Rice bran hydrolysates induce immunomodulatory effects by suppression of chemotaxis, and modulation of cytokine release and cell-mediated cytotoxicity
Suphanthip Phusrisom, Laddawan Senggunprai, Auemduan Prawan, Sarinya Kongpetch, Upa Kukongviriyapan,Supawan Thawornchinsombut, Ronnachai Changsri, Veerapol Kukongviriyapan✉
1Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
2Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
3Department of Food Technology, Faculty of Technology, Khon Kaen University, Khon Kaen 40002 , Thailand
4Chumphae Rice Research Center, Ministry of Agriculture and Cooperatives, Khon Kaen 40130, Thailand
ABSTRACT
KEYWORDS: Rice bran; Immunomodulatory; Non-MHC-restricted cell cytotoxicity; Quercetin glycoside; Ferulic acid;Immunoadherence
1. Introduction
Natural compounds are important sources of drug discovery. A number of phytochemicals such as quercetin and its glycosides are lead compounds for the treatment of some tropical diseases, such as dengue virus infection[1]. Rice (Oryza sativa L.) is an important staple food for about half of the world population, particularly those in Asian countries. Protein from rice bran is accountable for 10%-15% by weight, and it has been suggested to be useful as it is hypoallergenic[2]. Lipids from rice bran are rich in unsaturated fatty acids, particularly, oleic acid and linoleic acid[3]. Rice bran contains a significant amount of nutritive and non-nutritive chemicals that confer a number of health benefits. Bioactive phytochemicals in rice bran comprise phytosterol, γ-oryzanol,tocopherols, tocotrienols, small phenolic compounds such as ferulic acid, catechin, p-hydroxybenzoic acid, and flavonoids such as quercetin and rutin[2-5]. These compounds are responsible for the observed pharmacological activities including antioxidant potential,improvement of insulin sensitivity, alleviation of dyslipidemia and hypertension, and anti-inflammatory and immunomodulatory activities[6-9].
Phenolic compounds are secondary metabolites in plants that play a role in the growth and host defense against pathogens. Several studies have therefore investigated the ability of polyphenols such as ferulic acid, p-coumaric acid, rutin and quercetin present in rice bran in modulating the immune system, for instance, in responses against inflammation, production of cytokines, chemotaxis and activation of the immune system against cancer cells[10,11]. Other components from rice bran have been studied for their therapeutic properties.Rice bran proteins and glycoproteins restore immunocompetence in cyclophosphamide-induced animals[12], and the water-soluble polysaccharide from rice bran enhances the cytotoxic activity of Raw264.7 monocytes in association with inducing nitric oxide and TNFα secretion, which kills melanoma cells[13]. However, precise mechanisms associated with these effects are still lacking.
In this study, we investigated rice bran hydrolysates, which were derived from pigmented rice, Tubtim Chumphae. Rice bran was modified by hydrothermolysis to improve its functional properties, e.g. loosening complex structure of carbohydrates and proteins and increased water soluble[14]. This study examined the immunomodulatory effects of rice bran hydrolysates on cultured T lymphocytes and monocytes using Jurkat and THP-1 cells, and peripheral blood mononuclear cells (PBMCs). The work aimed at evaluating the effect of rice bran hydrolysates on cell proliferation,migration, infiltration, cytokine release, as well as the effect on cytotoxic lymphocytes in cancer cells.
2. Materials and methods
2.1. Chemicals and reagents
RPMI 1640 media, Ham’s F12 media, and fetal bovine serum albumin were purchased from Gibco®Life Technologies(Grand Island, NY, USA). MCDB131 media, endothelium cell growth supplement, phytohemagglutinin-L (PHA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)were obtained from Sigma-Aldrich (St. Louis, MO, USA). Goat polyclonal antibody against β-actin and anti-VCAM-1 were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The antibodies:anti-phospho-AMPKα (Thr172), anti p-mTOR (Ser2448), anti-phospho NF-κB p65, were purchased from Cell Signaling (Danvers, MA,USA). Monocyte chemoattractant protein 1 (MCP-1) was purchased from ProSpec-Tany TechnoGene Ltd. (Hamada, Israel). Human IL-2, IL-4, TNF-α and IFN-γ ELISA kits were purchased form InvitrogenTMThermo Fisher Scientific (Vienna, Austria).
2.2. Preparation of rice bran hydrolysates
Rice bran was obtained from nonglutinous pigmented rice of the variety Tubtim Chumphae (RD69) cultivated by Chumphae Rice Research Center, Rice Department, Thailand. Hydrolysates of rice bran were prepared essentially according to a previously described method[15]. Briefly, defatted rice bran was dispersed in water at pH 9.5 for 18 h, subjected to hydrothermolysis by autoclaving for 40 min, followed by digestion with protease G6 at pH 8.0 for 8 h. After termination of the reaction, the supernatant was ultrafiltered (MW cut-off at 50 kDa) and freeze-dried into a powder.
2.3. Analysis of rice bran hydrolysates and antioxidant assay
Phenolic acids in rice bran hydrolysates were analyzed by high performance liquid chromatography (HPLC) as the method described previously using HPLC equipped with a UV photodiode detector[16].Radical scavenging capacity was performed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and the reducing potential of rice bran hydrolysates was assessed by the ferric reducing antioxidant power(FRAP) assay according to methods previously described. Ascorbic acid was used as a standard reference[17]. Total phenolic content was determined using the Folin-Ciocalteu reagent method and gallic acid was used as a standard.
2.4. Assay of arabinoxylans
Water extractable arabinoxylan content was estimated by the phloroglucinol colorimetric method as described previously using xylose as a standard[18].
2.5. PBMC preparation
Blood was collected from healthy donors at Central Blood Bank of Srinagarind Hospital, Faculty of Medicine, Khon Kaen University.The PBMCs prepared by density gradient centrifugation were cultured in RPMI 1640 medium supplemented with 10% heatinactivated fetal bovine serum, 100 U/mL penicillin, and 50 mg/L gentamicin.
