Achillea biebersteinii extracts suppress angiogenesis and enhance sensitivity to 5-fluorouracil of human colon cancer cells via the PTEN/AKT/mTOR pathway in vitro
Mehmet Kadir Erdoğan, Can Ali Ağca, Hakan Aşkın
1Department of Biology, Faculty of Arts and Sciences, Bingol University, 12000, Bingol, Turkey
2Department of Molecular Biology and Genetics, Faculty of Arts and Sciences, Bingol University, 12000, Bingol, Turkey
3Department of Molecular Biology and Genetics, Faculty of Sciences, Ataturk University, 25000, Erzurum, Turkey
ABSTRACT
KEYWORDS:Achillea biebersteinii; Colorectal cancer;Chemosensitization; Apoptosis; Angiogenesis; mTOR
1.Introduction
The aromatic and medicinal properties of plants, which are reliable and readily available sources, have been used for centuries.Almost three-quarters of the world's population relies on herbal resources for treatment of diseases, but only about 30% of currently known plant species are used for medical purposes[1].The natural compounds obtained from plants have anticancer potency and they increase the activity of chemotherapeutic drugs when used in combination.Therefore, several studies are carried out on natural compounds and plant materials[2,3].The Achillea genus contains approximately 85 species and many of these species are endemic to the Middle East and Europe.Turkey's flora is hosting 23 endemic Achillea, a total of 42 species[4].In folk medicine, some Achillea species are used for ethnopharmacological purposes, such as healers of hemorrhoids, wounds, and pains[5].The anti-microbial, anti-allergic, spasmolytic, anti-diabetic, anti-oxidant, anti-inflammatory,anti-ulcer, hepatoprotective, choleretic, and antitumor activities of various Achillea species have been reported before[6,7].Achillea biebersteinii (A.biebersteinii) Afan.(family Compositae) is a perennial herb, 30-60 cm high, with leaves up to 10 cm, and flowering period April-May.A.biebersteinii is taken orally as herbal tea in folk medicine to treat injuries, abdominal and stomach pain, and cancer[8].
After cardiovascular diseases, cancer is the second most important cause of death worldwide.Although there have been significant improvements in the treatment of cancer in the last 50 years, this disease remains a major health problem for humanity.Therefore,great efforts are being made to develop new therapeutics in cancer treatment[9].In recent years, the therapeutic potential of medicinal plant extracts in cancer prevention and treatment has gained scientist's attention.In this context, the plants are seen as a significant source of cancer treatment.Approximately 75% of approved cancer drugs have been developed with inspiration from natural source agents[10].Plant-derived compounds that support the treatment of cancer in all stages are multi-targeted and nontoxic, and usually have synergistic effects with drugs, so all of these properties make them very important in cancer treatment[11].
Apoptosis is a programmed cell death that occurs in a cell under normal physiological conditions and within genetic control.Abnormalities in cancer cells are usually caused by mutations in genes such as p53, p38 MAPK, PTEN, and Akt, which encode proteins regulating cell proliferation and apoptosis.Avoidance of apoptosis is a hallmark of cancer cells, so drugs and agents that direct the cancer cells to apoptosis should be at the center of new anti-cancer therapies[12].
Angiogenesis has critical importance in tumor maturation and growth.The increase in tumor mass triggers the formation of new blood vessels in the tumor microenvironment, probably due to the absence of nutrients and oxygen[13].For this reason, anti-angiogenic therapy offers a promising approach to suppress tumor development and metastasis.
Colorectal cancer is the third most common type of cancer worldwide and the fourth most important cause of cancer-related deaths.The survival rate of metastatic colorectal cancer cases is lower than 10%.The main treatments of colorectal cancer are 5-fluorouracil (5-FU), radiotherapy, and surgical intervention[14].5-FU is a broad spectrum antimetabolite chemotherapy agent and against many cancer types (colorectal, breast, liver, pancreas, brain,ovary, etc.).It is widely used either alone or in combination with natural compounds or other chemotherapeutics.The cytotoxic activity of 5-FU is caused by incorrect binding of fluoronucleotides to DNA and RNA and inhibition of thymidylate synthase, a nucleotide synthesis enzyme.The combination of 5-FU with other natural agents increases the response and survival rates of patients with neck, head, breast, and colorectal cancers[15]and may contribute to reducing toxicity and side effects in cancer treatment.Therefore,in this study, anticancer, anti-angiogenic, and apoptotic effects of extracts of A.biebersteinii (ABE) and the combined treatments of these extracts with 5-FU on HT-29 cells were investigated by in vitro cell culture analysis for the first time.
