Phytochemical profile, antioxidant activity and wound healing properties of Artemisia absinthium essential oil
A.Benkhaled, A.Boudjelal✉, E.Napoli, F.Baali, G.Ruberto✉
1Department of Microbiology and Biochemistry, Faculty of Sciences, University of M'sila, Algeria
2Istituto del CNR di Chimica Biomolecolare, Catania, Italy
3Department of Biological Sciences, Faculty of Nature and Life Sciences, University of BBA, Algeria
ABSTRACT
KEYWORDS:Artemisia absinthium; Essential oil; Antioxidant;Wound healing
1.Introduction
Artemisia absinthium (A.absinthium) popularly known as common wormwood belongs to the family Asteraceae (Compositae).The genus Artemisia consists of over 500 different species and is distributed in almost all temperate zones.Many Artemisia species have been studied for their phytochemical profiles and pharmacological properties.A.absinthium has a long history as a medicinal plant[1,2]and is native to temperate regions of Eurasia and Northern Africa but is rather rare in Algeria[3].A.absinthium contains an essential oil that is characterized by a particular color ranging from green to dark blue.One of the most peculiar features of this oil is the presence of large amounts of thujone,an oxygenated monoterpene, which can account for 40%-90% of the oil.This compound is normally present in two isomeric forms named α- and β-thujone and the latter is more abundant.Other important constituents of A.absinthium are the non-volatile sesquiterpene lactones, particularly absinthin, which are responsible for the bitter taste of its extracts.
Besides the use as a medicinal plant, A.absinthium was used between the end of the 19th and the beginning of the 20th century for the production of the spirit absinthe, whose production and consumption grew very largely during the aforesaid periods[4-6].However, this alcoholic beverage was successively banned since the presence of thujone was considered as the cause of the socalled ‘absinthism' syndrome, including convulsions, hallucinations and mental disorders.However, no correlation was found between thujone and health.Then the limit of thujone content has been set as 35 mg/L in alcoholic beverages[2,7].
As a medicinal plant, A.absinthium has been used as an antipyretic,antispasmodic anti-inflammatory and memory-improving agent and was used to treat abscesses, wounds and other skin diseases[1,8].In a recent study, the petroleum ether extract of A.absinthium collected in Turkey, which was particularly rich in volatile components, showed positive effects on in vivo induced pseudopregnancy[9].
A recent ethnopharmacological survey performed in Algeria reports that various phytotherapeutic preparations from Artemisia spp.(including A.absinthium) possess antidiabetic, antihypertensive, and analgesic properties[10].Furthermore, A.absinthium essential oil also showed pesticide, insecticidal, and antimicrobial activities[8,11-13].
The main aim of the present study was to evaluate the antioxidant efficacy and the in vivo wound healing properties of A.absinthium essential oil and to provide a reference for sustainable exploitation of Algerian flora.
2.Materials and methods
2.1.Reagents and standards
All solvents used were high-purity American Chemical Society solvents from VWR (Milan, Italy); reagents and reference standards were purchased from Sigma-Aldrich Products (Merck KGaA,Darmstadt, Germany), Extrasynthese (Lyon, France) and Fluka(Milan, Italy).
2.2.Plant material
The flowering aerial parts of A.absinthium L.were collected in May 2018, in M'sila (situated in the central part of Algeria) at 35 º12'36.97" N latitude and 4º10' 46.08" E longitude.The plant was authenticated by Dr.Sarri DJ, Department SNV/ M'sila University,and a specimen (AB-13) was deposited at the herbarium of the Department.
2.3.Essential oil extraction
The essential oil was extracted by hydrodistillation using a Clevenger-type apparatus (Clevenger D04000201, Somiver, Algeria)according to the European Pharmacopoeia[14].The oil was dried with anhydrous sodium sulfate, weighed, and stored at 4℃ until use.
