Systematic Evaluation of Maren Runchang Pill (麻仁润肠丸) in the Treatment of Patients with Senile Constipation
CAO Wen-jie (曹文杰), LV Jian (吕 健), SUN Meng-hua (孙梦华),LIU Huan (刘 峘), XIE Zhen-nian (谢振年)
1. Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China
2. Xiyuan Hospital, China Academy of Traditional Chinese Medicine, Beijing 100091, China
3. Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing 100700, China
Correspondence to: Prof. LIU Huan, Tel: 010-64093205, Email: huananne@sina.com Prof. XIE Zhen-nian, Tel: 010-64093205, Email:xiezhennian@163.com
Supported by: National Key Research & Development Plan (2018YFC1707400, 2018YFC1707410)
ABSTRACT To systematically evaluate the clinical efficacy and safety of Maren Runchang Pill (麻仁润肠丸) in the treatment of senile constipation. Randomized controlled trials (RCT) on the treatment of senile constipation with Maren Runchang Pill (麻仁润肠丸) were searched by computer in CNKI, CBM,WanFang, VIP, PubMed, EMbase and the Cochrane Library databases. The retrieval time limit was from the establishment of the database to May 2019. A total of 2 researchers independently screened literature and extracted data according to the inclusion criteria, and Meta-analyzed the RCT that met the standards by applying RevMan5.3 software. A total of 5 randomized controlled trials were included, including 395 patients with senile constipation, 199 in the experimental group and 196 in the control group. The treatment group was treated with Maren Runchang Pill (麻仁润肠丸), while the control group was treated with other Western medicine, including fruit guide tablets, lactulose oral liquid or kaisailu, etc. The results of Meta-analysis showed that there was no significant difference in the total clinical effciacy between Maren Runchang Pill (麻仁润肠丸) and other Western medicines [RR=1.02, 95%CI (0.88, 1.17),P=0.83]. In terms of improvement of abdominal distension and inappetence, there was no significant difference between the treatment with Maren Runchang Pill (麻仁润肠丸) and other Western medicine [RR=1.03, 95%CI (0.85, 1.24),P=0.78].Maren Runchang Pill (麻仁润肠丸). This study showed that the efficacy of Maren Runchang Pill (麻仁润肠丸) in the treatment of senile constipation was not significantly different from that of conventional Western medicine, and was safe, and effective, with few adverse reactions. Maren Runchang Pill (麻仁润肠丸), as a traditional Chinese patent medicine, can play the role of regulating qi and replenishing blood, and regulating bowel and bowel laxative, with fewer cost. It is more suitable for the elderly to use, with good compliance,and clinically it should be used with syndrome differentiation. However, due to the quantity and quality of the original study, multi-center, high-quality randomized controlled trials are still needed to further confirm it.
