Chemical characterization, docking studies, anti-arthritic activity and acute oral toxicity of Convolvulus arvensis L. leaves
Uzma Saleem, Shingraf Zaib, Sana Khalid, Fareeha Anwar, Muhammad Furqan Akhtar, Bashir Ahmad
1Department of Pharmacology, Faculty of Pharmaceutical Sciences, Government College University Faisalabad, Pakistan
2Riphah Institute of Pharmaceutical Sciences, Riphah International University Lahore, Pakistan
ABSTRACT
KEYWORDS: Convolvulus arvensis; Arthritis; Phytoconstituents;Molecular docking
1. Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune and degenerative disorder associated with pain, swelling, and warmness in joints. Joint inflammation, hyperalgesia, and cartilage destruction are the most common causes of the autoimmune RA[1]. The prevalence of the disease is high among females and depends upon age[2]. The actual pathology of RA is unknown, but it is believed to be a T-cell mediated autoimmune condition[3]. Hyperalgesia associated with the RA is regulated through the prostaglandins and other endogenous mediators[4]. Ayurvedic literature has described the herbal treatment of cancer, pain and different chronic inflammatory conditions like gout, arthritis, etc[5]. Anti-arthritic activities of different plant extracts have been studied. Some of the current studies included anti-arthritic effects of Fagopyrum cymosum, Gentiana kurroo, Cuscuta reflexa, Acacia auriculiformis,Berberis orthobotrys, Ephedra gerardiana and Moringa rivae[6,7].
Convolvulus arvensis (C. arvensis) L. is herbaceous evergreen weed and generally found in areas with mild, tropical or Mediterranean atmospheres. It is locally known as ‘Leli’ and belongs to family Convolvulaceae. The plant can develop 5 cm thick carpets-off the ground and the length of the stem can be up to 2 m. It has been used as food and traditional medicine since the eighteen century[8].Various activities of aerial parts of C. arvensis such as laxative,anti-spasmodic, antiangiogenic, wound healing and treatment of jaundice have been reported[9]. Its decoction is used for the treatment of cough and flu[10]. In addition, C. arvensis is used as a diuretic and used to cure skin issues i.e. dandruff, furunculosis,and spider bites[11]. Other reported pharmacological effects include antioxidant, cytotoxic, immune-stimulatory, antibacterial and hepatoprotective activities. C. arvensis has been extensively employed as a folk medicine for the management of rheumatism,skin disorders, microbial infections, diabetes and constipation and diarrhea[12]. The objective of the present study was to evaluate the anti-arthritic activity and acute oral toxicity of C. arvensis along with plant characterization.
2. Materials and methods
2.1. Collection of plant material
The leaves of C. arvensis were collected from Bhawana, District Chiniot, Pakistan, in March 2017. The plant was identified and authenticated from the Botany Department, University of Agriculture, Faisalabad. A voucher number 244-2-18 had been assigned for future reference.
2.2. Preparation of extract
The leaves of C. arvensis were thoroughly rinsed with tap water to remove any adulteration materials, followed by shade drying,pulverization, and extraction with 80% methanol by triple maceration for 14 d. The crude methanolic extract of the plant leaves obtained was stored in separate amber colored jars at 2-8 ℃.
2.3. Physicochemical analysis
The physicochemical properties including moisture contents, total ash, acid insoluble ash, water-insoluble ash, sulphated ash, alcohol soluble extractives, and water-soluble extractives of the powdered leaves were determined according to the standard methods of United States Pharmacopoeia-National Formulary (2003). The powdered material of the plant was examined for total lipids, total proteins, and carbohydrates.
2.4. Phytochemical analysis
The methanolic extract of C. arvensis was evaluated for quantification of primary and secondary metabolites such as total proteins, total polyphenols, total flavonoids, total alkaloids and total glycosaponins[13].
2.5. High performance liquid chromatographic (HPLC)analysis
The HPLC analysis was performed for determining flavonoid and phenolic compounds in the plant extract. The sample was run in an HPLC system (Shimadzu, Japan) equipped with UV-vis detector,LC-10AT pump and controlled by CSW 32 Software. A Shimpack CLC-ODS (C-18) column of dimensions 25 cm × 4.6 mm and detector SPD-10-AV was used for analysis. The mobile phase consisted of two solutions, i.e., Solution A [H2O; acetic acid (AA)-94:6, pH =2.27]and B; acetonitrile, which were eluted by gradient elution. The mobile phase was flown at a rate of 1 mL/min. The spectrum was observed at 280 nm using a UV-vis detector[14].
2.6. Fourier transformed infrared (FTIR) spectroscopic analysis
The methanolic extract of C. arvensis was subjected to FTIR spectroscopic analysis by potassium bromide (KBr) discs. For this purpose, 1 mg of sample and 100 mg of KBr were mixed together and shifted to a dye to produce the disc. The dye was pressed in a hydraulic press to attain FTIR spectra in IR range 4 000-400 cm-1. The spectra attained were analyzed for the identification of functional groups in the plant sample[14].
2.7. Gas chromatography-mass spectroscopy (GC-MS)analysis
The GC-MS analysis of the methanolic extract of C. arvensis was performed by using a Perkin Elmer-Clarus-600 instrument. The working of GC was carried out at 70 eV. Helium was used as a carrier gas with a stream rate of 0.7 mL/min. The temperature of the column was set 60 ℃ for 5 min, increased to 290 ℃ at 3 ℃/min and finally hold for 5 min at 290 ℃. Temperatures of the injector and indicators were adjusted to 250 ℃ and 290 ℃, respectively.The injection volume was 1 μL and split proportion was 1:53.A chromatogram showing different peaks was obtained and the names, molecular weight, molecular formulas and structures of the compounds were acquired by comparing with the National Institute of Standards and Technology library[15].