2.6. Study protocol
Study protocol using human subjects was approved by the Center for Ethics in Human Research, Khon Kaen University (HE621035).
2.7. Cell culture
Jurkat cells (TIB-152, ATCC, Manassas, VA, USA) and THP-1 cells(TIB-202, ATCC) were cultured in RPMI 1640 media supplemented with 10% fetal bovine serum albumin, 100 U/mL penicillin, 50 μg/mL gentamicin, and 10 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), at pH 7.35. Human umbilical vein endothelial cells (HUVEC) (JCRB Cell Bank, Japan) were cultured in MCDB131 media supplemented with 1.18 g/L NaHCO3, 30 μg/L endothelial cell growth supplement, 2 500 U/L heparin, 50 μg/mL gentamicin, 100 U/mL penicillin and 10 mM HEPES, at pH 7.35 and 10% fetal bovine serum. The cells were subcultured every 2 days by diluting the non-adherent cells with cultured media and using 0.25% trypsin-EDTA for HUVEC cells. KKU-452 cells (JCRB Cell Bank) were cultured in Ham’s F12 media supplemented with 10%fetal bovine serum, 50 mg/L gentamicin sulfate, 100 U/mL penicillin and 10 mM HEPES, at pH 7.35. The cultured cells were maintained under an atmosphere of 5% CO2in air at a 37 ℃ incubator and subcultured every 2 days.
2.8. Assay of cell proliferation
PBMC, Jurkat and THP-1 cells at a density of 1×105cells were seeded onto a 96 well plate in complete media containing varying concentrations of rice bran hydrolysates or cyclosporine A (CsA) (2 μM for PBMC and 1 μM for Jurkat and THP-1 cells) and PHA (10 μg/mL) and were incubated for 72 h. Cell viability was assessed by MTT assay.
2.9. Chemotaxis of Jurkat cells
Jurkat cells (5×106cells/mL) were pre-incubated in serum-free media with rice bran hydrolysates (200-1 200 μg/mL) or CsA (1 μM)for 3 h. Then, 100 μL of cell suspension was seeded onto a transwell insert with an 8-micron pore size, and the lower chamber was filled with full media. After incubation for 24 h, the cells that migrated through the membrane to the lower chamber were counted under a microscope. The number of migratory cells in the treatment groups was compared to the control group.
2.10. Migration and infiltration of THP-1 cells
THP-1 cells were pre-incubated in serum-free media containing rice bran hydrolysates (200-1 200 μg/mL) for 24 h. Then, 5×105cells in 100 μL media were seeded onto a transwell insert with an 8-micron pore size coated with 0.3 mg/mL Matrigel®. The lower chamber was filled with full media containing 20 ng/mL MCP-1 as a chemoattractant. THP-1 cells were allowed to migrate for 1 h, then cells that migrated through membrane to the lower chamber were counted under a microscope.
2.11. Adhesion of THP-1 cells on HUVECs
HUVECs at a density of 1×104cells/well were seeded onto a 96 well plate, incubated overnight, and then treated with rice bran hydrolysates (200-1 200 μg/mL) for 1 h, followed by 100 ng/mL lipopolysaccharide (LPS) for another 4 h to stimulate HUVECs.THP-1 cells, preloaded with a fluorescent probe dichlorofluorescein diacetate (DCF-DA) for 30 min, were layered on the HUVECs monolayer. After incubation for 1 h, the excessive non-adhered THP-1 cells were washed away. The images of the adherent THP-1 cells were captured and analyzed by Image J software.
2.12. PBMCs cytokines production
Two mL (1.5×106cells/mL) of PBMCs were seeded onto a 6 wellplate in full media containing rice bran hydrolysates (400-1 600 μg/mL)or CsA (2 μM) with PHA. PBMCs were cultured for 72 h, supernatant media were collected by centrifugation and assayed for cytokine IL-2, TNF-α, IL-4 and IFN-γ using an ELISA kit according to the manufacturer’s instruction.
2.13. Protein expression by Western blotting analysis
After treatment with rice bran hydrolysates (200-1 200 μg/mL),cell pellets were washed and lysed with RIPA cell lysis buffer containing protease inhibitor and phosphatase inhibitor. The protein concentration was determined using Bradford dye-binding reagent. Twenty five μg of whole-cell lysates were subjected to a 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE). The separated proteins were electrophoretically transferred onto a PVDF membrane. The blotted PVDF membrane was blocked with 5% (w/v) skim milk powder in PBS buffer saline containing 0.1% Tween-20 or with 5% bovine serum albumin (w/v) in tris-buffered saline containing 0.1% Tween-20 for phospho-proteins, and then were incubated overnight at 4 ℃ with primary antibodies including antip-NF-κB, anti-p-AMPK, anti-p-mTOR, anti-VCAM-1 and anti-βactin, followed by incubation with secondary antibodies. The specific protein bands were detected by a chemiluminescent method. Protein expression was normalized using β-actin as the loading control.
2.14. Co-culture of cholangiocarcinoma (CCA) cells with PBMCs
The CCA cells, KKU-452 was used as target cells (T) and were seeded onto a 96-well plate and cultured overnight. PBMC as effectors cells (E) were treated with rice bran hydrolysates(800-1 600 μg/mL) and PHA (10 μg/mL) for 72 h and washed, and the non-adherent cells were seeded onto cultured KKU-452 cells at T: E ratios of 1:7.5 and 1:15. Co-cultured cells were incubated for 24 h. The suspended PBMC cells were discarded and washed twice with cultured media. The viability of KKU-452 cells was measured by MTT assay.
2.15. Statistical analysis
All results were presented as mean ± SD. Comparison between the control and treatment groups was performed with an analysis of variance with the Student-Newman-Keuls post-hoc test. Results were considered to be statistically significant at P<0.05.