KEYWORDS Senile constipation; Maren Runchang Pill (麻仁润肠丸); System evaluation; Meta analysis
Constipation is a common digestive disease and a common digestive disease in the elderly. It is clinically characterized by dry stool, weak bowel movements, or prolonged bowel cycle, or poor stools[1,2]. Senile constipation is a common senile syndrome[3-5], and the morbidity increases with age[5].The results of a number of community-based largescale epidemiological surveys show that overall constipation morbidity in China is 3% to 11%. The morbidity among the elderly aged 60 and above is 15% to 20%[6], and 84 or above can reach 20.0%to 37.3%. Among the elderly receiving long-term care, it is even up to 80%[4-6]. Senile constipation not only changes the physiological function, but also reduces the quality of life of elderly patients, seriously affecting the quality of life and physical and mental health of elderly patients, and consuming a large amount of medical expenses[7,8]. It is an important public health issue, so it is important to strengthen the exploration of senile constipation treatment,which can not only relieve the patient's pain, but also reduce the incidence of other diseases. In China,the application of traditional Chinese medicine to the treatment of senile constipation has a long history and many methods. Maren Runchang Pill (麻仁润肠丸) was made fromFructus Cannabis(Huo Ma Ren),Semen Armeniacae Amarum(Ku Xing Ren)
Radix et Rhizoma Rhei(Da Huang), banksian rose(Mu Xiang),Pericarpium Citri Reticulatae(Chen Pi),
Radix Paeoniae Alba(Bai Shao) and other traditional Chinese medicines as the main raw materials. The formula was based on the damp and fat Fructus Cannabis and moistening dryness for relaxing bowels,which is the main medicine; supplemented withSemen Armeniacae Amarum(Xing Ren) to lower qi and moisture the intestines, whiteRadix Paeoniae Alba(Bai Shao) to nourish yin and strengthen spleen;also supplemented with banksian rose (Mu Xiang)andPericarpium Citri Reticulatae(Chen Pi) for qi stagnating in stomach and intestine, Radix et Rhizoma Rhei (Da Huang) for reducing heat and moistening the intestines and relaxing bowels;Melfor moistening and smoothing intestine. The medicines are combined into pills, which have the functions of clearing intestine,relaxing bowels, and slowing down: It has the effects of relaxing bowels and moistening intestines, and can be used to treat constipation caused by intestinal heat, and is used to treat constipation; It has the effect of promoting digestion and can be used to treat abdominal distension and abdominal pain caused by dyspepsia; it has the effects of clearing heat and can be used to treat syndrome of intestine fire exuberance[9-11]. Maren Runchang Pill (麻仁润肠丸) have the function of lubricating the intestines,stimulating intestinal peristalsis and secretion, which is beneficial to the exhaust of stool, accumulation of qi,and to prevent abdominal pain. Clinical studies have shown that its application to the treatment of senile constipation is simple and effective[12-15]. To date, there have been no systematic reviews related. This study included published randomized controlled trials with a view to providing evidence-based evidence for the application of Maren Runchang Pill (麻仁润肠丸) in the treatment of senile constipation.
MATERIALS AND METHODS
Inclusion Criteria
The type of study is a randomized controlled trial. There are no restrictions on whether to use allocation concealment or blindness. The language is limited to Chinese and English. The study subjects were patients with senile constipation,aged 60, and their race, gender, and duration were not limited. Intervention measures: In the case of a clear diagnosis and clear criteria for determining the efficacy, the treatment group was only treated with Maren Runchang Pill (麻仁润肠丸), and the control group was treated with general treatment or other Western medicine. Outcome indicators:stool frequency, stool characteristics, the degree of abdominal pain and abdominal distension.Definition of clinical efficacy: stool shape is classified according to Bristol, and was divided into 7 grades.Level 1: Scattered nut-like stools; Level 2: Sclerotic sausage-like stools; Level 3: Sausage-shaped stools with cracks on the surface; Level 4: Smooth shaped soft stools; Level 5: Dispersed dough-shaped soft stools; Level 6: Pasty stools; Level 7: Watery stools.Clinical cure: Defecation to level 4 or above, difficulty in defecation and inexperienced defecation, smooth defecation; Significant effects: defecation improved to level 2, difficulty in defecation and incomplete defecation were significantly improved; Effective:Feces improved to grade 1, defecation difficulty and incomplete defecation. There is improvement in , and defecation; ineffective: the stool is unchanged and the symptoms are not improved. The effective calculation formula is: (the number of cured cases + the number of significant cases + the number of effective cases) / the total number of cases ×100%; senile constipation cure time is the duration of treatment of cured patients. The included studies included at least one main outcome indicator.