2.8. Molecular docking studies
2.8.1. Structure retrieval
The molecular docking study was performed to assess the residue binding to selected proteins, COX-1 and COX-2. The structure was determined in the Protein Data Bank (PDB) database for the docking study. The X-ray crystal structure of COX-2 in Mus musculus (entry 5 COX in the PDB) was used. The protein structure was prepared prior to being used in the docking study. The preparation of protein structure included protonation and force field-based parameterization of atoms followed by energy minimization prior to the docking procedure. For protonation, the protonated 3D algorithm was implemented in MOE (Web link 2) and was selected to protonate the protein structures while the AMBER99 force field was applied for energy minimization[16]. It gave rise to the removal of the water molecule and co-crystallized bound ligand structure. As for COX-1,the crystal structure of Mus musculus was not available in PDB, so it was predicted using a structure prediction tool. The protein sequence was retrieved from Uniprot and was pasted in the Phyre2 program for its 3D crystal structure prediction. The 3D structure was modeled with 100% confidence. It was downloaded and prepared in the same way.
2.8.2. Ligand library preparation
It is imperative to prepare the ligand library prior to performing a docking study. The 2D conformation of ligands was retrieved from the PubChem database followed by the preparation of ligands which includes protonation and energy minimization by selecting the MMFF94x force-field.
2.8.3. Molecular docking
The potential binding sites of COX-1 and COX-2 proteins were selected through the MOE site finder tool. MOE Dock tool was used to dock ligands within the defined docking sites of COX-1 and COX-2 proteins. Triangular matcher algorithm was selected as a default ligand placement method to find 1 000 best poses of docked molecules[17]. Rescoring of poses was done via London dG scoring function and the top ten ranked best poses per ligand generated by London dG were further minimized by force field refinement algorithm. All the ligands were ranked based on score, binding affinity, and Root-Mean-Square Deviation (RMSD) values.
2.8.4. Analysis of ligand receptor interaction
In order to have a clear view of receptor ligand interaction of the best-docked complexes, the LigX tool of MOE was used for the generation of 2D plots of receptor ligand interactions. The 2D graph depicts electrostatic interactions, hydrogen bonding, Van der Waals forces and hydrophobic interactions within the active site of the selected protein.
2.9. Animals
Animals for the study were purchased from the Veterinary Research Institute, Lahore, Pakistan. Albino rats of both sexes weighing >150 g and female mice weighing > 25 g were selected for the present study.Animals were kept in polypropylene cages (5 animals per cage), under standard laboratory conditions [temperature (23 ± 2) ℃, humidity 60%,12 h light, and dark cycle]. All the animals were acclimatized for two weeks before experimentation with free access to food and water.
2.10. Ethical approval for animal studies
The experimental study was performed after getting approval from the Institutional Review Board (Reference No. GCUF/ERC/1981)ruled under the regulation of the Institute of Laboratory Animal Resources, Commission on Life Sciences University, National Research Council (1996).
2.11. Acute oral toxicity study
Ten healthy adult female rats (150-200 g) were used for acute oral toxicity testing as per the Organization for Economic Co-operation and Development guidelines 2001 (AOT-425). By using five rats in each group (one control and one treatment group), a limit test (2 000 mg/kg)was performed. After dosing, the animals were noticed up to 14 d for any marked changes. On 15th day, blood samples were collected by cardic puncture under anesthesia for hematological and biochemical (liver function tests, renal function tests, lipid profile) analyses. Then,all the rats were killed by painless method and vital organs (liver,heart and kidney) were removed and preserved in 10% formalin for histopathological analysis[18].
2.12. In vitro anti-arthritis studies
2.12.1. Effect on bovine serum albumin (BSA)
An aqueous solution of BSA (5% W/V) and different solutions of standard drug and the methanolic extract of C. arvensis were prepared at concentrations of 100, 200, 400 and 800 μg/mL as previously described[19]. The test solutions (0.5 mL) contained 0.45 mL of BSA and 0.05 mL of solutions of the methanolic extract of C. arvensis. Control solution (0.5 mL) contained 0.45 mL of BSA and 0.05 mL of distilled water. Product control solution (0.5 mL)contained 0.45 mL of distilled water and 0.05 mL of test solution.Standard solution (0.5 mL) contained 0.45 mL of BSA and 0.05 mL of ibuprofen at different concentrations. All the above samples were adjusted to pH 6.3 with 1 mol/L HCl and subjected to incubation at 37 ℃ for 20 min. After incubation, each mixture was heated up to 57 ℃ for 30 min. Then phosphate buffer (2.5 mL) was added and absorbance was measured at 660 nm. The percentage inhibition of protein denaturation was calculated by using the following formula;Inhibition percentage=(Absorbance test solution-Absorbance product control)/Absorbance test control×100
2.12.2. Effect on egg albumin protein denaturation
A previously reported method was followed with slight modifications[7]. Briefly, test control solution (5 mL) contained 0.2 mL of egg albumin, 2.8 mL of phosphate buffer and 2 mL of the methanolic extract of C. arvensis solutions while standard solution contained ibuprofen at concentrations of 100, 200, 400 and 800 μg/mL. The control solution contained double distilled water. All samples were incubated at (37±2) ℃ followed by heating for 5 min at 70 ℃. Absorbance was determined at 660 nm. The inhibition percentage of egg albumin denaturation was measured by the following formula;
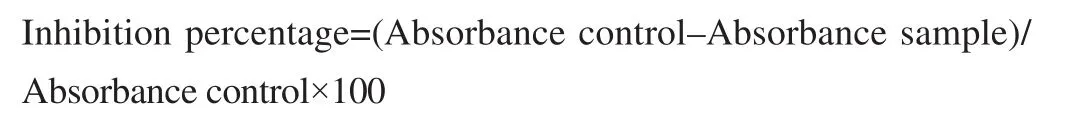
2.13. In vivo anti-arthritis studies
2.13.1. Complete Freund’s adjuvant (CFA) induced arthritis in rats
All the animals (except rats in negative control) were first anesthetized with ether and arthritis was induced by injecting 0.1 mL of CFA into hind paw following a standard protocol[20]. Animals were randomly divided into six groups (n=5) and retained in separate cages. The dose of the methanolic extract of C. arvensis was prepared in 2% w/v Tween 80. Group 1 (arthritic control) received CFA.Group 2 (negative control) received neither CFA nor any treatment.Group 3 received CFA and ibuprofen at a dose of 80 mg/kg. Groups 4, 5 and 6 received CFA and the methanolic extract of C. arvensis at doses of 100, 200 and 400 mg/kg/day, respectively.