2.Materials and methods
2.1.Chemicals, reagents, and kits
Dulbecco's Modified Eagle Medium, penicillin/streptomycin,fetal bovine serum, and Dulbecco's phosphate-buffered saline were obtained from Lonza.Trypan blue, Tris HCl, trypsinethylene diamine tetraacetic acid (EDTA), dimethyl sulphoxide,tetramethylethylenediamine, coomassie blue (G250), NaN3,luminol, skim milk powder, ponceau S, H3PO4, p-coumaric acid,phenylmethanesulfonyl fluoride, benzamidine, hexane, chloroform,methanol, ethanol, glycerine, glycerol, and sodium dodecyl sulfate were obtained from Sigma Aldrich.H2O2, NaOH, glycine, Tween-20, bromphenol blue, β-mercaptoethanol, NP-40, EDTA, ethylene glycol tetraacetic acid (EGTA), and β-glycerophosphate were purchased from Merck and 5-FU was also obtained commercially.Forward and reverse primers were obtained from Invitrogen.Primary and secondary antibodies were purchased from Cell Signaling.Cell Proliferation KitⅠ (MTT) and Cell Death Detection ELISA Kit from Roche, Human Vascular Endothelial Growth Factor (VEGF)ELISA Kit from Invitrogen, Pure Link RNA Mini Kit from Life Technologies, 2×qPCRBIO SyGreen Mix Lo-ROX Kit from PCR Biosystems and High Capacity cDNA Reverse Transcriptase Kit from Thermo Fisher were used and experiments were performed according to manufacturers' protocols.
2.2.Plant extraction
A.biebersteinii was collected from the east of Turkey, Bingol province, 1 150 meters altitude, in the flowering period.The plant samples were dried in non-moisture and sunlight free conditions.The aerial parts of the plant were used for extraction.As extraction processes, the sequential fractionation method[16]was carried out using hexane (ABH), chloroform (ABC), and methanol (ABM),which have different polarities.Shortly, 20 grams of dry plant samples were covered by filter paper thoroughly.Then filter paper was placed in the apparatus of the Soxlet device.A total of 400 mL of solvent (hexane, chloroform, and methanol, respectively)were placed in a 1 000 mL flask.The solvent that evaporates by temperature was condensed by the cooling part of the device and poured back into the flask.Then the solvents in the mixture, which collected in the flask, were removed by evaporator and vacuum oven.The obtained powdered extracts were stored at -20 ℃.
2.3.Cell culture
HT-29, colorectal adenocarcinoma cells were purchased from the American Type Culture Collection (USA).HT-29 cells were grown in culture at 37 ℃, 95% humidity, and 5%-6% CO2, using a high glucose Dulbecco's Modified Eagle Medium medium containing 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% L-glutamine.Cells were checked daily and the media were renewed.Cells were passaged by trypsin when they reached a density of 80%.In cell culture studies, HT-29 cells were treated with plant extracts and 5-FU, as well as combinations of plant extracts and 5-FU for 24 h.
2.4.Cell viability (MTT assay)
The effects of ABE, 5-FU, and combined treatments on the viability of HT-29 cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay[16].Briefly,96 well microplate, which contained 1×104cells in each well, was treated with increasing concentrations (50-1 000 µg/mL) of ABE and 5-FU, then incubated for 24 h under 37 ℃, 95% humidity and 5% CO2conditions.At the end of this period, 10 µL of MTT-labeled reagent was added to wells and incubated for 4 h.Then 100 µL of the solubilization solution was added into wells and incubated overnight.The color changes associated with formazan product were measured spectrophotometrically at 570 nm using an ELISA plate reader (SpectraMax 384 Plus, Molecular Devices, USA).Concentration-dependent cell viability was calculated according to absorbance values.The half-maximal inhibitory concentration (IC50)value of each sample was calculated and these values were used as the effective doses in the next steps of the study.
2.5.Isobologram analysis
Isobologram analysis allows quantitative determination of the effective doses of the drug, active substance or extracts, and the synergism between them when treated together.The synergism between extracts and 5-FU was assessed with a combination index(CI).CI<1, =1, and >1 indicate synergism, additive effect, and antagonism, respectively.The interaction between ABE and 5-FU was evaluated with the help of the isobologram and median-effect equality method[17].
2.6.Cell death detection ELISA (CDDE) assay
Apoptosis is characterized by blebbing on the membrane,cytoplasmic condensation, and endonuclease activity.This endonuclease activity causes internucleosomal breaks in DNA.These fractures form mono- and oligo-nucleosomes.The CDDE method allows the colorimetric determination of mono- and oligonucleosomes in each treatment group, so increase in apoptosis can be determined in the cells by enrichment factor.For the control group,enrichment factor was assumed as 1.0, and the increases of apoptosis in treated cells, which is depending on absorbance, were expressed as fold increase.The CDDE method allows in vitro qualitative and quantitative determination of the cytoplasmic histone that is cleaved as a result of cell death-induced DNA fragmentation.A total of 1×104cells were cultivated in each well of 96-well microplate and exposed to appropriate treatment for 24 h.Following treatment,the supernatant was collected, stored and the cells were trypsinized and lysed according to the previously described protocol[16].After lysis, the supernatants were taken to clean tubes and treated with anti-histone and anti-DNA peroxidase antibodies, followed by ELISA.The absorption of the wells was read at 405 and 490 nm for reference.When compared with un-treated cells, the intensive color formation was observed in the treatment groups, which showed the DNA fragmentation characteristic of apoptosis.The absorbances of treated groups were divided into the absorbance of the control,in order to calculate the enrichment factor, which is a colorimetric indicator of apoptosis.
