吡格列酮对高糖诱导的大鼠肾小管上皮细胞-间充质细胞转分化的影响及机制研究
孙兰 张帆 石明隽 田平平 郭兵


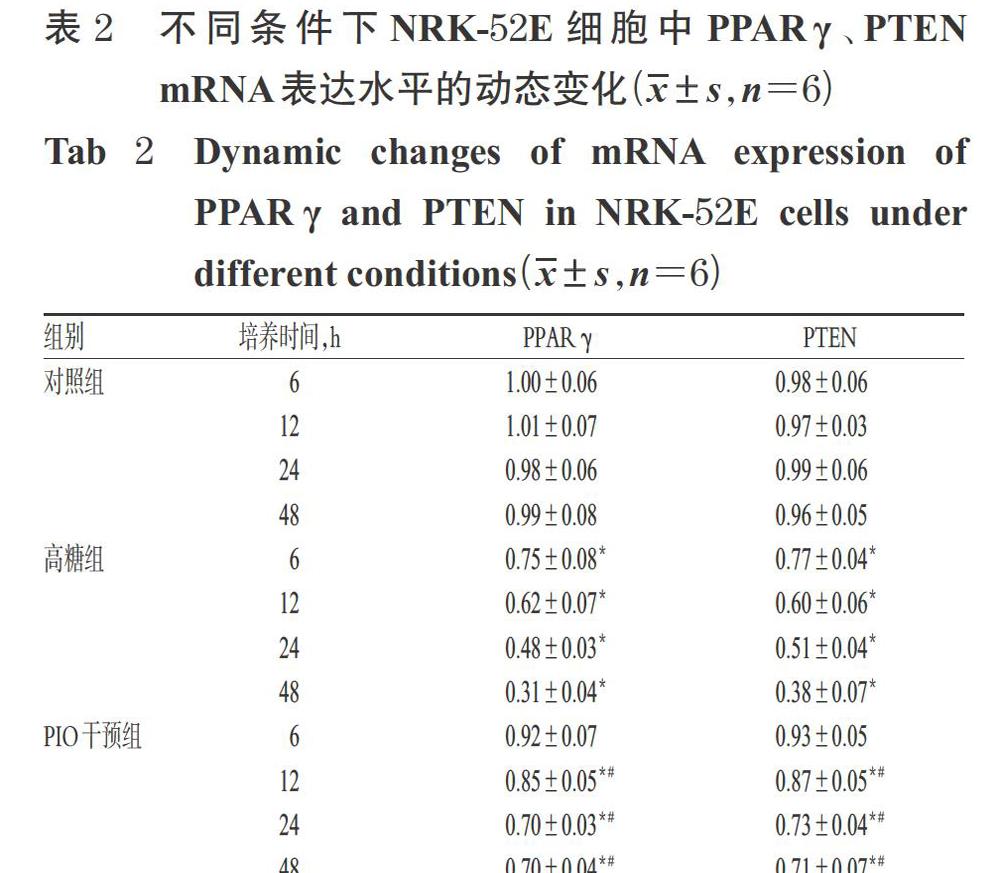
摘 要 目的:探討吡格列酮(PIO)对高糖诱导的大鼠肾小管上皮细胞-间充质细胞转分化(EMT)的影响及可能机制,为防治糖尿病肾病提供理论依据及新靶点。方法:将大鼠肾小管细胞NRK-52E随机分为对照组(5.5 mmol/L葡萄糖)、高糖组(30 mmol/L葡萄糖)、PIO干预组(30 mmol/L葡萄糖+5.0 μmol/L PIO)、GW9662干预组(30 mmol/L葡萄糖+5.0 μmol/L PIO+5.0 μmol/L特异性拮抗剂GW9662)。前3组细胞分别在培养6、12、24、48 h时进行动态检测,GW9662干预组细胞在培养48 h时进行检测。采用Real- time PCR法检测细胞中第10号染色体缺失的磷酸酶和张力蛋白同源基因(PTEN)、过氧化物酶体增殖物激活受体γ(PPARγ)mRNA的表达水平;采用Western blotting法检测细胞中PTEN、PPARγ、α-平滑肌肌动蛋白(α-SMA)、上皮钙黏素(E-cadherin)的表达以及磷脂酰肌醇-3-激酶(PI3K)/蛋白激酶B(AKT)信号通路的变化。结果:随培养时间的延长,与正常组比较,高糖组细胞中PPARγ、PTEN mRNA及蛋白(除PPARγ 6 h外)表达水平均显著降低,α-SMA、p-AKT(Thr308)蛋白表达水平均显著升高,E-cadherin蛋白表达水平显著降低(P<0.05),且呈时间依赖趋势;与高糖组比较,PIO组细胞中PPARγ(除6 h时的蛋白表达外)、PTEN的mRNA及蛋白表达水平均显著升高,α-SMA、p-AKT(Thr308)(除6 h外)蛋白表达水平均显著降低,E-cadherin蛋白表达水平显著增高(P<0.05),且呈时间依赖趋势。与高糖组比较,GW9662干预组细胞PTEN、PPARγ mRNA及蛋白表达水平,α-SMA、E-cadherin、p-AKT(Thr308)蛋白表达水平的差异均无统计学意义,PIO的作用效应被PPARγ拮抗剂GW9662阻断。结论:在高糖条件下PIO可能是通过激活PPARγ而实现对PTEN表达的调控,使PI3K/AKT信号通路受到抑制,进而抑制肾小管上皮细胞EMT的发生。
关键词 吡格列酮;肾小管上皮细胞;过氧化物酶体增殖物激活受体γ;第10号染色体缺失的磷酸酶和张力蛋白同源基因;磷脂酰肌醇-3-激酶/蛋白激酶B信号通路;上皮细胞-间充质细胞转分化;大鼠
ABSTRACT OBJECTIVE: To investigate the effects of pioglitazone(PIO)on high glucose-induced epithelial-mesenchymal transition(EMT) in renal tubular epithelial cells of rat and its possible mechanism, and to provide theoretic reference and new target for the prevention and treatment of diabetic nephropathy. METHODS: The rat renal tubular epithelial NRK-52E cells were randomly divided into control group (5.5 mmol/L glucose), high-glucose group (30 mmol/L glucose), PIO intervention group (30 mmol/L glucose+5.0 μmol/L PIO), GW9662 