All the treatments were administered orally 30 min before CFA administration and then the treatments were continued for 19 d. Paw edema was measured on alternate days by a digital vernier caliper and body weight of each group was recorded using a digital balance.On day 20, each animal was anesthetized, and blood was collected by cardiac puncture. The blood was then transferred into an EDTA and non-EDTA tubes. The quantitative rheumatoid factor (RF) was also calculated. For histopathological analysis, hind paws were amputated and preserved in 10% formalin. Decalcification was done and tissue segments were stained with hematoxylin and eosin[21].Then microscopic evaluation was carried out using binocular microscope. Cartilage destruction, granulation tissue, acute and chronic inflammation were evaluated. The severity was assessed using the scoring system i.e. 0-1: occasional cells were seen; 1, 2 and 3 showed severity.
2.13.2. Carrageenan induced inflammation model
Acute inflammation was induced by sub-plantar administration of 0.1 mL of 1% w/v carrageenan in normal saline into the hind paw.Thirty mice were allocated into 6 groups (n=5). The experimental dose was prepared in 2% Tween 80. Group 1 (carrageenan control)received carrageenan injection. Group 2 (negative control) received neither carrageenan injection nor treatment. Group 3 received carrageenan and ibuprofen at a dose of 80 mg/kg. Group 4, 5 and 6 received carrageenan and the methanolic extract of C. arvensis at doses of 100, 200 and 400 mg/kg, respectively. All treatments were given orally 1 h before carrageenan injection. Paw edema was measured hourly for 4 h by a digital Vernier caliper[22].
2.14. Antioxidant activity
2.14.1. Ferric reducing power
The reductive activity of the methanolic extract of C. arvensis was determined by direct electron donation in the reduction of potassium ferricyanide. The reaction mixture included 1 mL of the extract,2.5 mL of phosphate buffer (0.2 M with pH 6.6) and 2.5 mL of potassium ferricyanide (1% w/v). The solution without plant extract was used as the control. The mixture was subjected to incubation at 50 ℃ for 20 min. By the addition of 2.5 mL 1% w/v trichloroacetic acid in each solution, the reaction was stopped. Afterward,centrifugation was done at 3 000 rpm for 10 min. The 2.5 mL of the supernatant upper layer was mixed with 2.5 mL of distilled water and 0.5 mL of ferric chloride (0.1% w/v) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture showed the larger reductive potential of the sample[23].
2.14.2. DPPH scavenging activity
The DPPH scavenging capacity was evaluated using previously reported method[7]. One mL of 0.004% DPPH in methanol solution(freshly prepared) was added to 3 mL of plant extracts at different concentrations and mixture solutions were kept for 30 min in dark.Then absorbance was observed at 517 nm. A low absorbance of the reaction mixture showed high radical scavenging activity. The antioxidant activity of ascorbic acid was also determined. The solution without the extract of the plant was used as the control. All the experiments were done in triplicate.

2.14.3. Hydrogen peroxide scavenging capacity
Hydrogen peroxide scavenging capacity was measured using previously reported method[7]. A solution of hydrogen peroxide(40 mM) was made in phosphate buffer (pH 7.4). Extracts (100 μg/mL) in distilled water were added to a hydrogen peroxide solution(0.6 mL, 40 mM). The absorbance of hydrogen peroxide at 230 nm was calculated 10 min later against a blank solution containing the phosphate buffer without hydrogen peroxide.
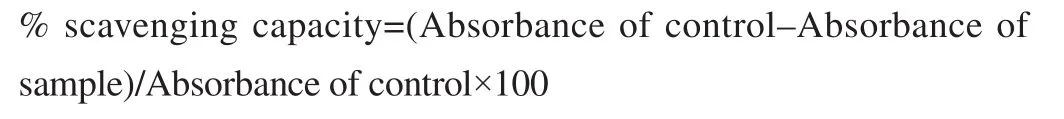
2.15. Statistical analysis
The results were expressed as mean ± SEM and statistical analysis was conducted by two-way ANOVA for in vivo and one-way ANOVA for in vitro studies followed by Bonferroni post hoc test utilizing Graph Pad Prism 5.0 and P < 0.05 was considered significant.
