Justicia secunda Vahl leaf fraction protects against acetaminophen-induced liver damage in rats by alleviating oxidative stress and enhancing membrane-bound phosphatase activities
Esther O.Aimofumeh, Godswill N.Anyasor, Ijeoma Esiaba
Department of Biochemistry, Benjamin S.Carson (Snr.) School of Medicine, Babcock University, Ilishan-Remo, Ogun State, Nigeria
ABSTRACT
KEYWORDS:Justicia secunda; Gas chromatography-mass spectrometry; Membrane-bound phosphatases; Hepatoprotection;Oxidative stress
1.Introduction
An overwhelming number of deaths around the world have been ascribed to non-communicable diseases, most of which are linked with oxidative stress[1].Non-approval drugs and withdrawal drugs by the Food and Drug Administration are one of the leading causes of drug-induced oxidative stress-related hepatotoxicity.Hepatic injury is a relatively common cause of acute hepatic disease with a mortality rate of about 10%[2].Several chemicals and medicines including acetaminophen have been reported to cause hepatic injury[3].
Acetaminophen (paracetamol, N-acetyl-p-aminophenol) is a drug of the para-aminophenol group used commonly for its analgesic and antipyretic purposes[3].Since the clinical introduction of acetaminophen, it has become the most popularly used over-thecounter analgesic-antipyretic drug all over the world.However, the overdose of acetaminophen has been reported as a leading cause of drug-induced hepatic failure in many countries[4].It has been demonstrated that cytochrome P450catalyzed the biotransformation of acetaminophen to a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI) that induces hepatotoxicity in rodents and humans.Mechanistically, excessive NAPQI depletes endogenous reduced glutathione (GSH) and forms adducts with proteins including mitochondrial proteins and induces mitochondrial oxidant stress and dysfunction.Consequently, nuclear DNA fragments and initiates necrotic cell death[5].
In the body system, there are mechanisms in place to maintain a balance between reactive oxygen species (ROS) and antioxidant systems.Cellular redox homeostasis is maintained by an elaborate endogenous antioxidant defence system including superoxide dismutase (SOD), catalase (CAT), and GSH[6].However, when the balance is tilted in favour of ROS, oxidative stress ensues, thereby eliciting oxygen free radical-induced cytotoxic effects.Consequently,membrane phospholipid peroxidation occurs leading to a change in membrane permeability, alteration in membrane phosphatase activity,and loss of membrane integrity[7].
Despite the tremendous progress in modern medicine, the prevention and treatment options for liver diseases remain scarce.Most of the therapies used in the treatment of acetaminophen toxicity have adverse effects.However, medicinal plants have always been considered as a relatively safe source of antioxidants including phenol and flavonoid compounds for the prevention of several oxidative stress-related diseases[8].Justicia secunda (J.secunda) Vahl (Family:Acanthaceae)is a perennial herb that grows up to 90 cm, with purplish-green stem,evergreen leaves, and pink flowers from South American origin.J.secunda is popularly known in the Venezuelan folk medicine as“Sanguinaria” and in Barbados as “bloodroot”.It grows in humid soils forming colonies beside rivers and creeks[9].It is usually collected from the wild for traditional use as a source of medicine.J.secunda is used in the management of numerous oxidative stress-induced disorders, including liver injury[10].J.secunda extract is also known to possess bioactive compounds with the potential to scavenge ROS and perhaps mitigate oxidative stress[10,11].Therefore, the present study was designed to investigate the protective effects of J.secunda leaf fraction against selected oxidative stress markers and membrane-bound phosphatases in acetaminophen-ROS induced hepatic damage in rats.
2.Materials and methods
2.1.Chemicals and reagents
Carboxymethyl cellulose and silymarin (Sigma-Aldrich, St.Louis,MO), alanine aminotransferase kit (Randox, United Kingdom) and aspartate aminotransferase kit (Randox, United Kingdom) were purchased.C-reactive protein ELISA kit (Labkit Barcelona, Spain)and rat oxidized low density lipoprotein ELISA kit (Mercodia Oxidized LDL ELISA kit, Mercodia, Inc.Uppsala, Sweden) were used.All other chemicals and reagents used were of analytical grade.