intervention group (30 mmol/L glucose+5.0 μmol/L PIO+5.0 μmol/L specific anta- gonist GW9662). The cells of the first 3 groups were detected at 6, 12, 24, 48 h of culture, while those in GW9662 intervention group were detected at 48 h of culture. mRNA expression of PTEN and PPARγ were detected by real-time PCR. The protein expression of PTEN, PPARγ, α-SMA and E-cadherin as well as the changes of PI3K/AKT signaling pathway were determined by Western blotting assay. RESULTS: With the extension of culture time, compared with control group, the mRNA and protein expression of PPARγ (except for protein expression at 6 h) and PTEN in high-glucose group reduced significantly, while the protein expression of α-SMA and p-AKT(Thr308) increased significantly, and the protein expression of E-cadherin reduced significantly(P<0.05), showing time-dependent trend. Compared with high-glucose group, the mRNA and the protein expression(except for 6 h) of PPARγ and PTEN were increased significantly in PIO intervention group, while the protein expression of α-SMA and p-AKT(Thr308) were decreased significantly, and the protein expression of E-cadherin was increased significantly (P<0.05), showing time-dependent trend. There was no statistical significance in mRNA and protein expression of PPARγ and PTEN, protein expression of E-cadherin,α-SMA and p-AKT(Thr308) between GW9662 intervention group and high-glucose group; the effect of PIO was blocked by PPARγ antagonist GW9662. CONCLUSIONS: PIO may up-regulate the expression of PTEN by activating PPARγ, inhibit PI3K/AKT signaling pathway so as to inhibit the occurrence of EMT of renal tubular epithelial cells.