2.7.ELISA for VEGF
VEGF is a significant promoter of angiogenesis in cancer formation.The amount of VEGF in treated cells was quantitatively determined by the Human VEGF ELISA method[16].A total of 5×103cells were seeded into each well of the microplate and incubated overnight under standard conditions.Cells were treated with IC50doses of 5-FU, ABE, and combinations for 24 h.After medium removed, cells were washed with cold PBS, gathered with the help of a scraper and transferred to tubes containing 50 µL of fresh medium.A total of 50 µL incubation buffer, 50 µL medium containing 5×103treated cells, and 50 µL standard diluent buffer were added to the wells of 8-well strips.After 2 h incubation at room temperature, the mixture was removed and the wells were washed 4 times with wash buffer.A total of 100 µL of HU-VEGF biotin conjugate solution was added to the wells.One hour later, at room temperature incubation,the solution was removed and the wells were washed 4 times with wash buffer.A total of 100 µL of streptavidin-horseradish peroxidase(HRP) was added to each well and incubated at room temperature for 30 min.The solutions were aspirated from the wells and the wells were washed 4 times with 1× washing buffer.A total of 100 µL stabilized chromogen was added to each well.The substrate solution began to turn blue.After incubation at room temperature and in the dark for 30 min, the solution was removed and 100 µL stop solution was added to each well.After homogenization was achieved, the color of the solution in the wells changed from blue to yellow.Two hours later, absorbances were measured by ELISA reader at 450 nm wavelength.The standard curve graph was plotted for each study, the results were compared with the standard curve, and the amount of VEGF for each sample was calculated as pg/mL.
2.8.Quantitative real-time PCR (qRT-PCR)
The effects of 5-FU, ABE, and combinations on mRNA expressions of p53, Bcl-2, Bax, mTOR, Akt, PTEN, and p38 MAPK genes in HT-29 cells were evaluated by qRT-PCR.A total of 1 mL of cold PBS was added to wells of a 6-well microplate.In each well, 1×106cells were treated with ABE, 5-FU, and combinations, and then the cells were collected with the help of a scraper.Total RNA extraction of these cells was performed using the Pure Link RNA Mini Kit (Invitrogen-Life Technologies, Carlsbad, CA, USA).Complementary DNA (cDNA)synthesis from total RNA was performed using random primers and the High Capacity cDNA Reverse Transcriptase Kit (Thermo Fisher,Austin, USA) according to the manufacturers' protocols.qRT-PCR was carried out using a real-time PCR device (Rotor-Gene Q, Qiagen,Hilden, Germany) and a master mix containing SYBR Green (2×qPCRBIO SyGreen Mix Lo-ROX Kit, PCR Biosystems, London, UK).QRT-PCR reaction conditions consisted of incubation at 95 ℃ for 2 min, followed by 40 cycles at 95 ℃ for 5 s, 64 ℃ for 20 s, 72 ℃ for 2 min, and then 1 cycle at 72 ℃ for 5 min.Forward and reverse primers were manually designed using the National Center for Biotechnology Information (Bethesda MD, USA) database and purchased from the manufacturer.The expression rates of mRNA were determined by the comparative threshold cycle (Ct) method for relative quantification(2-ΔΔCt), normalizing its Ct values to that ofβ-actin[18].
The forward and reverse primers were as follows:β-actin, F:5' CTCTTCCAGCCTTCCTTCCT 3',R:5' AG C AC T G T G T T G G C G TAC AG 3'; p 5 3,F:5' G T C C A A C A A C A C C A G C T C C T 3', R:5' C C T C AT T C A G C T C T C G G A A C 3'; B c l-2,F:5' G T G A A C T G G G G G A G G AT T G T-3', R:5' G G A G A A AT C A A A C A G A G G C C-3'; B a x,F:5' C C C G A G A G G T C T T T T T C C G A G-3', R:5' C C AG C C C AT G AT G G T T C T G AT-3'; A k t, F:5' C A C A C C A C C T G A C C A A G AT G 3', R:5'C T G G C C G A G T A G G A G A A C T G 3'; m T O R,F:5' T G T C C T G C T G G T C T G A AC T G 3', R:5'T T C A G C G AT G T C T T G T G A G G 3'; P T E N, F:5' G G T G AT G T G G C A G G A C T C T T-3', R:5'C AG C T T C C G AG AG G AG AG A A-3'; p 3 8 M A P K,F:5' T G G AT G C AT TAC A AC C AG AC A-3', R:5'GTCAACAGCTCGGCCATTAT-3'.