2.2.Experimental animals
Forty-two male albino rats (Wistar strain) weighing between 150-200 g were used in the study.Experimental animals were purchased from the Animal Facility, Babcock University, Ilishan-Remo, Ogun State.The rats were acclimatized under laboratory conditions preceding the experiment for 2 weeks, housed in a polypropylene cage lined with wood shaving, and provided with commercial pelleted diet and water ad libitum.
2.3.Plant collection and identification
J.secunda leaves were collected from farmland at Usaka Umuofor,Isiala Ngwa North, South-East of Nigeria.It was identified,authenticated, and assigned a voucher specimen number as 112177 at the Forestry Research Institute of Nigeria, Ibadan, Oyo State.
2.4.Extraction and partitioning procedures
Fresh leaves of J.secunda were plucked off the plant, washed,and oven-dried at 40 ℃ for 48 h.The dried leaves were pulverized using a mechanical grinder.Pulverized leaves (520 g) were extracted using 4 480 mL 70% methanol and the extraction process lasted for 72 h with intermittent shaking.The obtained extract was filtered using Whatman No.1 filter paper and the filtrate was subsequently concentrated using a rotary evaporator (Buchi Rotavapor RE-3,Buchi Labortecknic AG, Switzerland) at 40 ℃.Concentrates of J.secunda crude leaf extract (JSCRE) were reconstituted with distilled water in ratio 1:2 and further partitioned using a modified method of successive solvents to obtain the following fractions:J.secunda hexane leaf fraction (JSHLF), J.secunda ethyl acetate leaf fraction (JSELF), J.secunda butanol leaf fraction (JSBLF), and J.secunda aqueous leaf fraction (JSALF).Fractions were subsequently concentrated using the rotary evaporator at 40 ℃ and stored at 4 ℃until further use.
2.5.Quantitative phytochemical screening of J.secunda leaves extract/fractions
2.5.1.Determination of total phenol content
Total phenol contents of J.secunda leaves extract/fractions were determined following a modified Folin-Ciocalteu method as described by Singleton et al.[12].An aliquot of 0.5 mL each of J.secunda extract/fraction (1 mg/mL) was mixed with 2.5 mL Folin-Ciocalteu reagent (which was previously diluted with distilled water 1:10 v/v) and 2 mL 7.5% w/v of sodium carbonate.The tubes were vortexed for 15 s and allowed to stand for 30 min at 40 ℃ for color development.Gallic acid was used as a standard.Absorbance was measured at 750 nm using the ultraviolet-visible (UV-Visible)spectrophotometer (JP Selecta®, UV-2005, Abrera, Barcelona,Spain).Total phenol content was expressed as mg/g gallic acid equivalent extrapolated from the calibration curve.
2.5.2.Determination of total flavonoid content
Total flavonoid contents of J.secunda leaves extract/fractions were determined using the aluminum chloride method as described by Ordonez et al.[13].Half a milliliter of J.secunda extract/fractions (1 mg/mL) was mixed with 0.5 mL of 2% aluminum chloride (AlCl3)which was prepared in ethanol.The resultant mixture was then incubated for 60 min at room temperature for the development of a yellow color which indicated the presence of flavonoid.The absorbance at 420 nm was measured using a UV-visible spectrophotometer (JP Selecta®, UV-2005, Abrera, Barcelona,Spain).Total flavonoid content was expressed as mg/g quercetin equivalent extrapolated from the calibration curve.
2.5.3.Determination of tannin content
The tannin content of J.secunda leaves was determined following the modified vanillin-HCl methanol method as described by Noha et al.[14].The vanillin-HCl reagent was prepared by mixing equal volumes of 8% HCl and 1% vanillin in methanol.The reagent was mixed prior to use.Pulverized J.secunda leaves (0.2 g) were placed in a small conical flask.Thereafter, 10 mL of 1% concentrated HCl in methanol was added.The flask was capped and shaken continuously for 20 min and the content was centrifuged at 2 500 rpm for 5 min.The supernatant (1.0 mL) was pipetted into a test tube containing 5 mL of vanillin-HCl reagent.The absorbance was measured at 450 nm with a UV-visible spectrophotometer (JP Selecta®, UV-2005, Abrera, Barcelona, Spain) after 20 minutes of incubation at 30 ℃.A standard curve was prepared to express the result as a catechin equivalent as follows:

where C = concentration corresponding to the optical density; 10 =volume of the extract (mL); 200 = sample weight (mg).