Exclusion Criteria
Studies with very low quality literature, that are unable to extract data or missing data; select the report that has the most comprehensive data for republished studies; the control groups used other Chinese patent medicines; the studies are nonrandomized controlled trials. Random design, or semi-randomized controlled design is not mentioned
Document Retrieval
The database was searched by computer,which are as follows: CNKI, China Biomedical Literature Database (CBM), WanFang Database,VIP Database, PubMed, EMbase, and The Cochrane Library databases. Chinese search terms are: ("麻仁润肠丸") AND ("老年性便秘"or "便秘"),English search term: ("Maren Runchang wan Pills")AND ("senile constipation"). Based on the subject words of each database combined with free words,the search time limit is from the establishment of the database to May 2019.
Literature Screening
Import the preliminary inspection documents into NoteExpress for management, eliminate duplicate titles among databases, read the document titles and abstracts for preliminary screening according to the inclusion criteria, obtain the full text of the documents that meet the inclusion criteria or are uncertain whether to include, read the full text, and finally determine the included literature.
Data Extraction
Excel was used to establish data extraction form to collect information and data to be analyzed.Specific projects include: (1) basic information about research studies, including research ID,author, publication time, sample size, interventions,methodological characteristics, demographic baseline, outcome indicators; (2) study object features, including age, gender etc.; (3) RCTs bias risk assessment evaluation list, including random sequence generation method, allocation concealment, blinding set, ending the report was not complete, reports and other end selectively bias;extracting (4) extraction of the outcome indexes,including total clinical effectiveness, stool frequency,traits, bloating and loss of appetite; (5) safety outcomes: adverse events.
Assessment of risk of bias in included
A total of 2 researchers independently assessed the quality of the included literature based on the "bias risk assessment" tool recommended by the Cochrane Collaboration Network. The evaluation content included the generation of random serial numbers, the allocation and hiding of random schemes, the implementation of blind methods, incomplete data reporting, selective reporting of research results, and other biases.Finally, the judgments of "high risk of bias", "low risk of bias", and "uncertain risk of bias" were made for the literature. A total of researchers independently evaluated the methodological quality of the literature back-to-back, and when disagreement was reached, they were discussed or resolved by a third researcher.
Statistical Analysis
Data analysis was performed using RevMan 5.3 provided by the Cochrane network. Count data uses relative risk (RR) to express effects statistics;continuous variable data used mean difference(MD) when measuring results with the same unit of measurement, and gave a 95% confidence interval( confidence interval, CI). The heterogeneity of the included studies was analyzed using the Q test and the size was evaluated usingI2: ifI250%,statistical homogeneity will be considered good,and a fixed effects model will be used; ifI2>50%,statistical heterogeneity is larger, and sensitivity analysis or subgroup analysis will be performed to identify the causes of heterogeneity, such as age,sex, severity of disease, and the type, dosage,dosage form, treatment course, etc. Of the drug,and then a random effect model will be used. If the study does not meet the requirements of the Metaanalysis, a descriptive analysis will be performed. If the outcome index for the subgroup is greater than 10 papers, a funnel plot will be drawn to determine whether there is publication bias in the included studies.
RESULTS
Literature Search and Selection Process
A total of 254 literature were retrieved during the preliminary inspection. After the NoteExpress check, 89 papers were screened. After reading the titles and retrieving abstracts, a total of 25 documents were selected for full-text evaluation, including 14 CNKI papers, 7 WanFang papers, and 2 VIP papers, SinoMed 2. During the full-text evaluation, 2 reviewers read the paper, and after cross-checking,5 studies were finally included, all of which were Chinese literature, with a total of 595 patients. The literature screening process and results were shown in Figure 1, and the basic information of the included studies was shown in Table 1.

Figure 1. Literature Screening Flow Diagram
Basic Research Features Adopted
A total of 5 studies involving Maren Runchang Pill (麻仁润肠丸) for senile constipation were included in this study, all of which were in Chinese and were conducted in China. A total of 5 randomized controlled trials included a total of 395 elderly patients with constipation, with a maximum sample size of 120 cases, a minimum sample size of 43 cases, a test group of 199 cases, and a control group of 196 cases. In terms of outcome indicators, total clinical effectiveness was reported, as shown in Table 1.