2.9.Western blotting
The effects of ABE, 5-FU, and combination treatments on p53, Bcl-2, Bax, mTOR, Akt, PTEN, and p38 MAPK protein expressions in HT-29 cells were determined by Western blotting method.Membrane images, which were obtained by Western blotting analysis, were evaluated using the Image J program.β-actin protein expression was used as a reference.HT-29 cells were treated with either alone ABE or combination with 5-FU for 24 h and were harvested by scratching without trypsinization.The cells were lysed in the lysis buffer [20 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, 5 mM EDTA (pH 8.0), 0.5 mM EGTA (pH 8.0), 50 mM NaF, 20 mM β-glycerophosphate,1 mM Na3VO4, 1 mM benzamidine, 4 µM leupeptin, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol]containing a proteinase and phosphatase inhibitor cocktail, and proteins were extracted for 60 min on ice.Protein concentrations were determined manually with Bradford solution:Coomassie Blue G250, absolute ethanol, and 85% phosphoric acid (Sigma-Aldrich, USA).To detect p53, Bax, Bcl-2, p38 MAPK, PTEN, mTOR, Akt, and β-actin protein levels, equal amounts of protein samples were run on 8% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresi and transferred to polyvinylidene difluoride membranes (Millipore,Cat# IPVH00010).The membrane was rinsed with purified water and stained with Ponceau solution to check the transfer quality.The Ponceau solution was removed and the membrane was washed three times with Tris Buffered Saline Tween (TBS-T).Membranes were blocked with TBS-T (50 mM Tris, 150 mM NaCI, 0.5 Tween 20) containing 5% skim milk powder (Sigma, cat# 70166-500G).Membranes were then incubated overnight with the corresponding antibody, mouse monoclonal anti-p53 (1:1 000), rabbit polyclonal anti-Bax (1:1 000), mouse monoclonal anti-Bcl2 (1:1 000), rabbit polyclonal anti-p38 MAPK (1:1 000), rabbit polyclonal anti-PTEN (1:1 000), rabbit polyclonal anti-mTOR (1:1 000), mouse monoclonal anti-Akt (1:200), and mouse monoclonal anti-β-actin(1:200) diluted in 5% skim milk powder overnight at 4 ℃.After the incubation, the membrane was washed with TBS-T and incubated with corresponding HRP-linked secondary antibodies (antimouse IgG-HRP, sc-2781, Santa Cruz, USA; anti-rabbit IgG-HRP,Ab97051, Abcam, UK).Proteins were visualized by an enhanced chemiluminescence method using X-ray films.Densitometric analysis of the immunostaining band was performed by using ImageJ software (USA).The same protein loading of the samples was confirmed by the β-actin band.
2.10.Statistical analysis
All experiments were performed in triplicate and standard deviation values were calculated.Statistical analysis of the findings was evaluated by t-test and One Way ANOVA test using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA).CompuSyn 1.0 program was used for isobologram analysis of combined doses of 5-FU and plant extracts.P values <0.05 were assumed statistically significant.
3.Results
3.1.Cell viability
It was observed that cell viability decreased depending on the concentration in all treatment groups.Percent survival rates of cells treated with 5-FU at 100 and 1 000 µg/mL concentrations were 51.4% and 7.7%, respectively (Figure 1).ABE significantly decreased the viability of HT-29 cells.Hexane, chloroform, and methanol extracts at 200 µg/mL decreased cell viability to 72.1%,52.0%, and 44.1%, respectively.At 1 000 µg/mL concentration, cell viability was 23.1% for hexane extract, 17.5% for chloroform extract and 12.0% for methanol extract (Figure 1).
The IC50value was (107.4±3.8) µg/mL for 5-FU.The IC50values of ABH, ABC, and ABM were (473.5±7.6), (211.2±4.1), and(184.6±3.3) µg/mL, respectively.The IC50doses of 5-FU and plant extracts were used in the next steps of the study.The combined treatment of 5-FU with ABH, ABC, and ABM at IC50doses for 24 h decreased the cell viability of HT-29 cells to (26.0±0.6)%,(19.1±0.9)%, and (14.9±0.6)%, respectively.The differences between the combined treatment of ABE with 5-FU and only 5-FU treated cells (51.3%) were statistically significant (P<0.001).
3.2.Determination of synergism by isobologram analysis
The CI value for the combination of 5-FU and ABM was 0.65±0.03.The finding indicated that the combined treatment of 5-FU and ABM had synergistic effects on HT-29 cells.5-FU+ABC combination exhibited moderate synergism (CI=0.78±0.05) and 5-FU+ABH combination showed an additive effect (CI=1.07±0.03)on HT-29 cells.
3.3.Colorimetric analysis of apoptosis (cell death detection ELISA)
The cells treated with 5-FU showed a 5.2 fold increase in apoptosis compared with the control group (P<0.001).Compared with the control group, the apoptotic increase rates in cells treated with ABH,ABC, and ABM were 5.6, 5.9, and 6.9 fold, respectively, but the differences were not significant compared with the 5-FU treatment group.In addition, the apoptotic rates were significantly increased in combination treatment groups when compared with control and 5-FU treated groups (all P<0.001) (Figure 2).