3.3.2 对蛋白表达的影响 Western blotting法结果显示,与高糖组比较,PIO干预组细胞中PPARγ、PTEN和E-cadherin蛋白的相对表达水平均显著升高,α-SMA、p-AKT(Thr308)蛋白的相对表达水平均显著降低(P<0.05);而与高糖组比较,GW9662干预组细胞中各蛋白相对表达水平的差异均无统计学意义(P>0.05),表明PPARγ拮抗剂GW9662可以阻断PIO的作用效应。PIO和GW9662对高糖条件下NRK-52E细胞中PPARγ、PTEN、E-cadherin、α-SMA、p-AKT(Thr308)蛋白表达影响的电泳图见图3,对蛋白表达水平的影响见表5。
4 讨论
DN是糖尿病最常见的并发症,主要有肾小球硬化和肾小管间质纤维化等病理表现,但其发病机制中的很多具体环节目前尚不甚清楚。近年来研究认为,肾小管间质纤维化在DN的发生发展中具有更为重要的作用[10-11]。而已有研究表明,促进肾间质纤维化并进一步导致DN肾脏损伤的主要机制是EMT[1-2]。目前认为,上皮细胞标志物E-cadherin表达于上皮细胞中,在EMT过程中其表达减少甚至消失,而间质细胞标志物α-SMA在EMT早期则表达增多[12]。PTEN是第一个被发现具有磷酸酶活性的抑癌基因,可通过负性调控PI3K/AKT信号通路而发挥抗纤维化效应[3]。本研究结果也验证了随着高糖处理时间的逐渐延长,体外培养的肾小管上皮细胞NRK-52E中PPARγ和PTEN的mRNA及蛋白表达水平,以及上皮细胞标志物E-cadherin的蛋白表达水平均较对照组进行性降低,而间质细胞标志物α-SMA和PI3K/AKT通路因子p-AKT(Thr308)的蛋白表達水平均较正常对照组进行性升高。这提示,PI3K/AKT信号通路可以在高糖条件下被激活,同时高糖培养的NRK-52E细胞中PPARγ和PTEN的表达较正常时明显降低,表明肾小管上皮细胞发生了明显的EMT。
噻唑烷二酮类药物是PPARγ的选择性激动剂,有研究发现,其抗肾脏纤维化病变的机制是通过活化PPARγ 而实现的[9]。研究表明,PPARγ对PTEN表达进行调控的机制可能是其可以与位于PTEN上游的2个反应元件直接结合[6-7]。然而,噻唑烷二酮类药物是否通过活化PPARγ而具有上调高糖诱导的NRK-52E细胞中PTEN表达的功能,从而发挥抗EMT的作用,尚未有研究阐明。本研究选用噻唑烷二酮类抗糖尿病药物PIO,通过MTT检测显示,PIO对NRK-52E细胞增殖的影响具有双相性,浓度为2 μmol/L时能促进细胞增殖,10 μmol/L时则抑制细胞增殖。本次试验选择5 μmol/L浓度的PIO,该浓度既可对NRK-52E细胞产生一定抑制作用,又对细胞增殖无明显影响。
本研究结果显示,PIO干预组细胞在不同作用时间(除少数时间点外)下,其PPARγ、PTEN mRNA及蛋白表达水平均较高糖组显著升高,α-SMA和p-AKT(Thr308)蛋白表达水平均较高糖组显著降低,上皮细胞标志物E-cadherin蛋白表达水平较高糖组显著升高,且呈时间依赖趋势。这提示,PIO可一定程度地激活PPARγ、上调PTEN并抑制PI3K/AKT信号通路的过度激活,从而表现出抑制NRK-52E细胞EMT发生和进展的作用。
为了进一步确定PIO是否通过PPARγ途径上调PTEN来发挥抑制EMT的作用,笔者应用PPARγ特异性拮抗剂GW9662进行反向验证。GW9662能不可逆性地与PPARγ的285位半胱氨酸位点共价结合,有体外细胞试验已证实,1~10 μmol/L的GW9662具有特异性拮抗PPARγ活化的功能[13]。因此,选择GW9662作为验证药物来确定PIO是否通过PPARγ依赖性途径对PTEN的表达产生影响。本研究结果显示,与高糖组比较,PIO确能使PPARγ和PTEN mRNA及蛋白的表达水平以及E-cadherin蛋白的表达水平明显升高,使α-SMA、p-AKT(Thr308)蛋白的表达水平明显降低;而加入PPARγ特异性拮抗剂GW9662干预后,PPARγ、PTEN mRNA及蛋白的表达水平以及其余各蛋白的表达水平与高糖组均无显著性差异,即GW9662阻断了PIO对NRK-52E细胞的上述所有保护性作用。这进一步明确了PIO是通过PPARγ/PTEN/PI3K/AKT通路来发挥对NRK-52E细胞发生EMT的抑制作用。
综上,在高糖条件下PIO可能是通过激活PPARγ而实现对PTEN表达的调控,使PI3K/AKT信号通路受到抑制,进而抑制肾小管上皮细胞EMT的发生。本研究结果可为明确DN的发病机制以及寻找DN防治的有效药物提供理论依据。
参考文献
[ 1 ] LOEFFLER I,WOLF G. Epithelial-to-mesenchymal transition in diabetic nephropathy:fact or fiction? [J]. Cells,2015,4(4):631-652.