3. Results
3.1. Physicochemical and phytochemical analyses of C.arvensis
Physicochemical investigation showed that leaves of C. arvensis had different moisture and ash contents (total ash, water-insoluble ash, acid insoluble ash, sulphated ash). The water-soluble extractives were found higher in contrast to alcohol soluble extractives. A significant amount of lipid, protein and carbohydrate contents was quantified in leaves (Table 1). Total protein, glycosaponins,polyphenolic, flavonoid and alkaloid contents (64%, 20%, 52.4%,6.5%, and 7.8%, respectively) were determined in the plant extract.
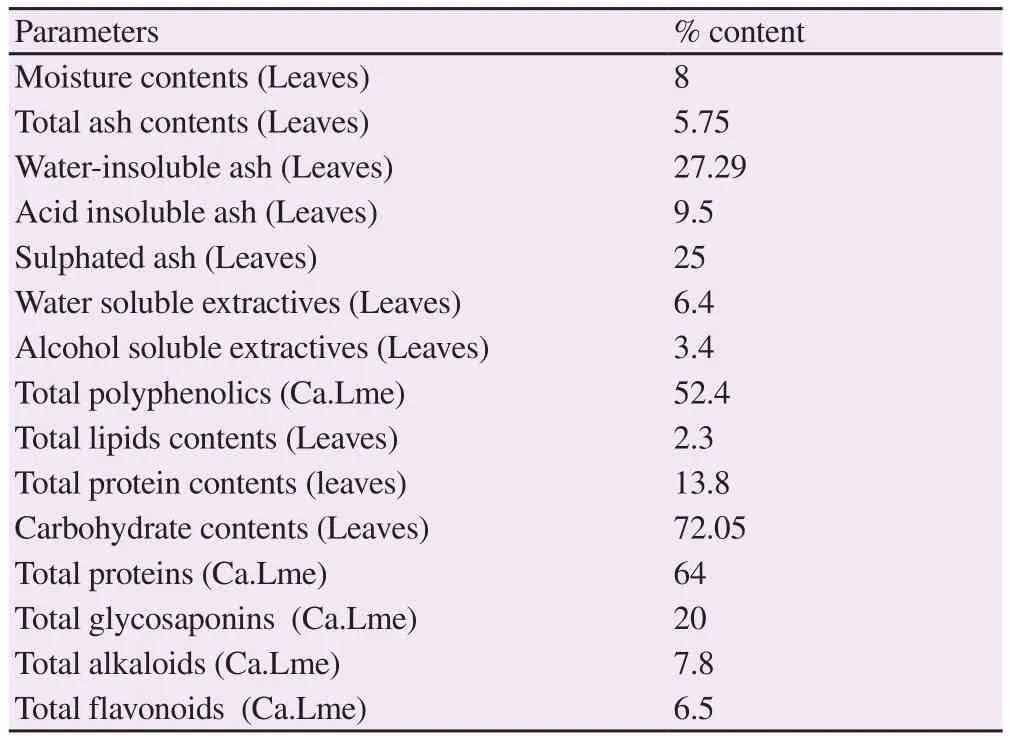
Table 1. Physicochemical and phytochemical analysis of powdered material and extract of Convolvulus arvensis leaves.
3.2. HPLC analysis
The HPLC analysis was performed for the determination of compounds present in the methanolic extract of C. arvensis. The peaks of all constituents were compared with those of the standards(Supplementary Figure 1A). Table 2 shows the compounds identified along with their retention time, quantity and area. The HPLC analysis identified quercetin, gallic acid, caffeic acid, vanillic acid,syringic acid, and sinapic acid. Vanillic acid and sinapic acid were found in higher amounts as compared to other compounds.
3.3. FTIR spectroscopic analysis
The FTIR spectroscopic analysis was performed on the plant extract. The FTIR spectrum of the plant extract showed the presence of alcoholic (3 200 cm-1), methyl (2 900 cm-1), methylene (2 650 cm-1and 1 815 cm-1), carboxylic acid (1 700 cm-1) and cyanide (1 050 cm-1) functional groups in the extract (Supplementary Figure 1B).
3.4. GC-MS analysis
The chromatogram of the methanolic extract of C. arvensis showed eight peaks (Supplementary Figure 1C). The library of compounds was used for comparing the fragmentation of chemicals. The name of compounds, retention time, molecular formula and molecular weight and peak area percentages were summed up in Table 2.
3.5. Molecular docking
Table 3 shows the residues involved in the interaction of COX-1 with the chemical constituents identified in the methanolic extract of C. arvensis. The chemical constituents including quercetin, gallic acid, caffeic acid, vanillic acid, syringic acid, and sinapic acid showed -13.989, -9.120, -10.218, -9.232 , -14.579 and -10.798 interaction scores, respectively, while ibuprofen showed -10.112 score. The residues involved in the interaction of COX-2 with the chemical constituents identified in the methanolic extract of C.arvensis are also demonstrated in Table 3. The chemical constituents including quercetin, gallic acid, caffeic acid, vanillic acid, syringic acid, and sinapic acid showed -12.371, -11.715, -10.172, -8.692,-13.907, and -10.886 interaction scores, respectively, while ibuprofen showed -9.190 interaction score. The highest score of interaction with COX-1 and COX-2 was found in syringic acid when compared with standard ibuprofen. The interactions between ligand,and COX-1 and COX-2 enzymes are shown in Supplementary Figures 2 and 3.