2.5.4.Determination of saponin content
The saponin content of J.secunda leaves was determined by following a modified procedure described by Okwu and Josiah[15].Five grams of pulverized J.secunda leaves were dispersed in 50 mL of 20% v/v ethanol which was prepared in distilled water.The suspension was heated over a hot water bath for 4 h with continuous stirring at 55 ℃.The mixture was filtered and the residue was reextracted with 50 mL of 20% ethanol.The combined extracts were reduced to 20 mL over a hot water bath at 9 ℃.The obtained concentrated solution was shaken vigorously with 10 mL of diethyl ether in a 250 mL separating funnel; the aqueous layer was collected and the ether layer was discarded.The purification process was repeated.Subsequently, 20 mL of butanol was added to the filtrate and the resultant butanol extract mixture was extracted twice by adding 10 mL of 5% w/v aqueous sodium chloride and filtered.After discarding the sodium chloride layer, the remaining whole solution was then heated to evaporation on the hot water bath and was later oven-dried at 40 ℃ to obtain a constant weight.The percentage saponins content in the sample was calculated using the formula below:% Saponins = (Weight of final filtrate)/(Weight of sample)×100
2.5.5.Determination of alkaloid content
The alkaloid content of J.secunda leaves was determined following a modified method described by Onyilagha and Islam[16].Five grams of pulverized J.secunda leaves were weighed into a 250 mL beaker and 200 mL of 20% acetic acid in ethanol was added.This was covered, allowed to stand for 4 h, and then filtered.The extract was concentrated with the use of a water bath.Concentrated ammonium hydroxide was added dropwise to the extract until precipitation was complete.The whole solution was allowed to settle and the collected precipitates were washed with dilute ammonium hydroxide and subsequently filtered.The residue was dried and weighed.The alkaloid content was determined using the formula below:
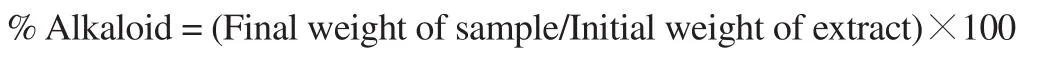
2.6.Determination of in vitro antioxidant activities of J.secunda leaves extract/fractions
2.6.1.2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
DPPH radical scavenging activities of J.secunda leaves extract/fractions were determined following the procedure described by Brand-Williams et al.[17].Two milliliters of the crude extract and fractions at ranging concentrations of 50, 100, 150, and 200 µg/mL were separately mixed with 0.5 mmol/L DPPH which was prepared in methanol.This was incubated in the dark for 30 min and was read with the aid of a UV-Visible spectrophotometer (JP Selecta®, UV-2005, Abrera, Barcelona, Spain) at a wavelength of 517 nm.
The percent of inhibition was calculated using the following method:

where Ablankis the absorbance of the control reaction (containing all reagents except the test compound), and Asampleis the absorbance of the test compound.Sample concentration providing 50% inhibition(IC50) was calculated from the graph plotting inhibition percentage against extract concentration.
2.6.2.Total antioxidant potential assay
The total antioxidant capacity of J.secunda leaves extract/fractions was determined using the method described by Prieto et al.[18].An aliquot of 0.1 mL of 1 mg/mL test fractions of J.secunda was combined with 1 mL of molybdate reagent solution (0.6 mol/L sulfuric acid, 28 mmol/L sodium phosphate and 4 mmol/L ammonium molybdate) in a tube.The tubes were capped and incubated in a boiling water bath at 95 ℃ for 90 min.Immediately after cooling the sample to room temperature, the absorbance of the reaction mixture was measured at 695 nm with the aid of a UV-Visible spectrophotometer (JP Selecta®,UV-2005, Abrera, Barcelona, Spain).
