.At 0.4 mg/mL, leaf and stem extracts showed a radical scavenging activity of 96%, whereas the root extract showed a slightly lower activity of 92%.The 70% ethanol extract of O.japonicus, with a high content of total phenolic and flavonoid compounds, showed a high antioxidant activity due to the presence of the hydroxyl groups in phenolic compounds and radicalscavenging activity of flavonoids present in vegetables via hydrogen donation to free radicals[36].ABTS, DPPH, and superoxide radical scavenging activities of the O.japonicus extracts showed that 70% ethanol extracts with a higher amount of phenolic compounds were more active than water extracts.Antioxidant activities of 23 commercial vegetable juices including grape, carrot, tomato,and beetroot juices were correlated with their phenolic contents,which is in line with our results[7,37].Phenolic components of O.japonicus extracts were previously considered to donate hydrogen and electrons to active oxygen, thereby destroying the active oxygen chain and exhibiting a high reducing power[38].
MTT assay results of 0-400 µg/mL O.japonicus extracts indicated no cytotoxicity.NO is a free radical widely distributed in living organisms and regulates various biological functions such as vasodilation and immunoregulation[39].Excessive NO production can have detrimental effects including inflammatory reactions and cancer progression[40].Here, NO production was significantly reduced upon O.japonicus extract treatment in a dose-dependent manner.
Inflammation defends the body against many harmful stimuli and helps repair tissue damage.In contrast, excessive inflammation can cause disease, and thus function as a double-edged knife[20].As major inflammatory mediators, cytokines are secreted to regulate the amplification and duration of the inflammatory response[21].If acute inflammation is not treated and progresses to chronic inflammation,inflammatory cells infiltrate and secrete various inflammatory factors TNF-α and IL-6[22].They damage or mutate the DNA,produce tumors, and secrete excessive amounts of inflammatory cytokines or enzymes[21,22].This accelerates tumor growth, renders tumor cells capable of infiltrating surrounding tissues, and enhances metastasis[22].TNF-α is a polypeptide synthesized in macrophages and is involved in infection and inflammation[41].IL-6 is a cytokine produced early during the inflammatory response and was shown to promote cancer development in human and animal models[42].IL-6 and TNF-α have been reported to inhibit inflammatory responses by inhibiting cytokine production[41,42].O.japonicus extract likely prevents inflammatory reactions by reducing cytokines involved in NO production and inflammatory reactions such as TNF-α and IL-6.The O.japonicus extract contains polyphenols, flavonoids, and anthocyanins, and 70% ethanol has a higher effect on the extraction of bioactive substances than water.70% ethanol extract also showed stronger antioxidant activity than water extract.The antioxidant activities including DPPH, ABTS, and superoxide radical scavenging abilities increased with the increasing concentration of O.japonicus.In the range of O.japonicus extract (0-400 µg/mL) used in this experiment, the viability of RAW 264.7 cells was not affected; while NO, TNF-α, and IL-6 production were inhibited in a concentrationdependent manner.Through the above experimental results, O.japonicus extract is thought to inhibit the oxidation reaction and inflammation.
Conflict of interest statement
We declare that there is no conflict of interest.
Authors' contributions
EH designed the study and made the original, revision and final version of the manuscript, as well as supervised the project.NT performed in vitro experiment and analytical calculation.
 Asian Pacific Journal of Tropical Biomedicine2020年11期
Asian Pacific Journal of Tropical Biomedicine2020年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Justicia secunda Vahl leaf fraction protects against acetaminophen-induced liver damage in rats by alleviating oxidative stress and enhancing membrane-bound phosphatase activities
- Polygonatum kingianum rhizome extract alleviates collagen antibody-induced arthritis by modulating proinflammatory cytokine production in mice
- Phytochemical profile, antioxidant activity and wound healing properties of Artemisia absinthium essential oil
- Achillea biebersteinii extracts suppress angiogenesis and enhance sensitivity to 5-fluorouracil of human colon cancer cells via the PTEN/AKT/mTOR pathway in vitro