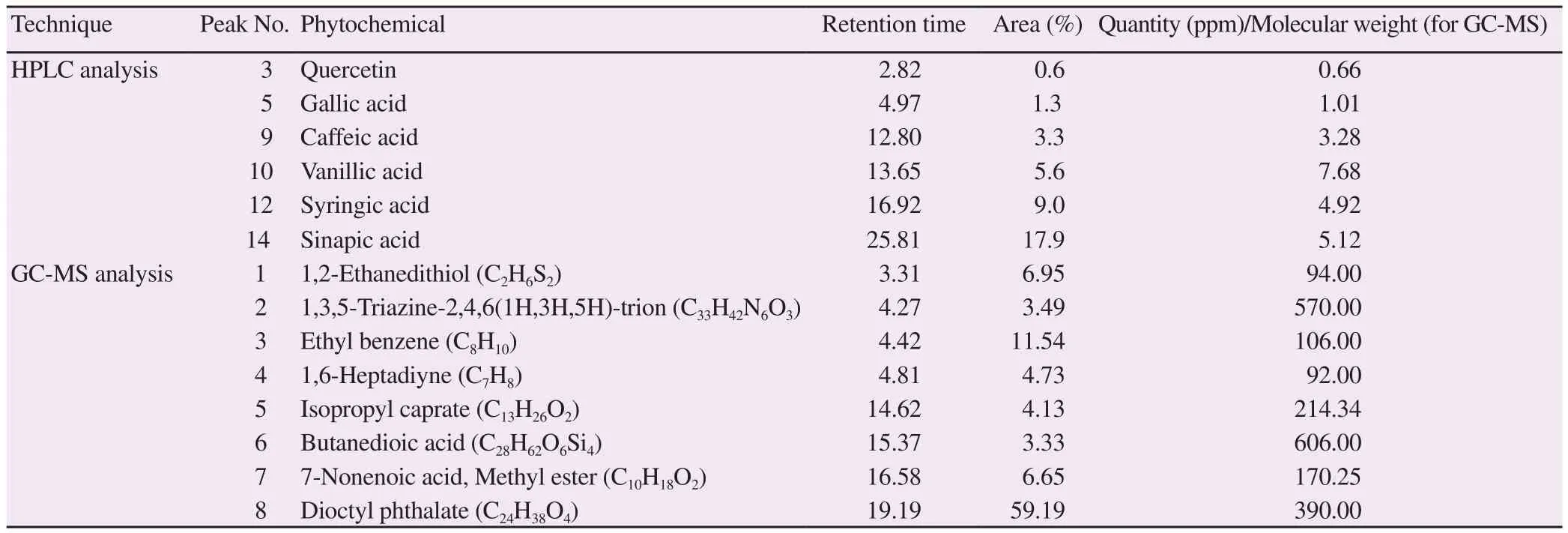
Table 2. Phytochemicals detected in methanolic extract of Convolvulus arvensis by HPLC and GC-MS analysis.
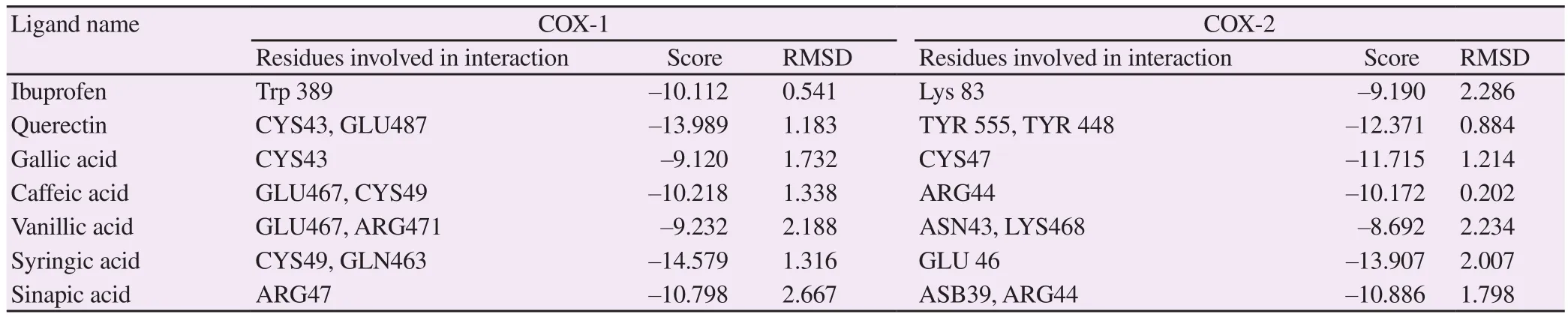
Table 3. Molecular docking of ligand with COX-1 and COX-2.