2.6.3.In vitro lipid peroxidation assay
The determination of J.secunda leaves extract/fractions effect on lipid peroxidation was carried out according to the procedure described by Janero[19].Egg homogenate (250 µL, 10%) and varying concentrations of extract/fractions (10, 20, 50, and 100 µg/mL)were mixed in a test tube with the volume made up to 500 µL with distilled water.Twenty-five microliters of 0.07 mol/L FeSO4were added to the above mixture and incubated for 30 min to induce lipid peroxidation.Thereafter, 750 µL of 20% acetic acid (pH 3.5) and 750 µL of 0.8% thiobarbituric acid (w/v) (prepared in 1.1% sodium dodecyl sulfate) and 25 µL 20% trichloroacetic acid were added,vortexed and heated in a boiling water bath (95 ℃) for 60 min.After cooling, 3.0 mL of n-butanol was added to each tube and was centrifuged at 3 000 rpm for 10 min.The absorbance of the organic upper layer was measured at 532 nm with the aid of the UV-visible spectrophotometer (JP Selecta®, UV-2005, Abrera, Barcelona,Spain).For the control, 50 µL of distilled water was used in place of the extract/fractions.

where A0is the absorbance of the control and Atis the absorbance of the test sample/standards.
2.6.4.Ferric reducing antioxidant potential (FRAP) assay
FRAP of J.secunda leaves extract/fractions was determined using the method described by Benzie and Strain[20].The freshly prepared 2 mL FRAP reagent [500 mL acetate buffer (300 mmol/L, pH 3.6),50 mL 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ) (10 mmol/L), and 50 mL FeCl3·6H2O (50 mmol/L)]was mixed with 75 µL each of the crude extract and fractions to form an intense blue Fe2+-TPTZ complex and the optical density was read using the UV-Visible spectrophotometer (JP Selecta®, UV-2005, Abrera, Barcelona, Spain)after two min at 593 nm against the blank with ascorbic acid serving as a standard.
2.7.Gas chromatography-mass spectrophotometry (GC-MS)analysis
GC-MS analysis was carried out using the Agilent Technology model GCMS-QP2010SE SHIMADZU, Japan.One microliter of JSELF was injected.Viscosity compensation time was 0.2 s,injection mode:normal, washing volume:8 µL, column oven temperature:60 ℃, injection temperature:250 ℃, pressure:144.4 kPa, total flow:38.7 mL/min, column flow:3.22 mL/min, linear velocity:46.3 cm/s, purge flow:3.0 mL/min.The constituent compounds were determined by comparing the retention times and mass weights with those obtained from the GC as well as the mass spectra National Institute of Science and Technology version 2.0 MS database and literature.
2.8.Acute oral toxicity test
The acute oral toxicity test was carried to determine the safe dose of JSELF following the up and down method described by the Organization for Economic Cooperation and Development guidelines[21].Six rats were randomly selected, weighed, and placed in cages.Three rats were individually treated with 2 g/kg b.wt.JSELF while the remaining three rats were given an equal volume of distilled water, orally by gastric gavage.After 48 hours of observation, no visible signs of toxicity, as well as mortality, were recorded.
2.9.Animal experimental design
Thirty-six male albino rats (Wistar strain) were randomly distributed into 6 groups of 6 rats each.All test agents were orally administered using 1% carboxymethyl cellulose as the vehicle.GroupⅠ(normal):rats were administered with carboxymethyl cellulose alone.GroupⅡ (untreated control):rats were administered with 2 g/kg body weight (b.wt.) acetaminophen once on the 14th day[22].Group Ⅲ(standard):rats were administered with 2 g/kg b.wt.acetaminophen and 100 mg/kg b.wt.silymarin[23].Group Ⅳ, Ⅴ, and Ⅵ:rats were administered with 100, 300, and 500 mg/kg b.wt.JSELF respectively, followed by 2 g/kg b.wt.acetaminophen.The time interval between JSELF and acetaminophen administration was 60 minutes on the 14th day.On the 15th day, the rats were anaesthetized using diethyl ether and sacrificed.Blood samples were collected via ocular puncture and processed to estimate liver function, C-reactive protein (CRP), and oxidized low-density lipoprotein (oxLDL), while isolated liver samples were used to analyze for in vivo antioxidants,membrane-bound phosphatases.
2.10.Determination of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities
Effects of JSELF on ALT and AST were determined using the protocols as provided by Randox diagnostic kit manufacturer(Randox, United Kingdom).