3. Results
3.1. Composition and antioxidant property of rice bran hydrolysates
The main component of rice bran hydrolysates was carbohydrate which accounted for (61.5 ± 0.4)%, followed by fiber (22.9 ±1.1)%, protein (8.5 ± 0.8)%, and lipid (2.30 ± 0.25)%. Rice bran hydrolysates prepared by hydrothermolysis and protease digestion had a high yield of total phenolic contents of (29.1 ± 2.7) mg gallic acid equivalent/g rice bran hydrolysates. The antioxidant activity of rice bran hydrolysates, assessed by the FRAP method, was (24.8 ±2.5) mg ascorbic acid equivalent/g rice bran hydrolysates. In DPPH assay, the IC50of rice bran hydrolysates was (0.96 ± 0.21) mg/mL while the IC50for a reference compound, ascorbic acid was (0.020± 0.001) mg/mL. The concentrations of major phenolic acids were ferulic acid (579 μg/g), catechin (530 μg/g), isoquercetin (494 μg/g),rutin (401 μg/g), p-hydroxybenzoic acid (279 μg/g), protocatechuic acid (213 μg/g), p-coumaric acid (136 μg/g) and caffeic acid (137 μg/g). In preparation for rice bran hydrolysates, the concentration of water-extractable arabinoxylans, a hemicellulose from the cell wall in cereal grains, was 13.2 mg/g rice bran hydrolysates.
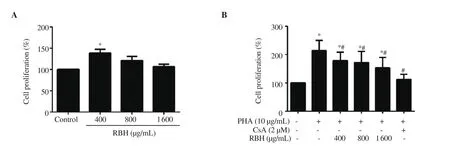
Figure 1. Effect of rice bran hydrolysates on PBMC proliferation. PBMCs were cultured in RPMI 1640 containing (A) rice bran hydrolysates (400-1 600 μg/mL) alone, and (B) with PHA (10 μg/mL) or PHA with or without CsA (2 μM) for 72 h. Cell viability was determined by MTT assay. Each bar represents mean± SD of 3 experiments. *P < 0.05 vs. Control, #P <0.05 vs. PHA. PBMC: peripheral blood mononuclear cell, CsA: cyclosporine A, PHA: phytohemagglutinin,RBH: rice bran hydrolysates.
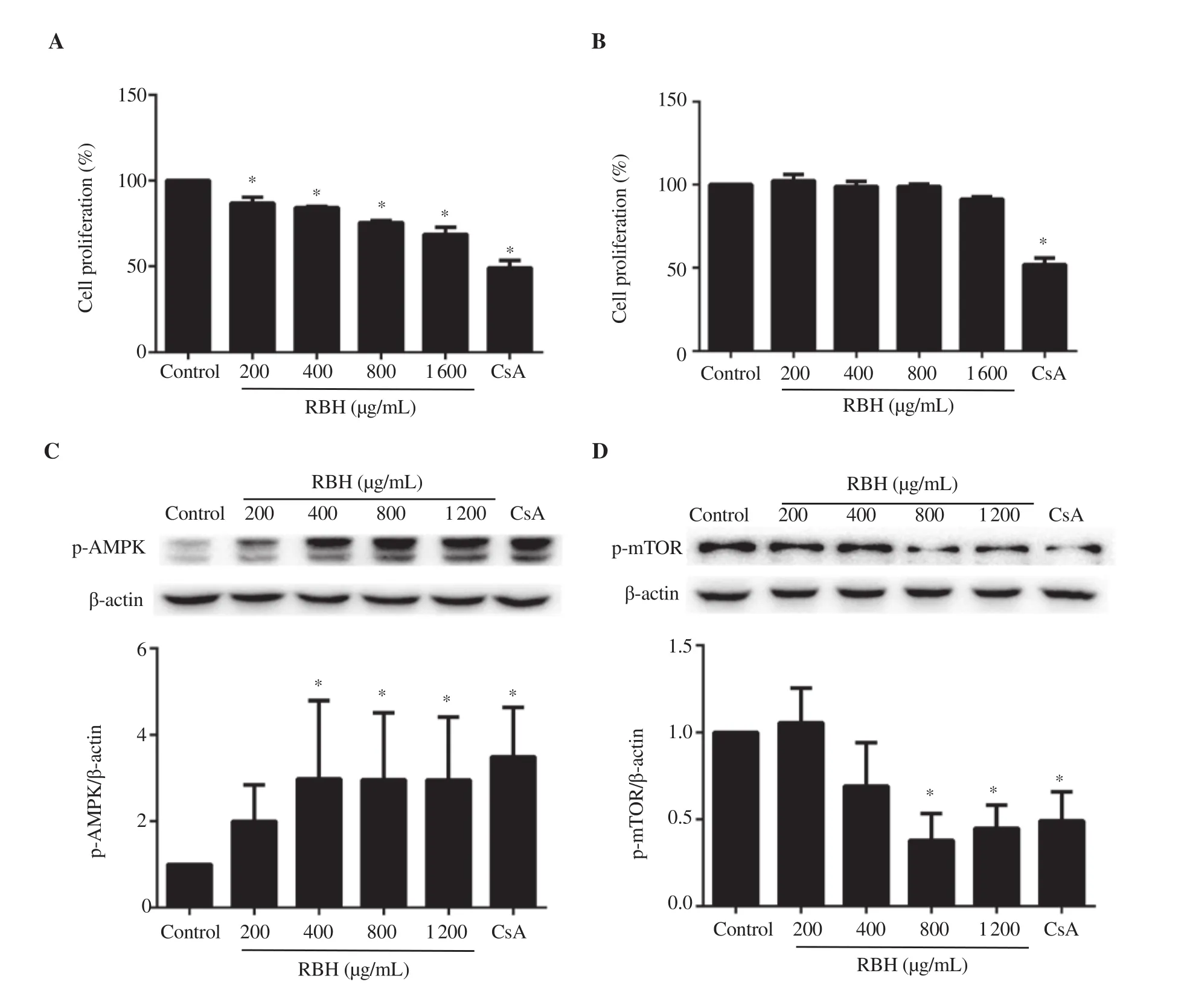
Figure 2. Effect of rice bran hydrolysates on Jurkat and THP-1 cell proliferation and proteins associated with proliferation. (A) Jurkat cells were cultured in RPMI 1640 containing rice bran hydrolysates (200-1 600 μg/mL) or CsA (1 μM) for 72 h or (B) THP-1 cells cultured for 24 h. Protein lysates from Jurkat cells treated for 6 or 24 h were determined for (C) p-AMPK or (D) p-mTOR, respectively by Western blotting analysis. Each bar represents mean ± SD of 3 experiments. *P <0.05 vs. Control group.