Bias Risk Assessment
The 5 studies included all had varying degrees of risk of bias. Among them, 2 of them[13,14]adopted a more reliable random grouping method and a blind method for subjects, implementers and evaluators, but no allocation concealment was set and there may not have selective reporting,so it was considered to have relatively low risk bias; 3 studies[12,15,16]adopted a more credible random method, and there may be no selective reporting, and no allocation concealment. There was no effective blind method and no follow-up was designed, so it was considered to have a moderate risk of bias. All studies have fully reported the indicators of their expectations. See Figure 2.

Figure 2. Evaluation of Bias Risk
Meta Analysis Results
Total clinical effectiveness
A total of 5 studies[12-16]was included. After heterogeneity testing, the heterogeneity among the studies was small [RR=1.02, 95% CI (0.88, 1.17),P=0.83], and fixed effect model for Meta-analysis was adopted, the result of which showed that there was no significant difference in the total effective rate between the Maren Runchang Pill (麻仁润肠丸) group and the conventional Western medicinetreatment group. See Figure 3.

Table 1. Basic Information Included in the Study
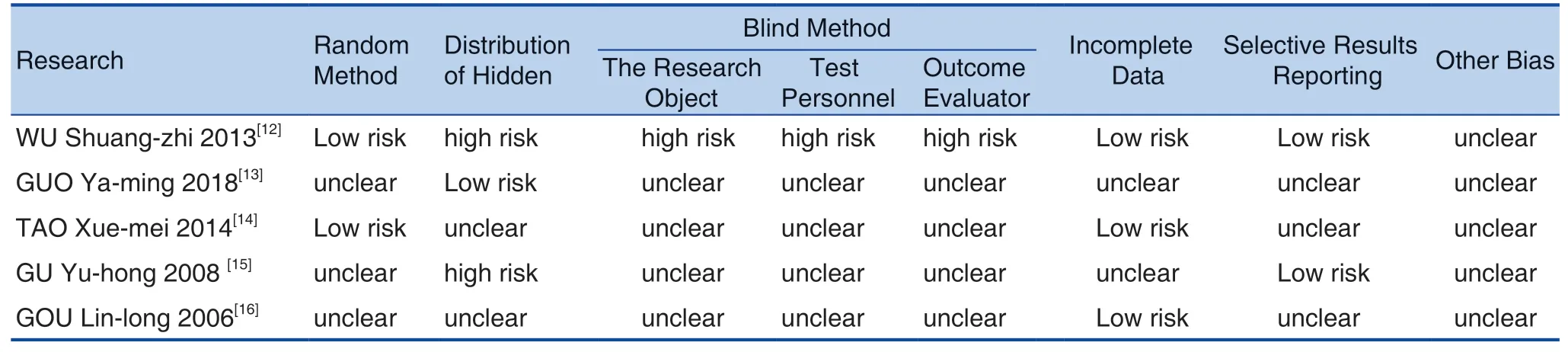
Table 2. Risk Assessment Table of the Included Studies

Figure 3. Meta-analysis of the Total Effective Rate of Western Medicine Treatment VSMaren Runchang Pill (麻仁润肠丸) Treatment
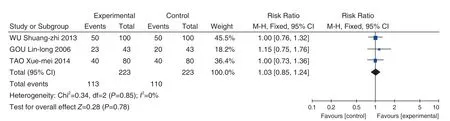
Figure 4. Meta-analysis of Relief of Abdominal Distension and Inappetence by Western Medicine VS Maren Runchang Pill (麻仁润肠丸)
Abdominal distension and the relieving of loss of appetite
A total of 3 studies were included. After the heterogeneity test, the heterogeneity among the studies was small [RR=1.03, 95% CI (0.85, 1.24),P=0.78]. Meta analysis showed that there was no statistically significant difference in the total effective rate between the Maren Runchang Pill (麻仁润肠丸) group and the conventional Western medicine treatment group. See Figure 4.