2.11.Determination of in vivo antioxidant activities
JSELF effects on endogenous antioxidants were estimated as follows:SOD activity was determined according to the method described by Misra and Fridovich[24]; GSH activity was determined according to the method described by Jollow et al.[25]; CAT activity was determined according to the method described by Sinha[26].
2.12.Determination of serum CRP and oxLDL
JSELF effect on serum CRP concentration was measured using the method of enzyme-linked immunosorbent assay (ELISA) kit (Labkit Barcelona, Spain); while serum oxLDL concentration was measured by using rat oxLDL ELISA kit (Mercodia Oxidized LDL ELISA kit,Mercodia, Inc.Uppsala, Sweden).
2.13.Determination of membrane-bound phosphatase activities
JSELF effect on total ATPase activity in the hepatic membrane was determined by using the method described by Evans[27].Ca2+ATPase activity in the hepatic membrane was determined using the method described by Hjerten and Pan[28].Mg2+ATPase activity in the hepatic membrane was determined using the method described by Ohnishi et al.[29].Na+K+ATPase activity in the hepatic membrane was determined using the procedure described by Bonting[30].
2.14.Statistical analysis
Experiments were performed in triplicates and data were expressed as mean ± standard error of mean.Data analysis was performed using GraphPad Instant software (GraphPad Prism®, Standard version 7.0).Analysis of Variance (ANOVA) followed by Duncan's new multiple range test was used to analyze the data.Statistical significance was set at P<0.05.
2.15.Ethical statement
The animals were carefully handled following ethical standards and protocols for the care and use of laboratory animals by the National Institute of Health in 2011 and study protocol was approved by Babcock University Health Research and Ethics Committee(BUHREC) with certificate number BUHREC684/18.
3.Results
3.1.Quantitative phytochemical screening of extract and fractions of J.secunda leaves
Table 1 shows that JSELF exhibited a significantly higher total phenol and total flavonoid contents (P<0.05) compared with JSBLF,JSCRE, JSHLF, and JSALF.Alkaloid, saponin and tannin contents in J.secunda leaves were (2.93±1.41)%, (25.13±0.12)% and(11.16±0.00)%, respectively.
3.2.In vitro antioxidant activities of J.secunda leaves extract/fractions
Figure 1 shows that the crude extract and fractions of J.secunda leaves and ascorbic acid scavenged DPPH radical in a concentrationdependent manner.At 50 µg/mL and 150 µg/mL, JSBLF and JSELF had higher DPPH scavenging activity and there was no significant difference between them (P>0.05); while at 100 and 200 µg/mL,JSBLF was highest.The IC50of JSBLF was the lowest (168.50 µg/mL) among all fractions followed by JSELF (201.70 µg/mL), JSCRE(205.90 µg/mL), JSHLF (361.30 µg/mL), and JSALF (388.30 µg/mL).The IC50of ascorbic acid was 5.58 µg/mL.
Result of total antioxidant potential assay showed that JSELF[(1.49±0.01) mg AAE/g]had the highest total antioxidant capacity followed by JSBLF [(1.04±0.03) mg AAE/g], JSCRE [(0.86±0.05)mg AAE/g], JSHLF [(0.65±0.01) mg AAE/g]and JSALF[(0.44±0.02) mg AAE/g]at 100 µg/mL.
Figure 2 shows that gallic acid, JSELF, and JSBLF significantly(P<0.05) inhibited lipid peroxidation.Gallic acid had the highest inhibition of lipid peroxidation at 50 and 100 µg/mL, while JSCRE had the highest inhibition of lipid peroxidation at 10 and 20 µg/mL.However, at 100 µg/mL, JSELF had the highest inhibition of lipid peroxidation while JSALF had the least.
Figure 3 shows that the test extract/fractions of J.secunda leaves had elevated FRAP in a concentration-dependent manner.JSCRE had the highest FRAP at 100 and 200 µg/mL; while JSBLF had the highest FRAP at 50 µg/mL.The JSALF had the least FRAP at all concentrations.