3.2. Effect of rice bran hydrolysates on cell proliferation
To evaluate the immunomodulatory activity of rice bran hydrolysates, the effect of rice bran hydrolysates on the proliferation of PBMC was determined. Results showed that rice bran hydrolysates alone at low concentration stimulated cell proliferation (Figure 1A). However, at higher concentrations of rice bran hydrolysates, the stimulating effect disappeared. The effect of rice bran hydrolysates on mitogen-activated cell proliferation was evaluated. PHA alone strongly stimulated PBMC proliferation(Figure 1B), while the rice bran hydrolysates suppressed the stimulating effect of PHA. CsA, an inhibitor of the nuclear factor of activated T cells for activation of T cells, completely abolished the effect of PHA (Figure 1B).
3.3. Proliferation of Jurkat cells and THP-1 cells
As PBMCs consist of many cell types necessary in mounting immune responses, the effect of rice bran hydrolysates was evaluated in Jurkat T lymphocytes and THP-1 monocytes. Results showed that rice bran hydrolysates significantly inhibited Jurkat cells proliferation in a dose-dependent manner, but had no effect on THP-1 cells (Figure 2A & B). CsA strongly suppressed proliferating cells (Figure 2A & B).
3.4. Proteins involved in proliferation & migration
The molecular mechanism by which rice bran hydrolysates suppressed Jurkat cells was investigated. Jurkat cells were pretreated with rice bran hydrolysates in serum-free media for 3 h, then incubated in completed medium containing rice bran hydrolysates for 6 or 24 h. Cell lysates were subjected to Western blotting analysis. Results showed that rice bran hydrolysates as well as CsA significantly activated p-AMPK within 6 h (Figure 2C) and strongly suppressed its downstream p-mTOR at 24 h (Figure 2D).
3.5. Chemotaxis and infiltration of Jurkat and THP-1 cells
Effects of rice bran hydrolysates on Jurkat cells on migration, as well as THP-1 cells on migration and infiltration were evaluated using the transwell chamber assay for chemotaxis study. Results showed that rice bran hydrolysates significantly inhibited the migration of Jurkat cells (Figure 3A, B) and infiltration of THP-1 cells through the matrigel (Figure 3C, D).
3.6. Effect of rice bran hydrolysates on THP-1 cells adhesion on HUVECs
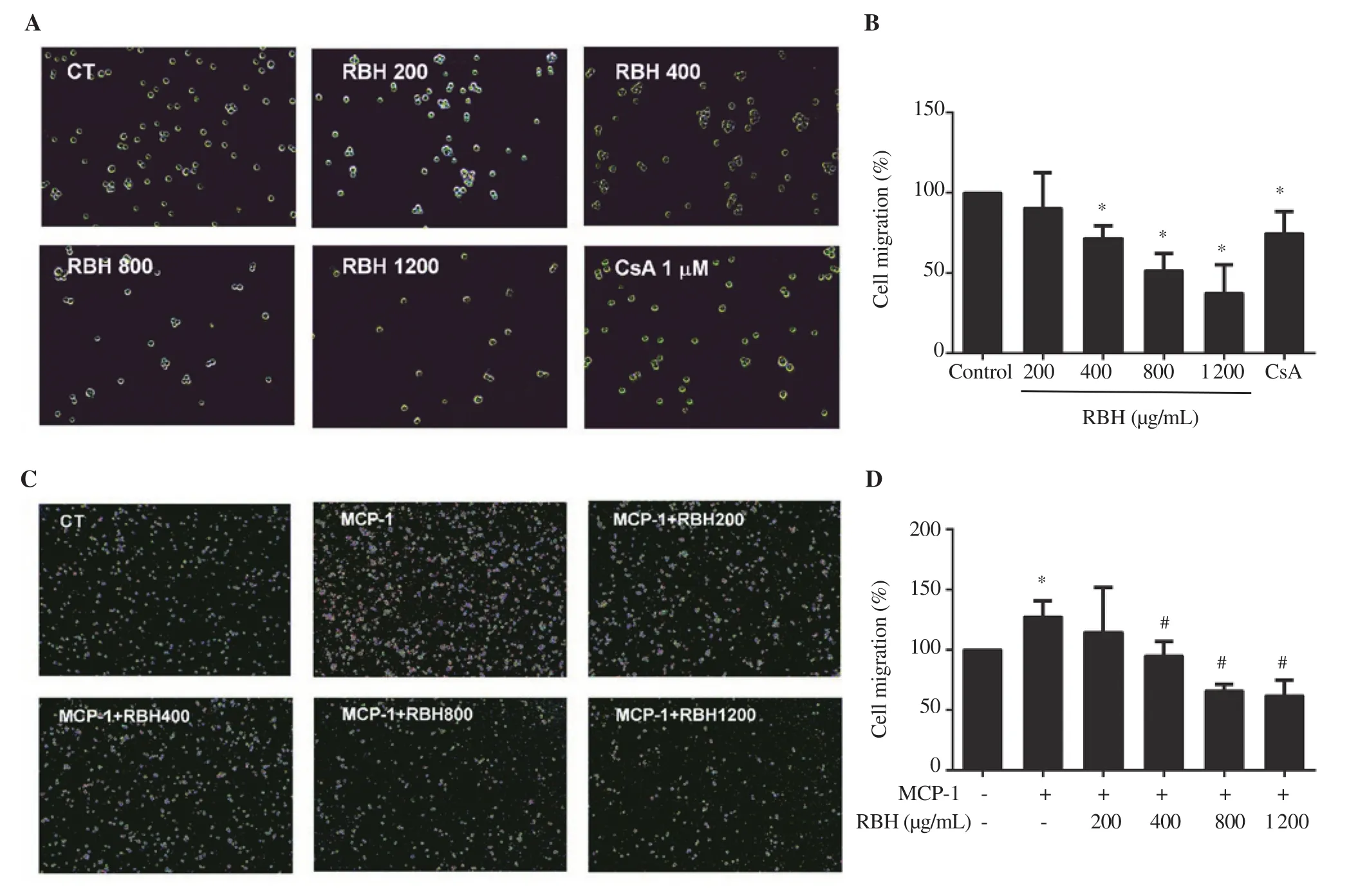
Figure 3. Effect of rice bran hydrolysates on chemotaxis of Jurkat cells and THP-1 cells. The cells were pretreated with rice bran hydrolysates (200-1 200 μg/mL) and then subjected to transwell chamber assay. Images and graph represent (A, B) migration of Jurkat cells and (C, D) THP-1 cells. Each bar represents mean ± SD of 3 experiments. *P < 0.05 vs. Control (CT) group, #P < 0.05 vs. MCP-1 group. MCP-1: monocyte chemoattractant protein-1.