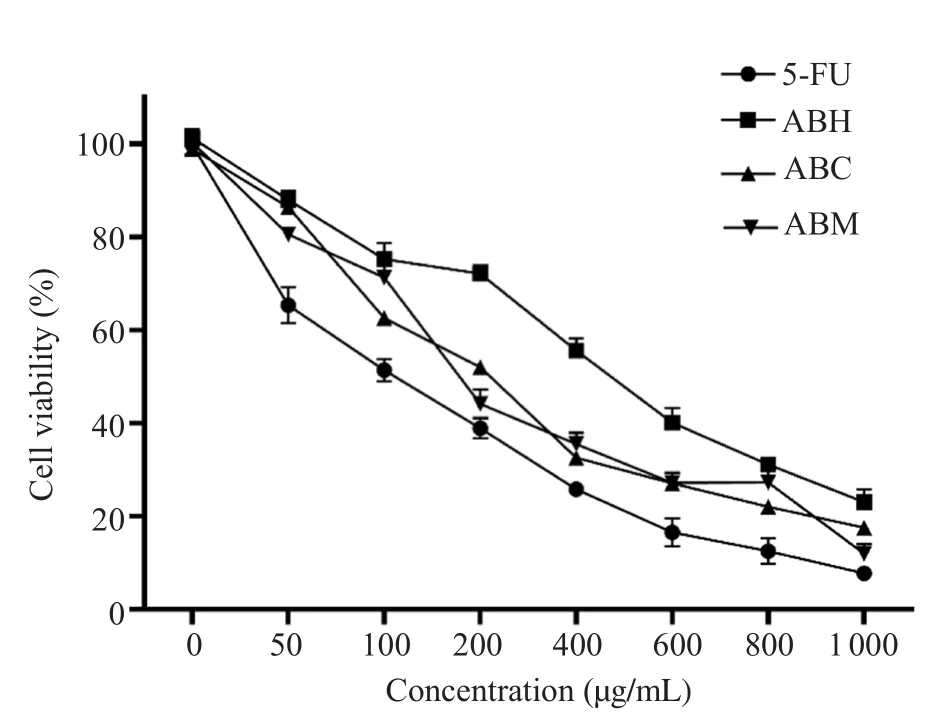
Figure 1.Effect of 5-FU and hexane, chloroform, and methanol extracts of Achillea biebersteinii (A.biebersteinii) on the viability of HT-29 cells at different concentrations.5-FU:5-fluorouracil, ABH:A.biebersteinii hexane extract, ABC:A.biebersteinii chloroform extract, ABM:A.biebersteinii methanol extract.
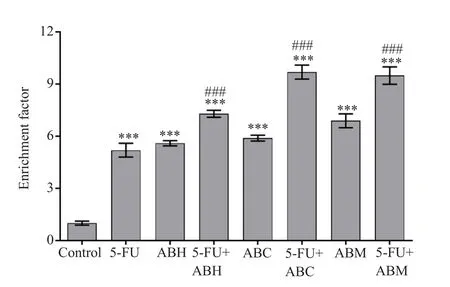
Figure 2.Apoptotic effect of A.biebersteinii extracts and combination treatments with 5-FU on HT-29 cells.5-FU:5-fluorouracil, ABH:A.biebersteinii hexane extract, ABC:A.biebersteinii chloroform extract, ABM:A.biebersteinii methanol extract.***compared with the control group, P<0.001;### compared with the 5-FU treated cells, P<0.001.
3.4.Human VEGF ELISA findings
VEGF amount was normalized as (100.0±1.1) pg/mL for the control (Figure 3A and 3B).The VEGF amounts of cells in all treatment groups were significantly lower when compared with the control group (P<0.001).In addition, VEGF amounts in cells treated with the combinations of 5-FU+ABC and 5-FU+ABM were significantly lower than the 5-FU group (P<0.001) (Figure 3B).
3.5.Gene expression
As seen in Table 1, compared with the control group, the p53 mRNA expressions were significantly increased in all treatment groups (P<0.001), except for the ABH group (P>0.05).The increases in p53 mRNA expression in the groups treated with ABH, ABC,and ABM in combination with 5-FU were more significant when compared with the 5-FU treated cells (P<0.001).
Bcl-2 and mTOR mRNA expressions in all treatment groups were decreased significantly (P<0.001) compared with the control group,and the difference was not significant in ABE treatment groups or combination treatment groups compared with 5-FU treated cells.The increases in the mRNA expressions of the Bax gene was significant in all treatment groups (P<0.001) compared with the control group,except for 5-FU+ABC treated cells.Compared with the 5-FU treated group, 5-FU+ABH and 5-FU+ABM combinations showed significant increases (P<0.001) (Table 1).
The Akt mRNA expression in all treatment groups was significantly lower than the control group (P<0.001) and the differences were significant in all combination groups compared with the group treated with only 5-FU (P<0.001) (Table 1).
The highest increase in the mRNA expression of the PTEN was seen in cells treated with ABM.In all treatment groups except for 5-FU treated cells, PTEN mRNA expression was significantly increased (P<0.001).And the differences were significant in all combination groups compared with the group treated with only 5-FU (P<0.001).Compared with the control, mRNA expression of p38 MAPK was significantly increased in all groups, except for ABC treated cells (P<0.001).The cells treated with 5-FU+ABM showed a significant increase (P<0.001) compared with only 5-FU treated group, but no significant change was observed in other combination groups (Table 1).
3.6.Western blot findings
The p53 protein expression levels were significantly increased in all treatment groups except for the ABH treated cells compared with the control group (P<0.001).When compared with the 5-FU treated cells, the increase in p53 protein expression levels of 5-FU+ABH and 5-FU+ABM combination groups were significant (P<0.001),but no significant change was observed in 5-FU+ABC treated cells(Figure 4A).