3.3.GC-MS analysis result
Table 2 shows twenty-two compounds tentatively identified in JSELF by GC-MS and their reported bioactivities.Phytol had the highest concentration (25.97%), while phenol, 2,6-bis(1,1-dimethyl)-had the least concentration (0.38%).Phenol, 2,6-bis(1,1-dimethyl)-,3,7,11,15-tetramethyl-2-hexadecen-1-ol, hexadecanoic acid, methylester, n-hexadecanoic acid, phytol, 1,2-15,16-diepoxyhexadecane,6-epi-shyobunol possess antioxidant properties, while 9,12-octadecadienoic acid, methyl ester, 9,12,15-octadecatrienoic acid, methyl ester, 9,12-octadecadienoic acid (Z,Z)-, 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- have hepatoprotective properties.

Table 1.Quantitative phytochemical screening of extract and fractions of Justicia secunda leaves.

Figure 1.DPPH radical scavenging activities of Justicia secunda crude extract and fractions.JSCRE:J.secunda crude leaf extract; JSHLF:J.secunda hexane leaf fraction; JSELF:J.secunda ethyl acetate leaf fraction; JSBLF:J.secunda butanol leaf fraction; JSALF:J.secunda aqueous leaf fraction; Different letters indicate significant difference at P<0.05.DPPH:2,2-diphenyl-1-picrylhydrazyl.Values are expressed as mean ± SEM (n=3).

Figure 2.Inhibition of lipid peroxidation activities by J.secunda crude extract and fractions.Different letters indicate significant difference at P<0.05.Values are expressed as mean ± SEM (n=3).

Figure 3.Ferric reducing antioxidant potential (FRAP) of J.secunda crude extract and fractions.AAE:Ascorbic acid equivalent; Different letters indicate significant difference at P<0.05.Values are expressed as mean ± SEM (n=3).
3.4.Effects of JSELF on liver enzymes and oxidative stress biomarkers
Table 3 shows that animal groups treated with 100 mg/kg b.wt.silymarin and JSELF at 100, 300, 500 mg/kg b.wt.significantlyALT:alanine aminotransferase; AST:aspartate aminotransferase; Group Ⅰ:normal (carboxymethyl cellulose alone); Group Ⅱ:untreated control (2 g/kg b.wt.acetaminophen only); Group Ⅲ:standard (2 g/kg b.wt.acetaminophen and 100 mg/kg b.wt.silymarin); Group Ⅳ-Ⅵ:test extract (100, 300 and 500 mg/kg b.wt.JSELF respectively, followed by 2 g/kg b.wt.acetaminophen); Different letters indicate values are significantly different at P<0.05.Values are expressed as mean ± SEM (n=6).reduced plasma ALT and AST activities compared with the untreated control group (P<0.05).The decreases of ALT and AST in JSELF treated group at 300 mg/kg b.wt.were more significant than silymarin treated group (P<0.05).

Table 2.Bioactivity of the compounds from ethyl acetate fraction of J.secunda leaves detected by GC-MS analysis.

Table 3.Effects of ethyl acetate fraction of J.secunda leaves on liver enzymes and oxidative stress biomarkers.

Table 4.Effects of J.secunda ethyl acetate leaf fraction on membrane-bound phosphatase activities in acetaminophen-induced rats.
Silymarin and JSELF at all doses significantly elevated SOD, CAT and GSH levels compared with the untreated control group (P<0.05).The increases in CAT and GSH levels of JSELF treated group at 500 mg/kg b.wt.were more significant than silymarin treated group(P<0.05).
In addition, silymarin and JSELF at all doses significantly reduced CRP and oxLDL concentrations compared with the untreated control group (P<0.05).The decrease of CRP in groups treated with 500 mg/kg b.wt.JSELF was more significant compared with silymarin treated group (P<0.05).
3.5.Effects of JSELF on membrane-bound phosphatase activities
Table 4 shows that silymarin and JSELF treated groups had improved total ATPase, Na+K+ATPase, and Mg2+ATPase activities compared with the untreated control group.In addition, groups treated with silymarin and 300 mg/kg b.wt.JSELF had improved Ca2+ATPase activities.