Anti-inflammatory activity of rice bran hydrolysates was examined by an in vitro monocyte-endothelial cell adhesion assay. Rice bran hydrolysates (800 and 1 200 μg/mL) significantly attenuated adhesion of THP-1 cells to HUVECs by 39.5% and 44.4%,respectively compared to the LPS-stimulated group (Figure 4A, B).The molecular mechanism of rice bran hydrolysates on HUVEC cells was also examined. LPS stimulated expression of NF-κB in HUVEC cells (Figure 4C) and rice bran hydrolysates pretreatment significantly inhibited NF-κB and VCAM-1 expression (Figure 4C,D).
3.7. Cytokine release in PBMCs
To investigate the immunomodulating effect of rice bran hydrolysates on PBMC, cytokines released by PBMC were determined. Background levels of IL-2, IL-4 and TNF-α were very low. Rice bran hydrolysates alone had a modest effect on the release of IL-2, TNF-α, IL-4, and IFN-γ when compared to the background levels in untreated cells (data not shown). A further study examined whether rice bran hydrolysates modulated the stimulatory effect of PHA. PHA induced the release of IL-2, TNF-α and IL-4 and IFN-γ in PBMCs (Figure 5A-D). Rice bran hydrolysates significantly enhanced the release of IL-2, TNF-α, IL-4, except IFN-γ. Moreover,CsA markedly inhibited the release of all cytokines to the background levels.
3.8. PBMC-mediated cytotoxicity on CCA cells
As rice bran hydrolysates could modulate PHA-induced inflammatory cytokine release in PBMCs by enhancing the release of cytokines,we investigated whether the activation of PBMCs could increase the cytotoxic effect of these cells on target cancer cells. PBMCs, incubated with rice bran hydrolysates and PHA before non-adherent mainly T cells