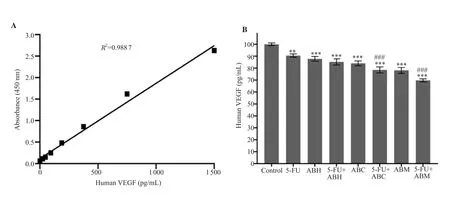
Figure 3.Human vascular endothelial growth factor (VEGF) standard curve (A) and VEGF amount changes in HT-29 cells (B).5-FU:5-fluorouracil, ABH:A.biebersteinii hexane extract, ABC:A.biebersteinii chloroform extract, ABM:A.biebersteinii methanol extract.Compared with the control group, **:P<0.01,***:P<0.001; compared with the 5-FU treated cells, ###:P<0.001.
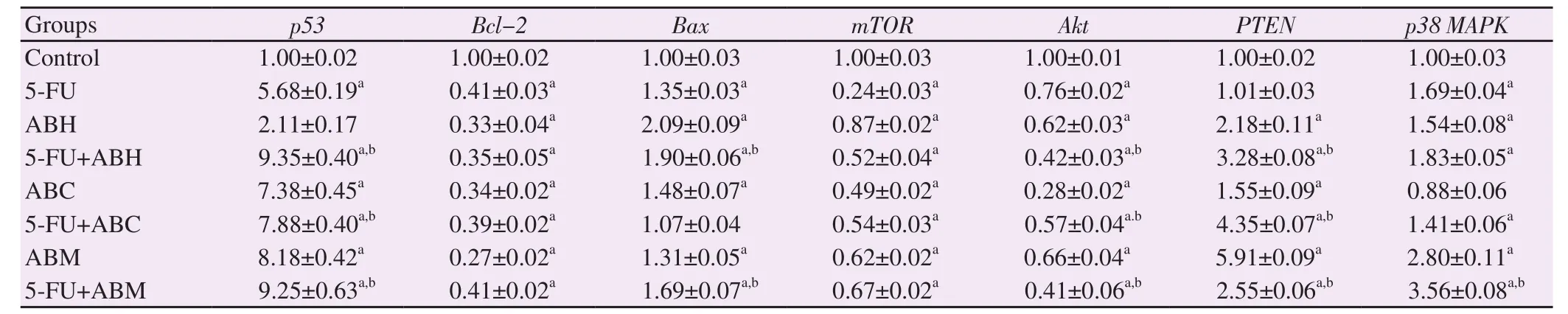
Table 1.mRNA expression of p53, Bcl-2, Bax, mTOR, Akt, PTEN, and p38 MAPK genes in HT-29 cells.
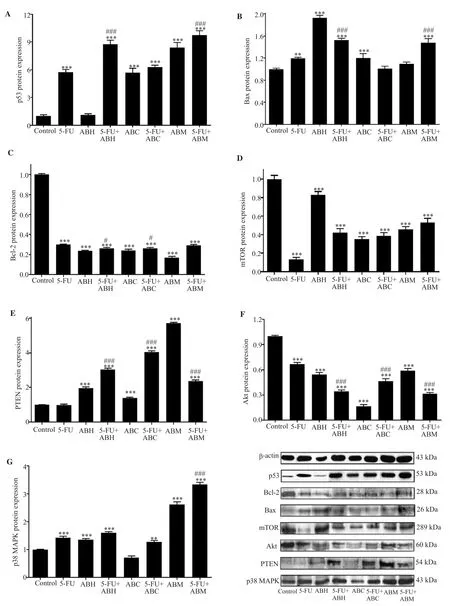
Figure 4.Western blot results of p53 (A), Bax (B), Bcl-2 (C), mTOR (D), PTEN (E), Akt (F), and p38 MAPK (G) proteins in HT-29 cells treated with 5-FU,A.biebersteinii extracts, and combinations for 24 h.The results were normalized using protein expression rates of β-actin.5-FU:5-fluorouracil, ABH:A.biebersteinii hexane extract, ABC:A.biebersteinii chloroform extract, ABM:A.biebersteinii methanol extract.Compared with the control group, *:P<0.05, **:P<0.01, ***:P<0.001; compared with the 5-FU treated cells, #:P<0.05, ###:P<0.001.
Bax protein expression showed no significant increase in 5-FU+ABC and ABM groups compared with the control group and significant increases were observed in all other groups (P<0.01).In 5-FU+ABH and 5-FU+ABM treated cells, the Bax protein levels were significantly higher than 5-FU treated cells (P<0.001) (Figure 4B).Protein expression levels of Bcl-2 and mTOR were significantly decreased in all treatment groups compared with the control group(P<0.001).Compared with 5-FU treated cells, Bcl-2 protein levels showed a statistically significant reduction in 5-FU+ABH and 5-FU+ABC treated cells (P<0.05) (Figure 4C), while the difference was not significant in all combination groups compared with the 5-FU group in terms of mTOR expression levels (Figure 4D).
Significant increases in PTEN protein levels were observed in all treatment groups (P<0.001) except for 5-FU treated cells and there were significant differences in all combination groups compared with the 5-FU treated group (P<0.001) (Figure 4E).Akt protein levels showed a statistically significant decrease in all treatment groups compared with the control group (P<0.001) with the lowest expression in the ABC treated cells and there were significant differences in all combination groups compared with the 5-FU treated group (P<0.001) (Figure 4F).
Compared with control group, p38 MAPK protein levels were significantly increased in all treatment groups (P<0.01) except for ABC treated cells.Compared with 5-FU treated cells, p38 MAPK protein levels were significantly higher in 5-FU+ABM groups(P<0.001), and no significant changes were observed in other combination treatment groups (Figure 4G).