4.Discussion
Oxidative damage is associated with the pathogenesis of numerous diseases.Plant bioactive compounds could mitigate the deleterious effects of oxygen radicals and play a critical role in the prevention and reversal of oxidative stress-induced disorders.In this study,the quantitative phytochemical evaluation showed that J.secunda crude extract and fractions contain considerable amounts of phenols,flavonoids, tannins, saponins, and alkaloids.It is reported that these bioactive compounds possess antioxidant properties and are capable of inhibiting deleterious metabolites including hydroxyl, peroxyl,superoxide anion radicals, and oxidative reactions triggered by ROS in biological systems[31].
Phenols, tannins, and flavonoids had been reported to exhibit hepatoprotective, nephroprotective, anti-inflammatory, and antioxidant activities[32].These beneficial biological effects might be due to the presence of structural hydroxyl functional groups[33].JSELF contained high flavonoid and phenol contents compared with the other fractions.Flavonoids and phenols are potent antioxidants that could prevent oxidative damage on tissues.Alkaloids have been reported to have anti-microbial, anti-fungal, anti-diarrheal, and antiinflammatory effects[34].Saponins have been demonstrated to have several pharmacological effects including anti-inflammatory, antimicrobial, and anthelmintic activities.The non-sugar moiety of saponins has a direct antioxidant action which could lead to reduced cancer risk and heart disease.Tannins are employed as diuretics in the treatment of diarrhea and duodenal tumors.Tannins also possess anti-inflammatory, antiseptic, and antioxidant properties[35].The presence of these phytochemicals in J.secunda leaves may account for its ethnomedical use in the treatment of several oxidative stressinduced disorders.
Crude extract and fractions of J.secunda leaves were subjected to in vitro antioxidant assays to determine the most active antioxidant fraction.The crude extract and test fractions of J.secunda leaves showed positive results as a scavenger of DPPH radical, evidenced by the decolorization of DPPH radical from purple to yellow color.When DPPH radicals react with an appropriate reducing agent, it loses color stoichiometrically depending on the number of electrons abstracted.JSBLF exhibited the highest DPPH scavenging activity in close proximity with JSELF compared with the other test fractions.This antioxidant effect could be attributed to the presence of the hydroxyl groups in the polyphenol and flavonoid ring structures of the antioxidant compounds present in J.secunda leaves.JSELF had the highest total antioxidant capacity compared with other fractions.Further study showed that JSELF exhibited the high FRAP and the most significant inhibition of lipid peroxidation compared with other test fractions.Hence, this study showed that JSELF was the most active antioxidant fraction.It has been previously demonstrated that plant extract with polyphenolic property could exhibit antioxidant activity in vitro[3].
GC-MS analysis of JSELF detected several compounds ranging from those employed as raw materials in industrial processes,agricultural production, cosmetics, plasticizer to compounds of medicinal value including antioxidant, hepatoprotective,nephroprotective, anticancer, antimicrobial, anti-inflammatory, antidiarrheal, and anti-diabetic.Bioactive compounds present in JSELF include phenol, 2,6-bis(1,1-dimethyl)-; 3,7,11,15-tetramethyl-2-hexadecen-1-ol; hexadecanoic acid, methyl ester; phytol, 1,2-15,16-diepoxyhexadecane; 6-epi-shyobunol; 9,12-octadecadienoic acid,methyl ester; 9,12,15-octadecatrienoic acid, methyl ester; 9,12-octadecadienoic acid (Z,Z) and 9,12,15-octadecatrienoic acid,(Z,Z,Z).Phenol, 2,6-bis(1,1-dimethyl)-, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, hexadecanoic acid, methyl ester, n-hexadecanoic acid, phytol, 1,2-15,16-diepoxyhexadecane, 6-epi-shyobunol were reported to have antioxidant properties; while 9,12-octadecadienoic acid, methyl ester, 9,12,15-octadecatrienoic acid, methyl ester, 9,12-octadecadienoic acid (Z,Z)-, 9,12,15-octadecatrienoic acid, (Z,Z,Z)-were reported to have hepatoprotective property[36].The suspected bioactive compounds could account for the use of J.secunda leaves in ethnomedical practice.