Adverse reactions
In the 5 included studies, there were no adverse effects in the treatment group of Maren Runchang Pill (麻仁润肠丸) compared with the normal treatment of the control group. The treatment group by Maren Runchang Pill (麻仁润肠丸) had mild abdominal pain compared with the control group of Fruit guide Tablet. In the treatment group, there was 1 case abdominal pain, 1 case of diarrhea, while there were 2 cases of mild abdominal pain and 2 cases of diarrhea in the control group;compared with the control group, Maren Runchang Pill (麻仁润肠丸) in the treatment group, compared with lactulose combined with Bacillus subtilis in the control group, had 2 cases of diarrhea and 1 case of upper abdominal discomfort. The control group had 5 cases of abdominal pain and 2 cases of diarrhea; There were no adverse reactions in the 2 groups compared with enema in the controlgroup. Compared with lactulose in the treatment group, Maren Runchang Pill (麻仁润肠丸) had no adverse reactions in the treatment group. There were 2 cases of abdominal distension and 1 case of diarrhea in the control group. See Table 3.

Table 3. Adverse Reactions
Publishing Bias Analysis
In view of the fact that the funnel chart was applicable to the case where the number of studies was more than 10, the number of studies included in this study was 5, so no funnel chart analysis and publication bias was performed. This study systematically and comprehensively searched all relevant studies on the treatment of senile constipation with Maren Runchang Pill (麻仁润肠丸), including the gray literature,but all studies were published with positive results, no negative results were found, and there was no guarantee whether there was a small sample and the negative results were not published; all studies included in the analysis were in Chinese, so there was some language publication bias. For this reason, this study cannot completely avoid the effects of small sample studies.
DISCUSSION
Constipation and Maren Runchang Pill (麻仁润肠丸)
Traditional Chinese medicine believes that the basic pathogenesis of constipation is large bowel conduction disorders, and is related to dysfunctions of the spleen, stomach, lung, liver, kidney etc. The clinical characteristics, etiology and pathogenesis of senile constipation are different from constipation of other ages. It has its own unique characteristics.The viscera and dysfunction of the elderly, such as spleen deficiency and dysfunction, conduction function of large intestine is stopped. Stomach is connected with intestine, and the exuberance of heat in the stomach and the large intestine is passed down, scorching fluid, leading to exuberance of heat in large intestine, and the dry feces inside;the lungs and the large intestine are in the exterior and interior. The heat and dryness-heat in lung move down to large intestine, leading to the dryness of the intestine and depletion of the fluid. If the liver dominates main the qi movement, with liver depression and qi stagnation, the viscera does not ventilate, with the stagnation of qi; kidney controls urine and stool. If kidney yin is insufficient, there is a lack of moistening and nourishing intestine,with dry stool. If kidney yang is insufficient, large intestine will be lost to warmth, with inability to transport. In the Maren Runchang Pill (麻仁润肠丸) prescription,Fructus Cannabis(Huo Ma Ren) is the main medicine,Fructus Cannabis(Ma Ren) is moist and fat, has the characteristics of smoothing down, can have the function of intestinal moisture and dispelling exogenous dryness, slow down constipation, being beneficial to large intestine qi knotting and constipation;Semen Armeniacae Amarum(Xing Ren ) can reduce qi and moisturize the intestine;Radix Paeoniae Alba(Bai Shao) can nourish yin, withPericarpium Citri Reticulatae(Mu Xiang) andPericarpium Citri Reticulatae(Chen Pi)for qi stagnating in stomach and intestine;Radix et Rhizoma Rheidiarrhea and laxative. Compound prescription has the functions of intestinal moistening, laxative and slowing down and is more suitable for senile constipation.