4.Discussion
The anti-cancer drugs directly induce death of cancerous cells by apoptosis via different signaling pathways.However, most types of cancer could avoid programmed cell death in different pathways.Co-targeting of several signaling pathways provides new ways for the discovery and development of new anti-cancer therapies.Several cellular functions such as cell cycle and metabolism, autophagy,cell proliferation, angiogenesis and apoptosis are important for tumorigenesis[19].In particular, genes and proteins that could upregulate or down-regulate apoptotic pathways are significant in explaining the molecular mechanism of cancer formation.PTEN/Akt/mTOR pathway plays a key role in numerous cellular functions including adhesion, migration, and invasion.Moreover, a recent study has shown that VEGF is one of the major initiators of angiogenesis and activates several signaling pathways including the Akt/mTOR pathway, which promotes cell proliferation and survival[20].In this study, we confirmed that ABE and 5-FU combination induced apoptosis and inhibited angiogenesis by regulating PTEN/Akt/mTOR signaling pathway in HT-29 cells.Moreover, ABE combinated with 5-FU showed more potent anticancer efficacy as compared with 5-FU.
Phytochemical studies have shown that Achillea species are rich in sesquiterpene lactones, flavonoids, alkaloids and especially monoterpene compounds.In a study on 10 different Achillea species(Achillea pannonica, Achillea biserrata, Achillea filipendula, Achillea clypeotala, Achillea macrophylla, Achillea crithmifolia, Achillea pyrenaica, Achillea taygetea, Achillea sibirica, and Achillea tenuifolia),eucalyptol and camphor have been reported to be among the major constituents[21].Monoterpenes were predominant in essential oil of A.biebersteinii as piperitone (34.9%), eucalyptol (13.0%),camphor (8.8%), and similarly in chloroform and methanolic fractions, piperitone, eucalyptol, camphor, and borneol were the major components[22].The major compounds of essential oils of A.biebersteinii samples, which were collected from different locations of Turkey, were piperitone (49.9%)[23]; eucalyptol (29.9%) and camphor (17.3%)[23]; piperitone (31.06%), camphor (12.46%),and eucalyptol (10.98%)[24].The main compounds of the n-hexane fraction were revealed to be piperitone (9.2%), camphor (4.2%),borneol (3.3%), α-terpineol (2.9%), and eucalyptol (2.5%)[25].Kordali et al.reported that the main components in the essential oil in A.biebersteinii plant samples were similar to hexane fractions and camphor, eucalyptol, and borneol were predominant[26].Terpenes are plant derived compounds, and they can induce apoptosis and inhibit the proliferation of cancer cells[27].The major compounds of A.biebersteinii have significant anticancer, antiapoptotic, and antioxidant potential.The antioxidant and anticancer activities of camphor[28], eucalyptol[29], piperitone[30], and α-terpineol[28]were reported previously.Also, the antiapoptotic and antitumorogenic effects of borneol were shown in another study[31].We found that the extracts alone and in combination with 5-FU inhibited cell viability in HT-29 cells.Our results were consistent with previous reports in which Achillea millefolium and A.biebersteinii ethanol extracts inhibit cell growth in different cancer cell lines, including SW742 human colorectal cancer cells[32].We thought that the antiproliferative effects result from several antioxidant chemicals in the ABE.Achillea teretifolia methanol extract showed strong antioxidant, cytotoxic,and apoptotic effects in DU145 and PC-3 prostate cancer cells[33].Supporting our data, Tohme et al.declarated that some compounds isolated from Achillea falcata have anticancer potential without toxic effects in HCT-116 colon cancer cells[34].In a study on mice,different doses of A.biebersteinii essential oil were given orally at 0.1-0.5 mL/kg, and clinical signs and symptoms were observed at half-hour intervals for the first 4 hours and at the end of the 72-hour period.In addition, mortality was followed daily for 14 d.At the end of these processes, even at the highest dose, no mortality and pathological symptoms were observed[35].Extracts obtained from 5 different Achillea species (Achillea wilhelmsii, Achillea setacea,Achillea vermicularis, Achillea phrygia, Achillea sipikorensis) have been shown to be safe at the administered doses (250-500 mg/kg) in mice[36].All these studies suggest that Achillea species, especially A.biebersteinii, have no acute toxicity in various doses.Several studies showed that the combinations of plant extracts with 5-FU may be more effective than chemotherapeutic agents alone[37,38].However,the potential molecular mechanisms underlying their anticancer activity are unclear.