2.4.Essential oil analysis
Gas chromatographic (GC) analyses were performed on a Shimadzu gas chromatograph, Model 17-A (Shimadzu, Japan) equipped with a flame ionization detector (FID) and with an operating software Class VP Chromatography Data System version 4.3 (Shimadzu).Analytical conditions:SPB-5 capillary column (15 m×0.10 mm×0.15 µm), helium as carrier gas (1 mL/min).Injection in split mode(1:200), injected volume 1 µL (4% essential oil/CH2Cl2v/v), injector and detector temperature 250 and 280℃, respectively.Linear velocity in column 19 cm/s.The oven temperature was held at 60℃for 1 min, then, programmed as reported previously[15].Percentages of compounds were determined from their peak areas in the GC-FID profiles.
Gas-chromatography-mass spectrometry (GC-MS) was carried out in the fast mode on a Shimadzu GC-MS mod.GCMS-QP5050A,with the same column and the same operative conditions used for analytical GC-FID and GCMS solution version 1.02 operating software (Shimadzu).Ionization voltage 70 eV, electron multiplier 900 V, ion source temperature 180℃.Mass spectra data were acquired in the scan mode in the m/z range 40-400.The same oil solutions (1 µL) were injected with the split mode (1:96).
2.5.Identification of components of essential oil
The identity of components was based on their GC retention index (relative to C9-C22n-alkanes on the SPB-5 column), computer matching of spectral MS data with those from NIST MS libraries[16],the comparison of the fragmentation patterns with those reported in the literature[17]and, whenever possible, co-injections with authentic samples.
2.6.Total antioxidant capacity (TAC)
The TAC of A.absinthium essential oil was evaluated by the phosphomolybdenum method[18].An aliquot of 0.3 mL of the essential oil was mixed with 3 mL of the reagent solution (0.6 mol/L of sulfuric acid, 28 mmol/L of sodium phosphate and 4 mmol/L of ammonium molybdate).The reaction mixture was incubated at 95℃ for 90 min and the absorbance measured at 695 nm.Butylated hydroxytoluene (BHT) was used as standard antioxidant.The antioxidant activity was expressed in µg equivalent of BHT per mg of essential oil (µg EBHT/mg essential oil).
2.7.DPPH radical scavenging activity
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging capacity was measured according to Li et al[19].A total of 1 mL of the freshly prepared 0.1 mM DPPH in methanol was added to the 3 mL of EO solution at different concentrations (0.05-1.5 mg/mL).The reaction mixture was left to stand at room temperature in the dark for 30 min and the absorbance was recorded at 517 nm.The ability to scavenge the DPPH radical was calculated using the following equation:

where Acand Asare the absorbances of the control and the sample,respectively.BHT was used as the reference compound.The assay was done in triplicate.
2.8.Scavenging activity of ABTS radical
The scavenging activity of ABTS radical (ABTS+) has been evaluated following the method of Re et al[20].An aliquot of 1 mL of essential oil was mixed with 2 mL of diluted ABTS+, after 30 min of incubation; the absorbance was measured at 734 nm.The percentage of ABTS+inhibition was calculated using the following formula:

where Acand Asare the absorbances of the control and the sample,respectively.Trolox was used as the reference antioxidant.All measurements were performed in triplicate.
2.9.Wound healing activity
2.9.1.Preparation of the ointment
The A.absinthium essential oil (AEO) was mixed with petroleum jelly (PJ) (Unilever, France) in two concentrations of 5% and 10% to obtain the essential oil ointments OAEO 5% and 10%,respectively[21].Cicatryl-Bio (CIC) (Pierre Fabre, France) was used as a reference drug.
2.9.2.Animals
Healthy adult male Wistar albino rats, weighing between 200-220 g, were obtained from Pasteur Institute of Algeria.Animals were fed with commercially pelleted rat diet and water ad libitum.