3.6. Acute oral toxicity study
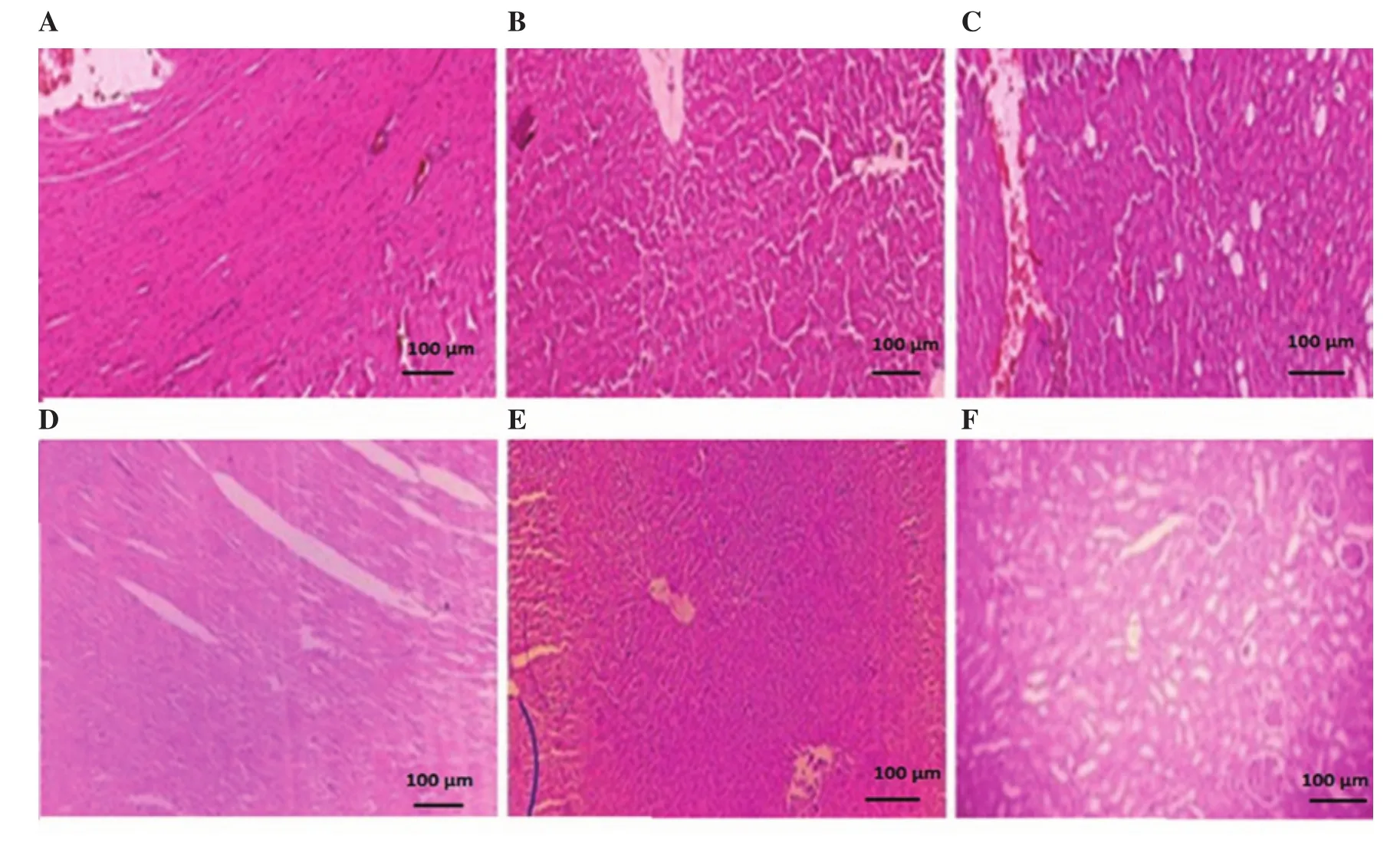
Figure 1. Morphological representation of heart, liver and kidney sections of control (A, B, C) and Convolvulus arvensis extract treated (D, E, F) animals,respectively. All the images were captured at 10×.
Administration of the methanolic extract of C. arvensis at 2 000 mg/kg did not cause any abnormalities and mortalities. The experimental animals were observed with special consideration throughout the time period (14 d). It was revealed that the bodyweight of rats treated with the vehicle [(193.20 ± 1.15) g]and 2 000 mg/kg methanolic extract of C. arvensis [(194.80 ± 0.58) g]did not vary significantly as compared to their corresponding body weights [(196.80 ± 1.06)g and (200.20 ± 1.03) g, respectively]after 14 d. The results showed that the acute administration of the methanolic extract of C. arvensis had no significant impact on the bodyweight of treated rats.
Acute toxicity study also showed that the administration of 2 000 mg/kg methanolic extract of C. arvensis had an insignificant effect on the weight of vital organs. The weights of heart, kidney, and liver in the control group [(0.65 ± 0.02) g, (1.71 ± 0.02) g and (6.36 ± 0.01)g, respectively]were similar to those of the group treated with the methanolic extract of C. arvensis [(0.66 ± 0.01) g, (1.75 ± 0.01) g and(6.58 ± 0.11) g, respectively].
The hematological profile of rats showed no significant variation in hematocrit and mean corpuscular hemoglobin with significant alteration in neutrophils, eosinophils, and lymphocytes. Levels of hemoglobin, total leucocyte count, red blood cells, mean corpuscular volume, mean corpuscular hemoglobin concentration and platelets of treated rats varied more significantly (P<0.001) as compared with the control group (Supplementary Table 1).
There were also significant (P<0.001) variations in biochemical indicators of liver function test. Levels of alanine aminotransferase,aspartate aminotransferase, and alkaline phosphatase were significantly decreased in the treatment group as compared to the vehicle control group. No significant changes were seen in total bilirubin. Blood urea and the serum creatinine levels were higher in the vehicle control group as compared with the plant extract. It was observed that there was a significant alteration in cholesterol,triglycerides and high-density lipoprotein cholesterol levels in comparison to the control group. However, rats showed no marked changes in low and very low-density cholesterol as compared to the control group (Supplementary Table 1). Histopathological analysis of heart, liver and kidney sections of extract-treated group exhibited no marked difference as compared with the vehicle control group(Figure 1).
3.7. Effect of the methanolic extract of C. arvensis on BSA protein and egg albumin denaturation
The present study showed BSA protein denaturation inhibition in a concentration-dependent manner. At higher concentrations,more pronounced effects were noticed. The methanolic extract of C. arvensis showed 93.11% inhibition while standard ibuprofen exhibited 90.81% inhibition at 800 μg/mL (Table 4).
The present study also showed egg albumin denaturation inhibition in a concentration-dependent manner. At higher concentrations, more noticeable effects were seen. Ibuprofen and the methanolic extract of C. arvensis both showed 88.88% inhibition at 800 μg/mL (Table 4).