and NK cells were co-incubated with KKU-452 cells. The result showed that PHA alone significantly activated PBMCs to suppress CCA cell proliferation to approximately 35% in both cell density ratios.Treatment with rice bran hydrolysates at 800-1 600 μg/mL increased the inhibition of KKU-452 cells by cytotoxic PBMCs to 50%-58% in two T:E ratios, respectively (Figure 6A, B). In a preliminary study, the treatment of KKU-452 cells with rice bran hydrolysates did not cause any cytotoxicity to the cancer cells (data not shown).
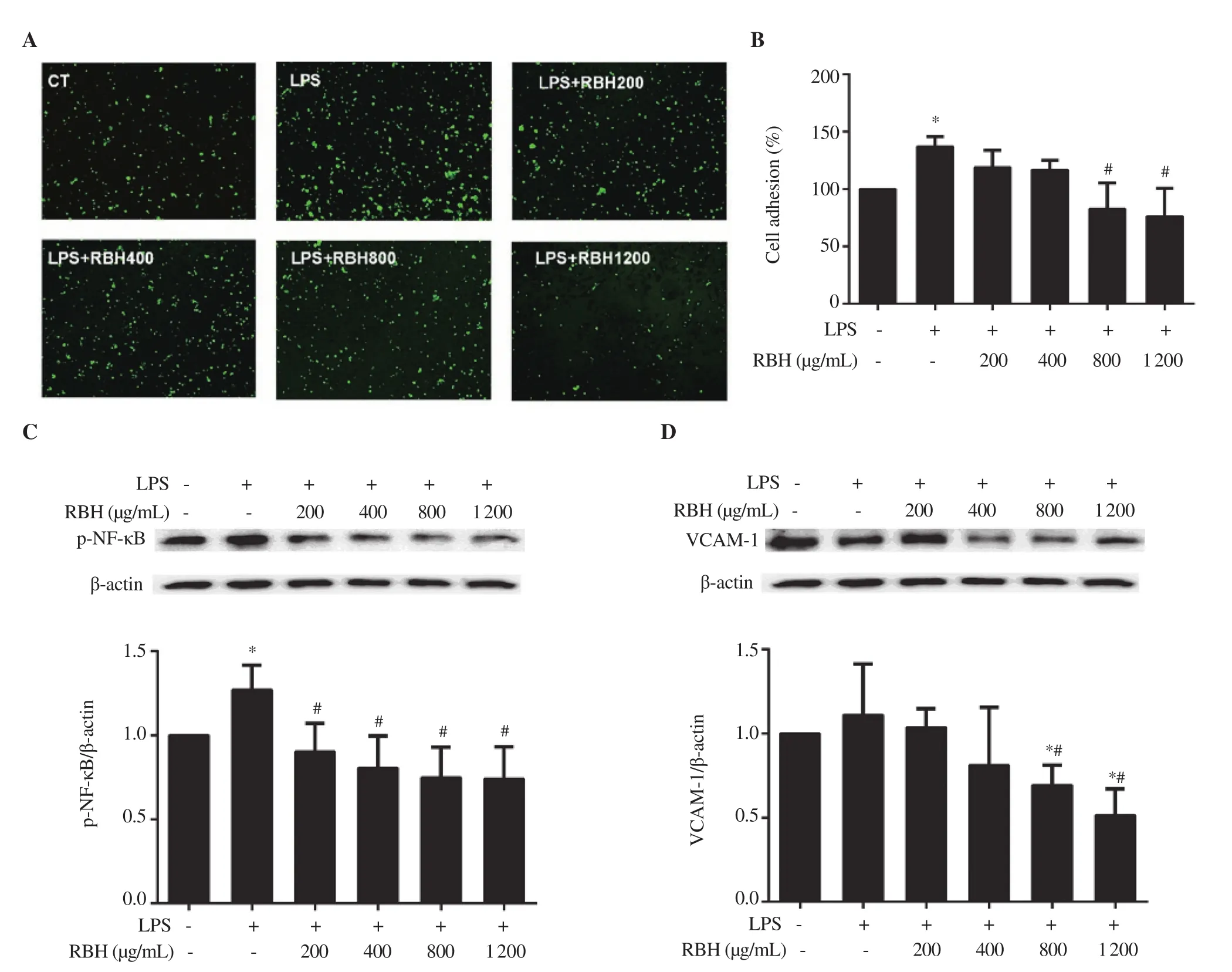
Figure 4. Inhibitory effect of rice bran hydrolysates on THP-1 cell adhesion on LPS-stimulated HUVECs. HUVEC cells were seeded to form monolayer,incubated with rice bran hydrolysates and stimulated with LPS (100 ng/mL). THP-1 loaded with DCF-DA cells were overlaid on HUVECs. (A, B) Images and graph represent cell adhesion of THP-1 on HUVECs. (C, D) Cell lysates from HUVECs were analyzed for p-NF-κB and VCAM-1 by Western immunoblotting. Each bar represents mean ± SD of 3 experiments. *P < 0.05 vs. Control, #P <0.05 vs. LPS. HUVEC: human umbilical vein endothelial cells,LPS: lipopolysaccharide, DCF-DA: dichlorofluorescein diacetate, VCAM-1: vascular cell adhesion molecule-1.
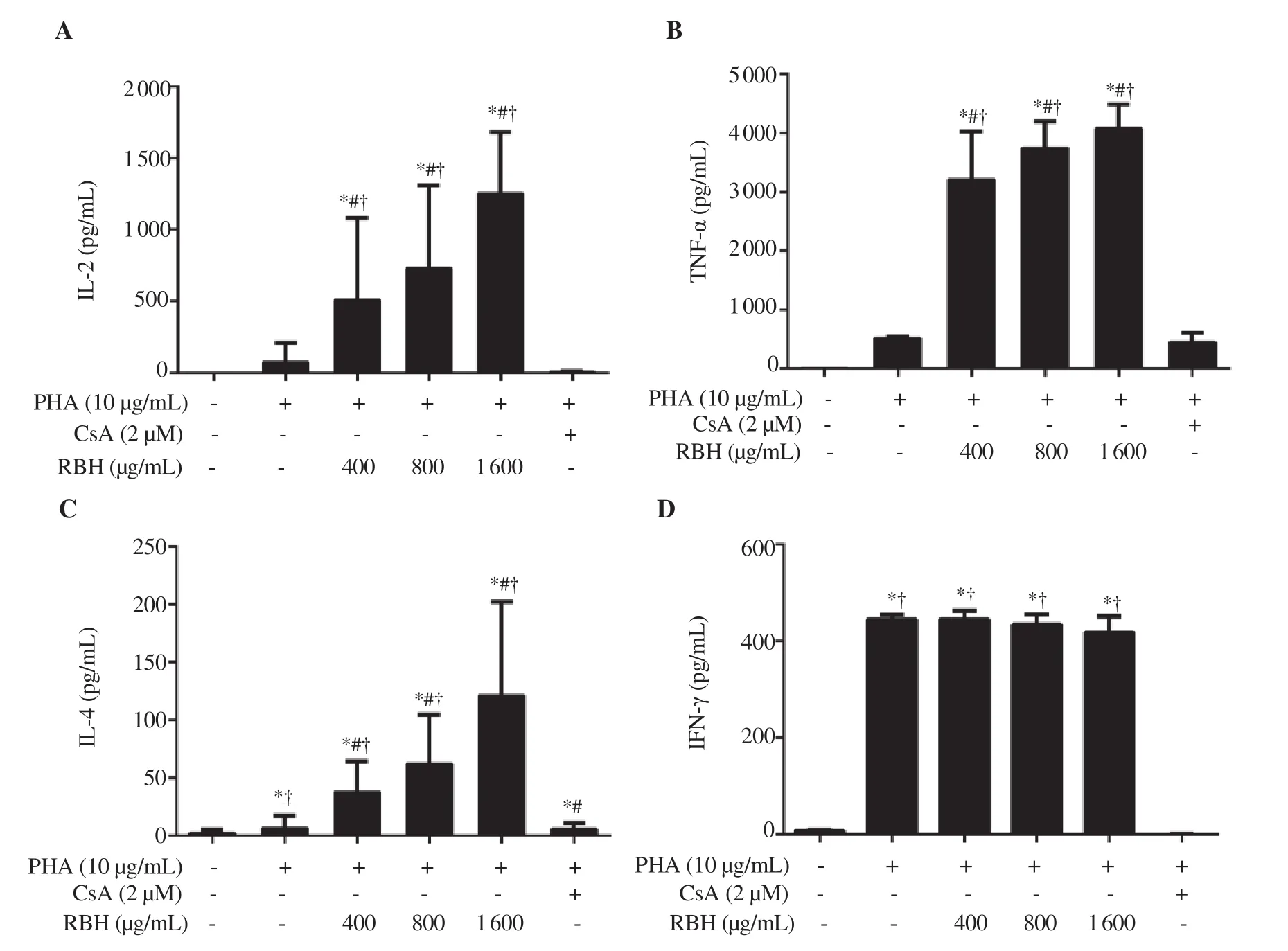
Figure 5. Effect of rice bran hydrolysates on cytokine production of PBMCs. PBMCs were cultured in media containing rice bran hydrolysates (400-1 600 μg/mL) or CsA (2 μM), and with PHA (10 μg/mL) for 72 h. Supernatant of media was assayed for (A) IL-2, (B) TNF-α, (C) IL-4 and (D) IFN-γ. Each bar represents mean ± SD of 4 experiments. *P < 0.05 vs. Control, #P <0.05 vs. PHA, †P < 0.05 vs. CsA.