In the animal study, groups treated with JSELF had reduced plasma ALT and AST activities compared with the untreated control group.This suggests that JSELF could possess hepatoprotective activity.Plant bioactive compounds with antioxidant potential could elicit hepatoprotective activity against ROS-induced hepatic damage[37].Hepatic injury induced by acetaminophen is widely employed as a model for the screening of hepatoprotective drugs.Hepatotoxic drugs such as acetaminophen have been well documented to cause a marked elevation in plasma ALT and AST activities.It has also been documented that N-acetyl-para-benzoquinone imine, a reactive intermediate of acetaminophen metabolism acts as an oxidative stressor that forms a covalent adduct with tissue macromolecules causing hepatic damage[5].
Further investigations revealed that silymarin and JSELF-treated groups had elevated hepatic SOD, CAT, and GSH levels compared with the untreated control group.This indicates that JSELF-treated groups could protect against acetaminophen-induced hepatic damage.It was thought that the presence of phenols and flavonoids in JSELF might have contributed to this hepato-protection, either by augmenting or complementing the endogenous antioxidant defence system thereby reducing oxidative stress.Research has shown that increased SOD, CAT, and GSH levels could serve as important indicators of the well-being of the body system against oxidative damage.In particular, GSH could serve as a sulfhydryl buffer in protecting thiol groups in macromolecules, most especially proteins,from the damaging effects of NAPQI[38].
Additional investigation showed that JSELF treated groups had reduced serum CRP and oxLDL concentrations compared with the untreated control group.This indicates that JSELF perhaps protected the hepatocytes against oxidative stressors.CRP is an acute-phase reactant that is synthesized in the liver in response to interleukin-6.It has been reported as a biomarker for tissue damage.High serum CRP level has been reported in patients with liver failure[39].Furthermore, biological lipids are known to be a consistent target for oxidative stressors.A study reported that accumulation of oxLDL is associated with liver fibrosis and studies on animals and humans have shown that there is a close relationship between lipid peroxidation and liver injury[40].
Moreover, JSELF-treated animals had improved total ATPase,Ca2+ATPase, Na+K+ATPase, and Mg2+ATPase activities.This suggests that JSELF could have protected the hepatocyte membrane from acetaminophen-induced oxidative damage.Plant-derived compounds have been demonstrated to influence membrane characteristics and stability against oxidative damage[41].Na+K+ATPase pump helps to maintain osmotic equilibrium and membrane potential in cells and it also maintains the gradient of a higher extracellular sodium concentration and a higher intracellular potassium concentration[42].Previous studies have revealed that ROS could act on the sulphydryl groups present in the active site of Ca2+ATPase impairing its function with the attendant release of inorganic phosphate ions[43].Mg2+ATPase is involved in energyrequiring processes in the cell and requires Mg2+as an essential cofactor for the activation of enzymatic ATP hydrolysis[44].
5.Conclusion
Thus, findings from this study indicate that JSELF exhibited protective action against oxidative stress and improved the membrane-bound phosphatases activities in acetaminophen-induced hepatic damage in rats.This protective action might be through the mitigation of oxidative stressors by bioactive compounds.It is recommended that JSELF could be further explored as a source of lead chemoprotective drug candidates in the management of oxidative stress-related disorders.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
This research was supported by a grant funded by the Remi Awode Foundation.We thank the Department of Biochemistry for the provision of laboratory facilities and space.We also thank Mr.Samson, Animal Facility Manager, Babcock University for his assistance throughout the animal experiment phase.contributed to research design, executed the research, analysed,interpreted data and write-up and revision of the manuscript.EI contributed to the conception, analysed, interpreted data, and revised draft and final manuscript.
Authors' contributions
AG designed the research concept, collected, analysed, interpreted data and contributed to drafting and revision of the manuscript.AE
 Asian Pacific Journal of Tropical Biomedicine2020年11期
Asian Pacific Journal of Tropical Biomedicine2020年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Polygonatum kingianum rhizome extract alleviates collagen antibody-induced arthritis by modulating proinflammatory cytokine production in mice
- Phytochemical profile, antioxidant activity and wound healing properties of Artemisia absinthium essential oil
- Achillea biebersteinii extracts suppress angiogenesis and enhance sensitivity to 5-fluorouracil of human colon cancer cells via the PTEN/AKT/mTOR pathway in vitro
- Antioxidant and anti-inflammatory activities of Orostachys japonicus