2.9.3.Experimental protocol
The rats were weighed, marked and divided into 5 groups of 5 rats each:Untreated group (UT group), Cicatryl-treated group (CIC group), ointment artemisia essential oil 5%-treated group (OAEO 5% group), ointment artemisia essential oil 10%-treated group(OAEO 10% group), and petroleum jelly-treated group (PJ group).An area of 250 mm2on the back of the rat was shaved.The animals were left in their cages 24 hours to verify the absence of irritation of the shaved zone[22].
2.9.4.Skin irritation test
The OAEO 5% and 10% were applied separately on the back of the rats.After 4 h, the skin of the animals was evaluated to detect the presence of inflammation signs[23].
2.9.5.Healing activity
Animals were anaesthetized using intraperitoneal injection of ketamine (90 mg/kg)-xylazine (10 mg/kg)[24].A circle approximately 2.5 cm in diameter was drawn on the skin of the lumbar region and was then excised.The animals were placed in individual cages with clean litters.
Preparations (CIC, OAEO 5%, OAEO 10%, and PJ) were applied locally at an amount of 0.5 g per rat of the different groups once per day until complete re-epithelialization[25].The dimensions of excision wounds were measured every 3 days during the trial period by tracing the wounds on transparent paper and measuring through the graph paper.The percentage of the evolution of wound contraction was calculated using the following formula[26]:

2.9.6.Histological sections
At the end of the experimentation, the rats were sacrificed, the skin healed and 0.5 cm of healthy skin were removed for histological study[25].The tissue slices were fixed in formalin (10%) for 72 h.These samples were dehydrated by passing it through three successive baths of ethanol.Then they were thinned in two baths of xylene and embedded in paraffin by two successive baths at 60℃each one.The obtained paraffin blocks were then cut by microtome,rehydrated, stained with haematoxylin-eosin[27]and analyzed by Optika B-500 microscope (Optika s.r.l., Italy) at×10 and ×40 magnifications.
2.10.Statistical analysis
All statistical analyses were performed using GraphPad Prism 7.0.Data were expressed as mean ± SD.Statistical significance between groups was analyzed by one-way ANOVA followed by Dunnett's test.Values for P<0.05 were considered statistically significant.
2.11.Ethical statement
All experimental protocols were approved by the National Committee for Evaluation and Programming of University Research of Algerian Ministry of Higher Education and Scientific Research(Registration N :DO1N01UN280120150001), according to International Council for Laboratory Animal Science[28].
3.Results
3.1.Yield and chemical composition
The extraction yield of essential oil from the flowering aerial parts of A.absinthium collected in Algeria was (0.29 ± 0.08)% as v/w.The oil showed an intense dark blue color.
A total of 75 components were identified in the oil (Table 1).Oxygenated monoterpenes were quantitatively the main constituents accounting for 59.64% of the total oil and including 19 components.Camphor with ca.47.59% was the main compound of this class and also of the total oil, followed by terpinen-4-ol (ca.6.36%).While linalool and cis-sabinene hydrate also exceeded 1%, the content of all other components was below this value.The second class of components was represented by sesquiterpenes accounting for 16.67% of the oil.It included more classes with 24 components.Chamazulene, which is responsible for the blue color of the oil reached a higher concentration (10.35%).Among the other components, only caryophyllene oxide and a dihydrochamazulene isomer slightly exceeded 1%.Monoterpene hydrocarbons accounted for 14.93% of the oil and included 13 components.The main compounds were γ-terpinene, camphene, p-cymene, α-pinene, and α-terpinene.The last group was represented by the so-called ‘other compounds', with a total content of 3.73% and 18 components.The composition was rather variegated and the main component was geranyl p-cymene.A typical GC profile of A.absinthium essential oil with some of the main components is shown in Figure 1.

Table 1.Chemical composition of the essential oil of Artemisia absinthium from Algeria.
3.2.In vitro antioxidant activity
The antioxidant activity of A.absinthium essential oil was evaluated by total antioxidant capacity, and DPPH and ABTS radical scavenging assays, using BHT and Trolox as standards.