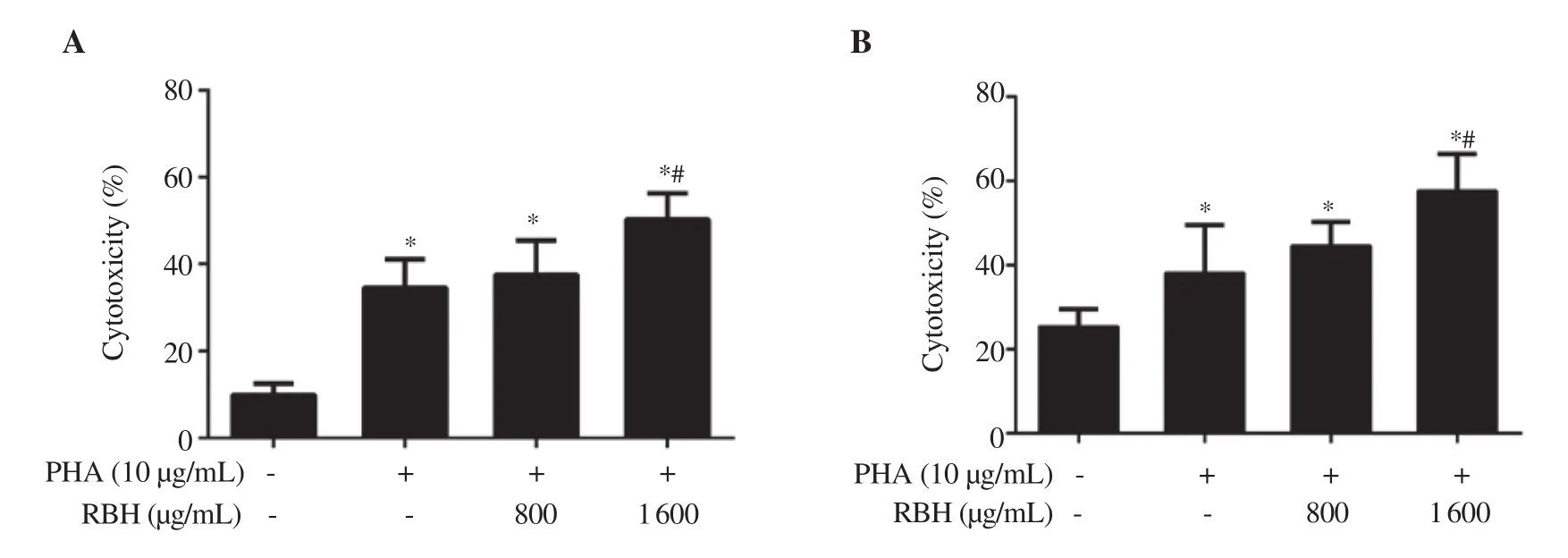
Figure 6. Effect of rice bran hydrolysates on cell-mediated cytotoxicity. PBMCs (Effector cells: E) were treated with PHA and rice bran hydrolysates for 72 h,washed and non-adherent lymphocytes were overlaid on KKU-452 cells (Target cells: T) with T:E ratios of (A) 1:7.5 and (B) 1:15. The cells were co-cultured for 24 h, washed extensively and cell viability of KKU-452 cells was assessed by MTT assay. Each bar represents mean ± SD of 3-4 experiments. *P < 0.05 vs.Control, #P <0.05 vs. PHA.
4. Discussion
Rice bran is rich in polyphenolic and several bioactive compounds.It possesses many pharmacological effects including antiinflammatory and immunomodulatory activities. Hydrolysates were prepared by which rice bran was modified by thermohydrolysis to increase its utilities. Rice bran hydrolysates contain both nutritive components and a significant amount of phenolic and other bioactive compounds. Rice bran hydrolysates showed anti-inflammatory effects by suppression of lymphocyte proliferation, chemotaxis,monocyte adherent, and infiltration. It also has an adjuvant effect on the activation of cytotoxic lymphocytes on non-MHC-restricted tumor killing.
Phenolic content in pigmented rice is always higher than nonpigmented rice and extraction with aqueous solvent yields higher content than less polar solvent[5,19]. Although rice bran hydrolysates had a relatively high content of total phenolic content, antioxidant activity, as assessed by DPPH and FRAP assays, is relatively low.It is uncertain whether the direct antioxidant effect plays any role in our observed effects. Rice bran hydrolysates have a high amount of ferulic acid, catechin and quercetin glycosides. Ferulic acid and caffeic acid have been shown to enhance splenocyte proliferation in response to LPS and lectin, and increased cytotoxic activity of natural killer (NK) cells and T-lymphocytes[11]. Quercetin and its glycosides, the abundant plant flavonoid, have shown to enhance the killing activity of NK cells against K562 cells in vitro stimulation[20].Feruloylated oligosaccharides, the ferulic esters, induce the release of TNF-α, IL-1β and PGE2in unstimulated macrophages. In contrast,the ferulic esters suppressed the release of the proinflammatory mediators in LPS-induced macrophages[9]. It is probable that multiple phenolic compounds act in concert on antiinflammatory and immunomodulatory effects.