3.8. Activity against CFA-induced arthritis
There was a significant (P<0.001) decrease in paw diameter in extracttreated rats as compared with the arthritic group. The methanolic extract of C. arvensis at doses of 100 mg/kg, 200 mg/kg and 400 mg/kg and ibuprofen at 80 mg/kg showed pronounced inhibition of edematous paw i.e. 70.74%, 72.67%, 78.04% and 75.90%, respectively on 19th day (Table 5). The arthritic group showed marked alteration in hematological parameters while the plant extract and ibuprofen significantly improved (P<0.001) these alterations (Supplementary Table 2). The RF, erythrocyte sedimentation rate and white blood cells were decreased as compared to the CFA control group. A significant weight gain (P<0.001) was found in groups treated with extract and ibuprofen as compared to arthritic group (Supplementary Table 3). Figure 2 exhibited histopathological examination of the ankle joint and showed that ankle joint of normal control presented intact synovium, no inflammation of the synovial lining and no cells of inflammation. The scoring of histopathology revealed that there was more protection in extract-treated (400 mg/kg) rats as compared to rats of the arthritic group by decreasing acute and chronic inflammation along with the decreased cartilage destruction(Supplementary Table 4). Figure 2 also showed a morphological representation of paw after the sub-plantar administration of CFA.
3.9. Effect on carrageenan-induced paw edema
The methanolic extract of C. arvensis showed a significant decrease in paw inflammation. The plant extract at 100 mg/kg, 200 mg/kg,400 mg/kg and standard drug at 80 mg/kg showed 48.10%, 55.44%,61.26% and 49.87% inhibition, respectively as shown in Table 6.
3.10. Antioxidant activity
The antioxidant activity was measured by performing DPPH,Ferric reducing power and hydrogen peroxide scavenging assays,and the plant extract showed varying DPPH and H2O2scavenging and reducing power capabilities when compared with the standardascorbic acid. It was found that ascorbic acid showed significantly higher % inhibition of DPPH (78.70%), reducing power (0.923) and H2O2scavenging activity (59.34%) in comparison to the those of the methanolic extract of C. arvensis (49.35%, 0.783 and 6.75%).
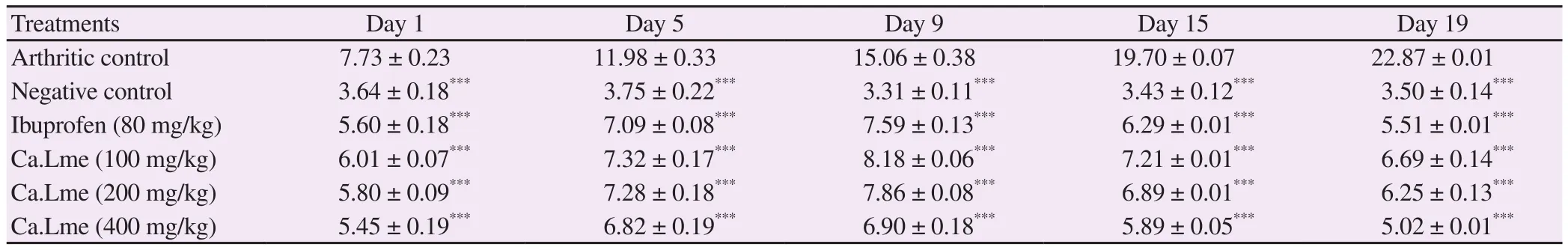
Table 5. Effect of Convolvulus arvensis extract on paw diameter in CFA induced arthritis rats.
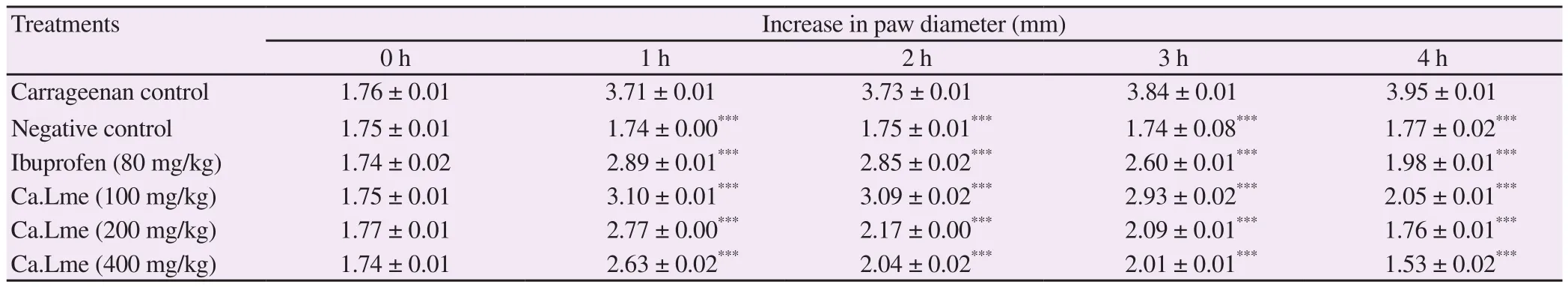
Table 6. Effect of Convolvulus arvensis on carrageenan induced paw edema in mice.