The results showed that the total antioxidant capacity of the essential oil was significantly (P<0.001) lower than that of the reference compound BHT [(228.99±6.06) µg EBHT/mg and (417.99± 1.31) µg EBHT/mg, respectively].
A.absinthium essential oil was able to reduce the stable free radical DPPH with an IC50of (139.93±7.81) µg/mL, but was significantly less active (P<0.001) than the synthetic antioxidant BHT [IC50(14.60±0.71) µg/mL].
In the ABTS assay, the anti-radical activity of the essential oil of A.absinthium showed higher IC50in comparison with the IC50of Trolox[(32.96±0.71) and (3.15±0.02) µg/mL, respectively](P<0.001).
3.3.Skin irritation test
No edema, erythema or signs of inflammation were observed after topical application of A.absinthium essential oil formulations on rats.
3.4.Evolution of healing process of wounds
During the healing period, and according to a specific interval of time of three days, the wounds were regularly measured.The evolution of the surface of each wound excision was assessed and the results are represented in Figure 2, which showed that the OAEO 10% treatment had the best results.
All treated animals showed a significant reduction in wound area when compared to the untreated group (P<0.05).There was no significant difference between groups treated with the A.absinthium essential oil ointment and the reference drug Cicatryl.An improved healing pattern with wound closure was observed in treated groups within 9-12 days (Table 2).