Effectiveness and Safety of Maren Runchang Pill (麻仁润肠丸)
The results of the study showed that there was no statistical difference in the total effective rate of Maren Runchang Pill (麻仁润肠丸) in the treatment of senile constipation [RR = 1.02, 95% CI (0.88,1.17),P=0.83], abdominal distension and appetite loss [RR = 1.03, 95% CI (0.85 1.24),P=0.78],compared with Western medicine treatment, so a descriptive statistical analysis was performed. In the 5 studies included, the total effective rate of the treatment group in Maren Runchang Pill (麻仁润肠丸), was 96% and 8% compared with the general treatment of the control group, and there were no adverse reactions in the 2 groups. The total effective rates were 91.67% and 76.67% in Maren Runchang Pill (麻仁润肠丸) and in fruit guide tablets. There were 2 adverse reactions in the treatment group, 4 adverse reactions in the control group, and 2 cases of shedding. Compared with the double viable bacteria in the control group, the total effective rate was 75% and 77.5% respectively in the observation group and in the control group. There were 3 adverse reactions in the observation group and 7 adverse reactions in the control group. The total effective rates of Maren Runchang Pill (麻仁润肠丸)in the treatment group and of lactulose in the control group were 86.95% and 75%, with no adverse reactions. There were 3 cases of adverse reactions in the control group. There was no significant difference between the 2 groups in terms of relieving the clinical symptoms of senile constipation (stool frequency, traits, abdominal distension, and loss of appetite).
At present, the drugs used to treat functional constipation in the elderly include irritant laxatives,bulk laxatives, osmotic laxatives and lubricating laxatives. Long-term use of irritant laxatives is not only easy to damage the intestinal wall nerves, but also may cause colon lesions; bloating caused by taking bulk laxatives is often unbearable for patients;lubricating laxatives not only have a poor taste, but also cause long-term use of fat-soluble vitamins obstacle[14], for example, if the main component of the guide is phenolphthalein, which is an irritating laxative, although it is effective, it is often caused by abdominal pain, diarrhea after long-term use, and it is easy to cause water and electrolyte disorders and further cause drug-dependent constipation.The observation results show that although the effects of taking Maren Runchang Pill (麻仁润肠丸)on senile constipation is similar to other Western medicines, Maren Runchang Pill (麻仁润肠丸)has fewer adverse reactions in the treatment of senile constipation. The damage to the intestinal wall tissue is less than that of Western medicine,and it is not easy to produce drug dependence.Therefore, the treatment of constipation has certain clinical advantages, especially suitable for elderly patients. In addition, Maren Runchang Pill (麻仁润肠丸), as a traditional Chinese patent medicine, can play a role in regulating qi and blood circulation,moisturizing intestinal laxative and relaxing bowels.The treatment cost is relatively low. Elder patients are easier to accept and have good compliance.To sum up, for the treatment of senile constipation,Maren Runchang Pill (麻仁润肠丸) has certain clinical advantages over simple Western medicine treatment.
Research Quality Evaluation and Limitations
① This study found that there were various diagnostic and curative standards for senile constipation, and there was a lack of a unified"gold standard". Therefore, the curative effects evaluations included in each study would be greatly different, and the treatment courses of each study would be quite different, which was not convenient for integration evaluation and would affect the consistency and accuracy of the results.
② The quality of the studies included in this systematic review was low. Most of the papers only mention "random", and did not specifically explain the specific implementation method of random grouping. As a result, most of the studies in the random sequence generation entries met the criteria for uncertain risk of bias.
③ The essence of randomized allocation was that those who generate random sequences and those who determine the allocation order cannot be adopted into subjects, and should not participate in the subsequent test process. Especially, they can not participate in the measurement of the results.Those were important measures to avoid selective bias due to various artificial factors affecting random grouping[19]. None of the included studies mentioned randomized concealment concealment, in which those who met the criteria for determining the risk of bias in the concealment entries, may have exaggerated their results.