4. Discussion
RA that is associated with symptoms of swelling of joints, the release of RF (autoantibody) and distortion of bone is a more continuous ailment that presents with major systemic complexities with a high death rate in patients compared with healthy individuals[24]. Herbal plants are being utilized for hundreds of years to treat different sicknesses. C. arvensis was reported to decrease inflammation and pain of joints[10]. Preliminary studies are important for the efficient utilization of herbal medicines. The preliminary physicochemical and phytochemical analysis was performed for the standardization of the herbal plant. HPLC was also performed that indicated the presence of quercetin, gallic acid, caffeic acid, vanillic acid, syringic acid, and sinapic acid. It has previously been stated that flavonoid and phenolic contents possess anti-inflammatory properties[25]. Previous studies suggested the anti-inflammatory potential of identified quercetin, gallic acid, caffeic acid, vanillic acid, syringic acid and sinapic acid[26-30]. The FTIR and GC-MS analyses have a fundamental role to ascertain functional groups and bioactive compounds. In the present study, the results of FTIR suggested the presence of different functional groups and GC-MS analysis further showed eight peaks of compounds[31,32]. The acute oral toxicity study is essential to decide the harmless dose range to accomplish the clinical signs and symptoms of drug toxicity[33]. In the present study, no mortality was observed, so it can be considered that the lethal dose could be above 2 000 mg/kg.
In the present study, the anti-arthritic activity of the methanolic extract of C. arvensis was evaluated by in vitro and in vivo methods.In vitro methods included protein denaturation using BSA and egg albumin. In vivo methods included two models i.e. carrageenaninduced paw edema and CFA model. The main purpose of the present study was to determine the effects of the methanolic extract of C. arvensis against arthritic edema and parameters associated with arthritis. Denaturation of proteins is a well-known reason for inflammation[34]. The secondary and tertiary structure of proteins in the cell membranes is lost by extrinsic stress, heat, organic solvents or strong acid and bases[35]. Mechanism of denaturation is associated with changes of electrostatic power, hydrogen, hydrophobic and disulphide bonds. Protein denaturation implies loses of natural properties of protein particles. Subsequently, counteractive action of protein denaturation may likewise help in progress of inflammation[36]. Some literature proposed that the denaturation of proteins was one of the reasons for inflammation of the rheumatoid joint that was linked to the generation of autoantigens in certain rheumatic illnesses[37]. In the present study, it was noticed that the extract and ibuprofen showed significant inhibition of protein denaturation. Thus, the methanolic extract of C. arvensis might be useful in preventing the inflammation associated with protein denaturation.
Different models of animals are utilized as a part of the preclinical research in arthritis[37]. The plant extract was examined against the carrageenan model. Carrageenan prompted paw edema has been extensively utilized to screen the anti-inflammatory drugs and plant extracts. Edema produced via carrageenan is found to be biphasic.The primary stage (60 min) includes the arrival of serotonin and histamine and the second stage (> 60 min) is intervened by cyclooxygenase substances. Congruity between the two stages is given by kinin. The methanolic extract of C. arvensis fundamentally restrained the edema development in both the primary and second stages. In the present study, the anti-edematous action of the plant persevered in the second stage with the maximal impact found at 4 h.As noted, there was pronounced percentage inhibition of paw edema at the fourth hour. The percentage inhibitions of inflammation by the extract and standard medication ibuprofen were 61.26% and 49.87%,respectively. Therefore, it can be concluded that the inhibitory impact of methanolic extract of C. arvensis in carrageenan-incited inflammation might be due to inhibition of COX that hindered the prostaglandin production.
The anti-arthritic action of the plant extract was further confirmed in CFA induced arthritis. The CFA was comprised of inactivated and dried Mycobacterium that induced autoimmunity and resulted in the generation of immunoglobulin. The CFA incited joint pain articulated with swelling in the hind paw (primary stage of arthritis) which continued for a week and where the production of prostaglandin happened. Following a couple of days, inflammation in the contralateral and in front paw was noticed (secondary stage of arthritis) along with the presence of arthritic nodules in the ear and tail[38,39].
The methanolic extract of C. arvensis displayed significant antiarthritic through amelioration of altered biomarkers in polyarthritis.A pronounced improvement in hematological and histopathological parameters showed the reduction in polyarthritis associated swelling by the methanolic extract of C. arvensis. The results were comparable to the standard anti-inflammatory drug. These findings propose the potential of the methanolic extract of C. arvensis for the treatment of clinical diseases like RA.
Molecular docking showed the interaction between ligands and two isoforms of COX i.e. COX-1 and COX-2. The plant constituents showed better interaction with the two COX isoforms as compared with ibuprofen. Hence, it can be concluded that these binding sites of the ligand with COX-1 and COX-2 residues could serve as future target sites for the action of drugs. The anti-arthritic potential of the methanolic extract of C. arvensis could be ascribed to the presence of steroids, tannins, flavonoids, alkaloids, glycosides, saponins and terpenoids found after fundamental phytochemical analysis.Nonspecific anti-arthritic action was perhaps due to the consolidated impact of the different phytoconstituents present in the methanolic extract of C. arvensis.
In conclusion, the study indicated that the methanolic extract of C.arvensis (100 mg/kg, 200 mg/kg and 400 mg/kg) shows protective effect against arthritis, which offered a pharmacological justification to the customary utilization of the plant against rheumatoid joint and inflammation.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
US designed the work and drafted the article. SZ and SK did data collection, FA performed data analysis and interpretation, MFA and BA did critical revision of article.
 Asian Pacific Journal of Tropical Biomedicine2020年10期
Asian Pacific Journal of Tropical Biomedicine2020年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-diabetic properties and bioactive compounds of Teucrium polium L.
- Mucus from different fish species alleviates carrageenan-induced inflammatory paw edema in rats
- Anti-MMP-2 and MMP-9 activity of Salsola komarovii Iljin extract and its solvent fractions
- Rice bran hydrolysates induce immunomodulatory effects by suppression of chemotaxis, and modulation of cytokine release and cell-mediated cytotoxicity