Daily visual observations indicated the presence of signs of inflammation around the wound in rats of different groups on the first day after the excision of the skin.These signs disappeared rapidly in the treated groups (CIC, OAEO 5% and 10%) and persisted for a few days for the rest of the groups (UT and PJ).There was a complete closure of wounds in the treated groups with OAEO and Cicatryl in comparison with the untreated group and the group treated with petroleum jelly.
3.5.Histological sections
Histology of the skin area of the excised wound on day 21 post wounding showed incomplete epithelization with more inflammatory cells, poorly formed granulation tissue and sparse distribution of collagen fibers for the UT and PJ groups.A wound gap was observed in the epidermis layers in the two groups.These observations explain the delay in the wound healing process (Figure 3A&B).
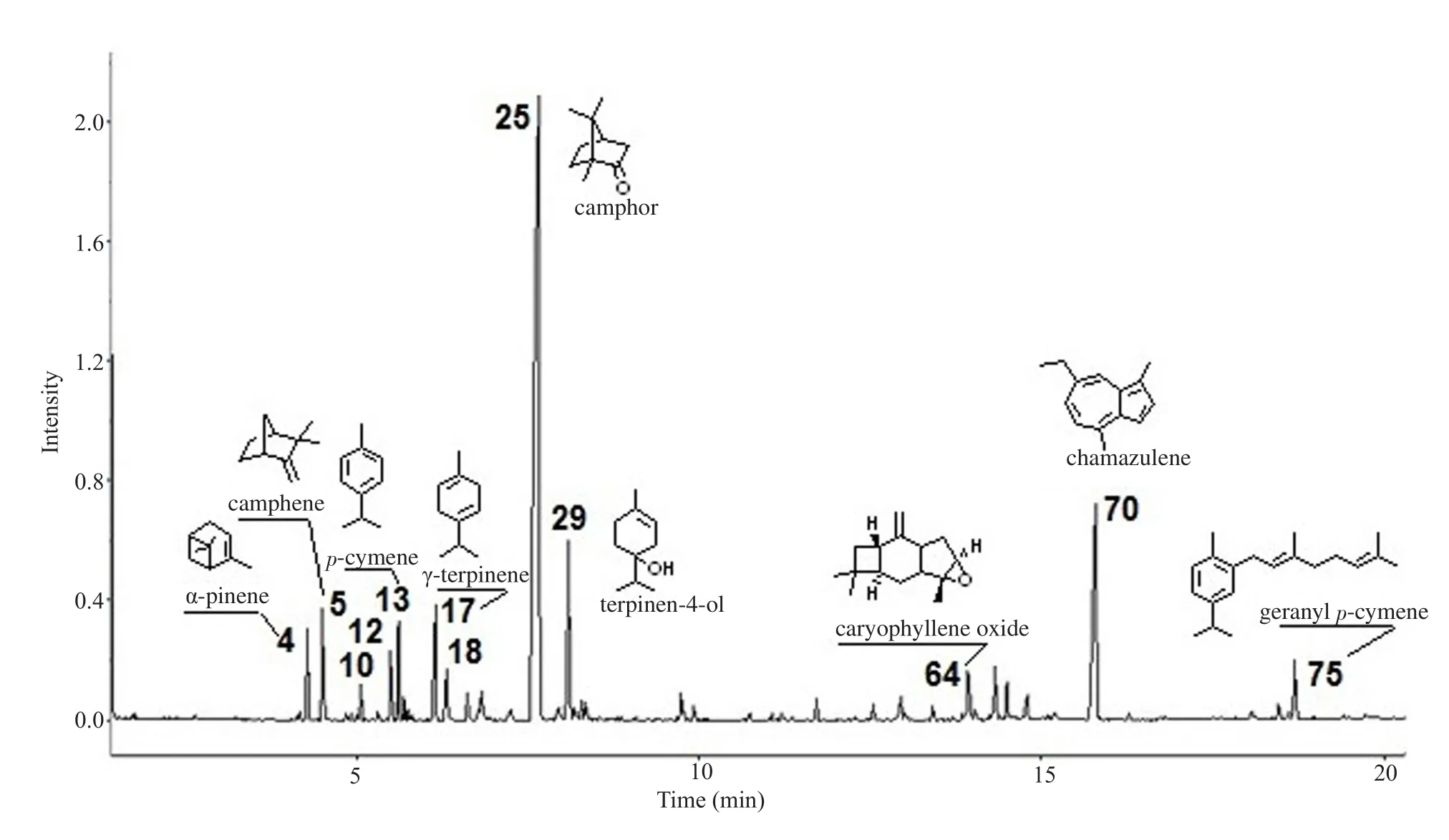
Figure 1.GC profile of the essential oil of Artemisia absithium from Algeria and some of the main components (for numbering see Table 1).
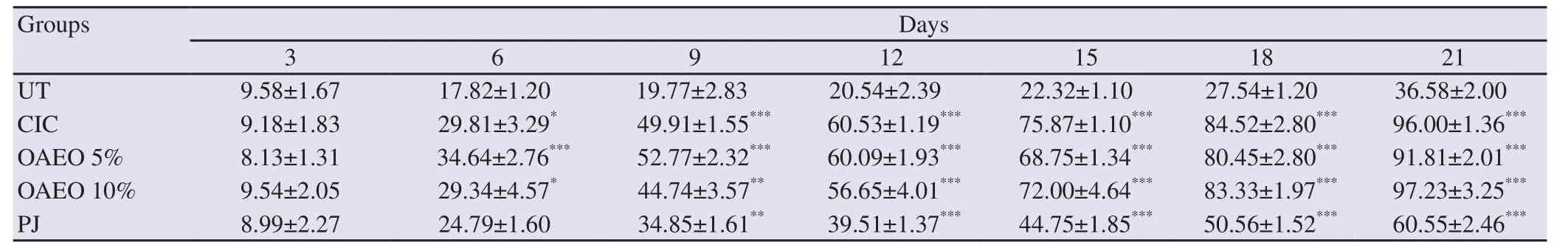
Table 2.Effect of different treatments on the evolution of the healing process of excision wounds in Wistar albino rats.
The histological sections of all treated groups (CIC, OAEO 5% and OAEO 10%) showed better healing resulting in more regular cell layers and more epidermal ridges.There was a complete reepithelialization with a visible thick neo-formative epidermis,abundant granulation tissue and higher collagen deposition.These histopathological observations provide additional evidence for the contraction value of wound areas in treated groups (Figure 3C, D,E).