④ All the included studies did not report the registration of the research protocol, the calculation of the sample size, conflicts of interest, and followup. At present, there are many case reports on senile constipation-related data, lack of well-designed,and strictly implemented randomized controlled trials with large sample sizes. This reduces the persuasiveness and representativeness of the trial.Therefore, in future research, it is recommended the design, implementation, and reporting should be standardized, such as clinical trial registration(U.S. Clinical Trial.gov, Chinese Clinical Trial Registry, ChiCTR, etc.) in the early stages of research, and methodological reference to the ROB tools developed by the Cochrane Collaboration Network. The report standardization[20]refers to the CONSORT standard (2010 edition)[21]. In addition,due to reduced activity of the elderly, lack of fiber in the diet, and many with systemic diseases such as diabetes, uremia, cerebrovascular accidents, etc.,often a variety of drugs such as antidepressants,diuretics, anticholinergics, analgesics medicine, etc.were taken, which can cause constipation. Clinically,it usually manifests as decreased stool frequency,less than 3 times of defecation per week, difficulty in defecation, incomplete defecation, and hard or balllike stool. Excessive defecation can induce transient ischemic attack or defecation syncope, complicated by cardiac infarction and cerebrovascular disease on the basic diseases. Therefore, the purpose of treating senile constipation is not only to defecate,but also to restore normal gastrointestinal transport and emptying functions, and establish normal defecation rules and defecation behavior. The elderly should avoid the use of irritant laxatives that can take effects within 2-8 hours, and should use drugs that take effects within 1-3 days to prevent dehydration, electrolyte imbalance, and fecal incontinence[15]. As a traditional Chinese medicine,Maren Runchang Pill (麻仁润肠丸) is different from Western medicine in terms of onset time and effects,symptom relief and relief duration, etc. Therefore, in the future clinical treatment, it is recommended that clinicians should further record Maren Runchang Pill (麻仁润肠丸) on the time and effects of onset,the degree, the duration of remission of symptoms etc. Secondly, patients with senile constipation have different types of syndromes. We should pay attention to observe whether there are differences in the efficacy of Maren Runchang Pill (麻仁润肠丸) for different types of senile constipation during clinical use, so as to further promote the clinical application of Maren Runchang Pill (麻仁润肠丸).
Clinical Significance
In summary, Maren Runchang Pill (麻仁润肠丸) has a certain clinical effects in the treatment of senile constipation, without serious adverse reactions. However, given that the quality of the included research methods is generally not high,the included studies have not conducted largescale multicenter clinical trials, and the quality of the methods is generally poor, which affects the reliability of the conclusions. In addition,in order to produce high-quality evidence to provide strong support to the application of Maren Runchang Pill (麻仁润肠丸) in clinical practice,more large samples, scientifically designed and strictly implemented randomized controlled trials are needed to study the clinical efficacy and safety of Maren Runchang Pill (麻仁润肠丸) in treating elderly people. Future clinical researchers should strictly design trials, follow the principles of evidence-based medicine and clinical trial management practices, conduct more high-quality clinical trials, formulate random sequence generation and random hiding schemes,strictly implement blind methods, improve quality control during the trial, register the research plan in advance to standardize the research implementation process, provide reliable research conclusions, and precise evidence for the clinical efficacy and safety of Maren Runchang Pill (麻仁润肠丸) in the treatment of senile constipation.
 World Journal of Integrated Traditional and Western Medicine2020年4期
World Journal of Integrated Traditional and Western Medicine2020年4期
- World Journal of Integrated Traditional and Western Medicine的其它文章
- A Brief History of the Development of Integrated Traditional Chinese and Western Medicine
- INSTRUCTION FOR AUTHORS
- A Study to View International Standard Terminology from the English Translation of Terms Related to Tongue Proper in an Original Foreign Work
- Effects of Electroacupuncture on Pain Threshold and Prostaglandin E2 in Spinal Cord in Postoperative Pain Rat
- Intradermal Acupuncture on the Prevention and Adjuvant Treatment of Coronavirus Disease 2019