[ 2 ] 周星丞,张帆,严瑞,等. SAA1在狼疮小鼠肾脏中的表达及其对小鼠肾小管上皮细胞EMT的作用[J].中国免疫学杂志,2019,35(21):2561-2565、2575.
[ 3 ] LI L,ZHU X,SHOU T,et al. MicroRNA-28 promote cell proliferation and invasion in gastric cancer via the PTEN/PI3K/AKT signaling pathway[J]. Mol Med Rep,2017,17(3):4003-4010.
[ 4 ] 王圆圆,刘瑞霞,郭兵,等.大鼠肾组织PETN表达下调在糖尿病肾病发展中的作用[J].生理学报,2011,63(4):325-332.
[ 5 ] 李霜,王圆圆,郭兵,等. 控制血糖对糖尿病大鼠肾PTEN表达及纤维化病变的影响[J].贵阳医学院学报,2012,37(1):14-19.
[ 6 ] LIU YW,DAI B,XU CG,et al. Rosiglitazone inhibits transforming growth factor-β1 mediated fibrogenesis in ADPKD cyst-lining epithelial cells[J]. PLoS One,2011. DOI:10.1371/journal.pone.0028915.
[ 7 ] ZAMBRANO S,BLANCA AJ,RUIZ-ARMENTA MV,et al. L-carnitine attenuates the development of kidney fibrosis in hypertensive rats by upregulating PPAR-γ[J]. Amer J Hypertens,2014,27(3):460-470.
[ 8 ] BONOFIGLIO D,GABRIELE S,AQUILA S,et al. Estrogen receptor alpha binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor gamma signaling in breast cancer cells[J]. Clin Cancer Res,2005,17(11):6139-6147.
[ 9 ] FANG HQ,FANG WT,CAO HW,et al. Di-(2-ethylhexyl)-phthalate induce apoptosis via the PPARγ/PTEN/AKT pathway in differentiated human embryonic stem cells[J].Food Chem Toxicol,2019. DOI:10.1016/j.fct.2019.05. 060.
[10] 彭君,彭家清,秦鵬,等. miR-27a-3p靶向SnoN抑制高糖诱导的人近端肾小管上皮细胞EMT的作用[J].免疫学杂志,2020,36(1):45-51.
[11] 孙小鸿,黄凯鹏,黄河清. Connexin43通过SIRT1-HIF1-α通路改善糖尿病肾小管间质纤维化的研究[J].中国药理学与毒理学杂志,2019,33(9):718.
[12] LAMOUILLE S,XU J,DERYNCK R. Molecular mechanisms of epithelial-mesenchymal transition[J]. Nat Rev Mol Cell Biol,2014,15(3):178-196.
[13] LEESNITZER LM,PARKS DJ,BLEDSOE RK,et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferators activated receptors by GW9662[J]. Biochemistry,2002,41(21):6640- 6650.
(收稿日期:2020-02-13 修回日期:2020-06-28)
(编辑:段思怡)