Apoptotic cell death or typeⅠprogrammed cell death is generally morphologically characterized by nuclear condensation, cell shrinkage, membrane overflow, DNA fragmentation, and the formation of apoptotic bodies.In cancer cells, impaired apoptotic signaling pathways are common and significantly contribute to tumorigenesis and metastasis.p53 plays a crucial role in the triggering of apoptosis either by affecting PTEN or the death receptor and mitochondrial pathway components, such as proapoptotic Bax and pro-survival Bcl-2[12].Radiotherapy or chemotherapy used for cancer treatment causes DNA damage in some cells, which can lead to apoptotic death by a pathway linked to p53.Formation of apoptosis is inhibited by anti-apoptotic Bcl-2,while it is induced by pro-apoptotic Bax[13].p38 MAPK is a member of mitogen-activating protein kinases, and it plays an important role in the regulation of apoptosis, arrest of cell cycle, growth inhibition,and differentiation[39].Most chemotherapeutic agents induce apoptosis via p38 MAPK activity.Conventional cancer therapy is based on therapeutic agents that could be toxic to healthy cells with multiple side effects.In this context, many plant-derived components have negligible toxicity on healthy cells, which make them a promising chemotherapeutic candidate, and play an important role in the induction of apoptosis in cancer cells.Targeting and activating the apoptotic pathway in a non-toxic manner is critical for colorectal cancer treatment[40].We further investigated whether ABE inhibited cell proliferation and induced apoptosis in colorectal cancer cells by triggering the mitochondrial signaling pathway.Our results showed that ABE stimulated apoptosis by mitochondrial pathway, in addition to inhibition of cell viability in HT-29 cell line.We observed that ABH and ABM significantly down-regulated pro-survival Bcl-2 family protein Bcl-2 and up-regulated pro-apoptotic Bcl-2 family protein Bax.However, upregulations of gene and protein expressions of p53 and p38 MAPK in treatment with ABM showed that apoptosis is active through another pathway.We have also confirmed that all extract treatments led to cell death as determined by cell death detection ELISA assay.Remarkably, these events for the first time showed that ABM and ABC exhibited a synergistic effect with 5-FU.Overall, our results indicate that ABE was able to inhibit cell viability and to induce apoptosis through the mitochondrial pathway.Akt is a serine/threonine kinase with three isoforms, also known as protein kinase B.In particular, Akt-1 is actively involved in oncogenic and apoptotic signaling pathways[41].mTOR (target of rapamycin in mammals) is a serine/threonine protein kinase,which is an important regulator of cell growth and proliferation[42].PTEN (phosphate and tensin homologue) has both lipid and protein phosphatase activity.Mutations in the PTEN gene represent an important step in the development of many cancer strains.PTEN has tumor suppressor function and regulates the pathway negatively.Loss of PTEN causes uncontrolled cell proliferation, avoidance to apoptosis and tumor angiogenesis[43].It is well-known that the PTEN/Akt/mTOR pathway has a role in several cellular functions that are also important for tumorigenesis including angiogenesis,cell cycle progression, and apoptosis.In recent years, great efforts are being made to discover small molecule inhibitors that bind to VEGF along with the PTEN/Akt/mTOR signaling pathway.VEGF is one of the most important indicators of angiogenesis and closely affects tumor progression and metastasis in many human cancers by triggering endothelial cell proliferation[13].It has been shown that VEGF has an important role in tumor angiogenesis.The tumor growth and vasculogenesis were slowed down by suppression of VEGF in tumour bearing mice[44].Baharara et al.showed that A.biebersteinii water extract prepared with silver nanoparticle caused a 50% reduction in the length and number of vascular-like structures at a concentration of 200 µg/mL, in the rat aortic ring model[45].In the current study, we observed that the levels of Akt and mTOR expression were dramatically reduced in HT-29 cells following exposure to ABE.More importantly, qRT-PCR and Western blot analysis confirmed that PTEN mRNA and protein expresion was increased in extracts alone and with 5-FU in HT-29 cells.According to our results, all extracts and combinations of chloroform and methanol extracts with 5-FU in HT-29 cells caused inhibition of VEGF by inducing PTEN/Akt/mTOR signaling pathway.
5.Conclusion
In conclusion, this study showed the anticancer effect of ABE on colorectal cancer and its synergistic efficacy with 5-FU at the molecular level.The findings of this study revealed that ABE exhibited anticancer effects in HT-29 cells by inhibiting the cell proliferation and angiogenesis, and inducing apoptosis.Taken together, it is concluded that these combinations may prove useful and decrease the side effects of 5-FU in the treatment of colorectal cancer.Revealing the anticancer potential of A.biebersteinii and its synergistic efficacy with 5-FU will contribute to the development of new and less toxic clinical treatment approaches.However,supporting these findings with further in vivo studies is required to prove the effectiveness of this combined therapy.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This work was supported by The Scientific Research Projects Coordination Unit of Ataturk University (Project no:AUBAP2013-292) and The Scientific Research Projects Coordination Unit of Bingol University (Project no:BAP-FEF.2017.00.010).
Authors' contributions
MKE and HA devised the project, the main conceptual ideas and proof outline.MKE and CAA carried out the experiments.MKE,CAA and HA analyzed data, wrote and revised the article, and finally approved of the version to be published.
 Asian Pacific Journal of Tropical Biomedicine2020年11期
Asian Pacific Journal of Tropical Biomedicine2020年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Justicia secunda Vahl leaf fraction protects against acetaminophen-induced liver damage in rats by alleviating oxidative stress and enhancing membrane-bound phosphatase activities
- Polygonatum kingianum rhizome extract alleviates collagen antibody-induced arthritis by modulating proinflammatory cytokine production in mice
- Phytochemical profile, antioxidant activity and wound healing properties of Artemisia absinthium essential oil
- Antioxidant and anti-inflammatory activities of Orostachys japonicus