Arabinoxylan in our preparation is relatively high. It can be classified into the water extractable and unextractable arabinoxylan.Water extractable arabinoxylan may exhibit most of the biological activities, particularly immunomodulation. Arabinoxylan shows a potential adjuvant activity in the stimulation of NK cells, Th2 immune response and dendritic cells[21]. In our study, the presence of arabinoxylan in cultured media was about 20 μg/mL which is in the range of arabinoxylan concentrations reported to exhibit immunomodulatory effects[9,22].
Immune cell proliferation, chemotaxis, infiltration and immunoadherent are the inflammatory response to noxious stimulus. The mechanism of rice bran hydrolysates was also investigated. The results of our study are consistent with recent studies which have shown that rice bran hydrolysates activate AMPK in HepG2 cells and stimulate insulinsensitizing effect[7]. Furthermore, activation of AMPK is associated with suppression of mTOR leading to antiproliferation of bile duct cancer cells[23]. Since mTOR plays a central role in the regulation of protein synthesis, cell growth, autophagy, cell motility, invasion and cancer metastasis[24], suppression of mTOR expression may be responsible for the observed suppression of cell proliferation, migration,and infiltration of Jurkat and THP-1 cells.
Immune adherence between vascular endothelium and monocytes or cancer cells is a complex process leading to atherosclerosis or metastasis of cancer[25]. NF-κB is activated by various stresses such as LPS, oxidized low density lipoprotein and inflammatory cytokines[26]. Activation of NF-κB, found in the endothelium,monocytes, and stromal cells, induces expression of its regulated genes, i.e. adhesion molecules including VCAMs, ICAMs, integrins,and selectins[26]. Our study showed that suppression of activation of NF-κB and its downstream VCAM-1 by rice bran hydrolysates may be associated with suppression of THP-1 adhesion on HUVEC monolayer. This effect may support an anti-inflammatory effect of rice bran hydrolysates.
Rice bran hydrolysates significantly enhanced PHA-stimulated cytokine release. PHA is a lectin that binds to multiple cell surface glycoproteins on T-cells, causing Ca2+influx, activation of T cells,and stimulation of cell proliferation[27]. Our result agrees with the previous report that PHA induced PBMC in the release of several cytokines[28]. It is noted that rice bran hydrolysates act as a strong adjuvant in enhancing PHA effect on the release of IL-2, IL-4, and TNF-α. The mechanism of rice bran hydrolysates in enhancing PHA-stimulated cytokine release was not investigated in this study,which is probably due to the adjuvant effects of arabinoxylan and quercetin glycosides present in the high amount[9,21]. Although levels of these cytokines, particularly IL-2, remarkably increased,the proliferation of PMBC actually decreased with increasing concentrations of rice bran hydrolysates. The probable explanation is that rice bran hydrolysates also strongly inhibited mTOR, an important downstream effector of the IL-2 receptor, resulting in suppression of T cell proliferation.
The immunomodulatory effect of enhanced cancer cell killing may be of great interest since the evasion of host immunosurveillance is a hallmark of cancer characteristics. Our study found that PHA could induce cell-mediated killing of cancer cells. The non-MHC-restricted tumor cell killing of activated PBMC is enhanced by copretreatment with rice bran hydrolysates. It should be noted that the enhanced cytotoxicity of tumor cells is not caused by the direct effect of rice bran hydrolysates or the released cytokines, as treated PBMC cells were washed before co-incubation with tumor cells.Moreover, as lymphocytes were cultured with tumor cells in the absence of monocytes, it is unlikely that CD8+T cells are induced through this short-term culture. A mixture of T cell and NK cell populations that are stimulated by cytokines are probably the effector cells in the non-MHC-restricted tumor cell killing including CD3+, CD56+, cytokine-induced killer and γδ T cells[29]. The type of cytotoxic cells in activated PBMC was not assessed in the current study. Rice bran hydrolysates could be useful as an adjuvant in cellbased immunotherapy and need further investigation.
In conclusion, rice bran hydrolysates are rich in polyphenolic and other bioactive compounds and show anti-inflammatory effects by suppression of lymphocyte proliferation, chemotaxis, monocyte adherence, and infiltration. Rice bran hydrolysates may have an adjuvant effect on the activation of cytotoxic lymphocytes on non-MHC-restricted tumor killing. The immunomodulatory effect of phytochemicals derived from rice bran may be clinically useful and warrants further investigation.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
We acknowledge Dr. Justin Thomas Reese for editing the manuscript via Publication Clinic KKU.
Authors’ contributions
VK conceived the study, SP, LS, AP, and SK designed and performed the experiments. UK and ST analyzed rice bran. ST and RC provided and prepared study materials. VK, SP, LS, SK, AP, and UK analyzed the data. SP, VK and UK wrote the manuscript.
Funding
This work was supported by Bureau of Rice Research &Development, Thailand, and Grant-in-aid from Faculty of Medicine(IN62133), Khon Kaen University, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2020年10期
Asian Pacific Journal of Tropical Biomedicine2020年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-diabetic properties and bioactive compounds of Teucrium polium L.
- Chemical characterization, docking studies, anti-arthritic activity and acute oral toxicity of Convolvulus arvensis L. leaves
- Mucus from different fish species alleviates carrageenan-induced inflammatory paw edema in rats
- Anti-MMP-2 and MMP-9 activity of Salsola komarovii Iljin extract and its solvent fractions