4.Discussion
The essential oil yield in our study was slightly higher than literature data which report a yield range between 0.2% and 1.5%[4,29-31].The chemical composition of A.absinthium oil can explain the biological activity of this oil and justify the use of this plant in folk medicine.A.absinthium from Algeria can be defined as a camphor chemotype,and in comparison with literature data, this sample appears partially similar to plants collected in Brazil and Tunisia, which show the following main components:camphor>caryophyllene>1,8-cineole>germacrene-D>α-cadinol in the Brazilian sample, and chamazulene>camphor>bornyl acetate>chrysanthenyl acetate>γterpinene in the Tunisinian sample[11,32].

Figure 3.Histological evaluation of wound skin sections stained with hematoxylin and eosin (40×magnification).A and B:UT and PJ treatments,respectively, showing fewer collagen fibers and more inflammatory cells.C, D, and E:animals treated with OAEO 5%, OAEO 10%, and CIC drug reference, respectively, showing better healing and complete re-epithelialization.
Oxygenated monoterpenes such as camphor and terpinen-4-ol, which are representative components in the investigated oil,were reported to exhibit many biological activities, comprising anti-leishmania, insecticidal and repellency activity, as well as antimicrobial, anti-infammatory and anti-respiratory tract infections[32-34].Among the minor components, γ-terpinene,camphene, p-cymene, gernanyl p-cymene and α-pinene are of an amount over 2%.
On the basis of a literature survey, many other important compounds have also been identified in A.absinthium oil from different origins, including epoxyocimene, 1,8-cineole, myrcene,trans-sabinyl acetate, sabinene, viridiflorol, and thuja-2,4(10)-diene[2,30,32,35].The predominance of one or two of the aforesaid components defines pure or mixed chemotypes[2,36].
The antioxidant/anti-radicals activity of A.absinthium essential oil was rather modest and probably ascribable to the presence of chamazulene.
Camphor is endowed with various pharmacological properties such as anti-bacterial, anti-fungal, and wound-healing activities[37].A recent study has shown the effect of camphor on skin health by inducing fibroblast proliferation, maintaining or recovering collagen and elastin production in UV exposed skin, and preventing thickening of the epidermis and subcutaneous fat layer[38].Camphor also showed a significant effect on epithelization and neovascularization on second-degree burn wounds in rats[39].Therefore, the presence of camphor in A.absinthium essential oil may explain the wound healing activity of the OAEO 10% ointment group.
The wound healing property of A.absinthium essential oil was also remarkable in comparison with the standard drug Cicatryl.This suggests that A.absinthium essential oil could be developed as a potential effective wound healing ingredient for topical medicaments.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
The authors are grateful to Mr.Antonio Greco (ICB-CNR, Catania)for his skilful technical support.
Funding
This work was supported by Algerian Ministry of Higher Education and Scientific Research/ Algeria (CNEPRU:DO1N01UN280120150001) and Consiglio Nazionale delle Ricerche, Rome, Italy.
Authors' contributions
Amel B.and GR conceived and designed the experiments,supervising all steps of research; EN conducted the essential oil analyses, organized and wrote the relative experimental section;Amel B., AB and FB performed the essential oil extrcation, the antioxidant activity evaluation, the in vivo experiments and wrote the pertinent portion; GR and Amel B.wrote the article with the contribution from all co-authors.All authors approved the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年11期
Asian Pacific Journal of Tropical Biomedicine2020年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Justicia secunda Vahl leaf fraction protects against acetaminophen-induced liver damage in rats by alleviating oxidative stress and enhancing membrane-bound phosphatase activities
- Polygonatum kingianum rhizome extract alleviates collagen antibody-induced arthritis by modulating proinflammatory cytokine production in mice
- Achillea biebersteinii extracts suppress angiogenesis and enhance sensitivity to 5-fluorouracil of human colon cancer cells via the PTEN/AKT/mTOR pathway in vitro
- Antioxidant and anti-inflammatory activities of Orostachys japonicus
