Mucus from different fish species alleviates carrageenan-induced inflammatory paw edema in rats
Mustafa Hitit, Orhan Corum, Mehmet Ozbek, Kamil Uney, Ertugrul Terzi, Gokhan Arslan, AY Sonmez
1Department of Genetics, Faculty of Veterinary Medicine, Kastamonu University, Kastamonu, 37200, Turkey
2Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Kastamonu University, Kastamonu, 37200, Turkey
3Department of Histology and Embryology, Faculty of Veterinary Medicine, Mehmet Akif Ersoy University, Burdur, 15030, Turkey
4Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Selcuk University, Konya, 42031, Turkey
5Faculty of Fisheries, Kastamonu University, Kastamonu, 37200, Turkey
6Faculty of Fisheries, Ataturk University, Erzurum, 25240, Turkey
ABSTRACT
KEYWORDS: Fish mucus; Inflammation; Carrageenan; Rat paw edema
1. Introduction
Inflammation is the body’s protective response to various harmful agents (bacterial infection, trauma, chemicals, etc.). However,prolonged and excessive inflammation induces tissue damage and leads to the progression of many diseases such as cancer, stroke,cardiovascular diseases, asthma, inflammatory bowel disease,arthritis, and neurodegenerative diseases[1,2]. Although steroids and nonsteroidal anti-inflammatory drugs are used in the management of inflammation, their side effects on the gastrointestinal, renal,cardiovascular and endocrine systems, metabolism, and platelets limit their use[3,4]. Therefore, natural anti-inflammatory constituents are needed to increase the pharmacologic response and reduce the side effects[5]. One of the most common methods used to evaluate the acute anti-inflammatory effects of various constituents is carrageenan-induced paw inflammation[6].
Cytokines and free radicals play important roles in the inflammation process. Cytokines are low-molecular-weight messenger proteins that aid in the communication between cells. Of the proinflammatory cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-1β,and IL-6 are primary cytokines that mediate inflammation[7,8].Cyclooxygenase (COX)-2 enzyme is induced by cytokines,lipopolysaccharides, or growth factors in the inflamed region and mediates the release of prostaglandins (PG) that play a role in the inflammation process[9]. Although free radicals and antioxidant defense mechanisms are balanced in a healthy body, the level of free radicals is increased and levels of antioxidant markers, such as catalase (CAT) and superoxidase dismutase (SOD) are decreased during the inflammation process[10].
Mucus produced by fish epidermal cells acts as the physiological and biochemical barrier. Fish mucus is of great physiological and ecological importance owing to its functions in respiration, osmotic regulation, reproduction, excretion, communication, and protection against diseases and pathogens[11,12]. Fish mucus exerts various pharmacological effects such as antimicrobial, antifungal, antiinflammatory and antinociceptive effects in addition to facilitating the wound healing process[13-16]. The contents of fish mucus depend on internal and external factors, such as fish species and sex,development stage, stress, hyperosmolarity, pH, and infection[17].In general, mucus consists of 95% water and glycoproteins. It also contains numerous other constituents such as fatty acids, amino acids, antimicrobial peptides, immunoglobulins, and lectins[17-19].
Although the analgesic effect of mucus obtained from different fish species on acute pain induced by a scalpel incision on the paw was investigated in rats[13], no information was available on the effects of fish mucus on acute inflammation. This study aimed to evaluate the anti-inflammatory effect of mucus obtained from brook trout,rainbow trout, European sea bass, and gilthead sea bream through edema formation, determination of gene expression (TNF-α, IL-1β,IL-6, IL-10, TGF-β, COX-2, CAT, and SOD), and histopathological evaluations in rats with carrageenan-induced acute paw edema.
2. Materials and methods
2.1. Animals
The forty-two male Wistar albino rats, aged 8-12 weeks and weighing (220 ± 15) g, were used in the study. Rats were maintained for a week before the study for acclimation purpose in an environment with 55% humidity and 22 ℃ ambient temperature under a 12/12-hour light/dark cycle. The animals were fed standard chow and water ad libitum.
2.2. Preparation of fish mucus
Mucus samples obtained from 4 different fish species, i.e., brook trout[Salvelinus fontinalis (S. fontinalis)], rainbow trout [Oncorhynchus mykiss (O. mykiss)], European sea bass [Dicentrarchus labrax (D.labrax)], and gilthead sea bream [Sparus aurata (S. aurata)], were used in the study. Mucus samples were collected under hypothermic stress as previously described[18]. Briefly, fishes were immersed in distilled water, cleaned, and weighed; one fish was placed in each plastic container. Distilled water at a volume equal to that of fish was added to the containers (v/v fish/distilled water, 1:1), and the containers were kept in a freezer at -20 ℃ for 1 h. Then, mucus samples were collected from the dorsolateral region using a clean plastic spatula by scraping gently. After centrifugation of the mucus samples at 5 000 rpm for 10 min, the supernatant was removed, lyophilized, and stored at 4 ℃ until the experiment.
2.3. Carrageenan-induced paw edema in rats
Rats were randomly assigned to 7 groups (n = 6) as follows:Group 1 (healthy control), Group 2 (carrageenan control), Group 3(carrageenan + diclofenac 25 mg/kg, p.o.), Group 4 (carrageenan + S.fontinalis mucus 25 mg/kg, p.o.), Group 5 (carrageenan + O. mykiss mucus 25 mg/kg, p.o.), Group 6 (carrageenan + D. labrax mucus 25 mg/kg, p.o.), and Group 7 (carrageenan + S. aurata mucus 25 mg/kg,p.o.).
Acute paw edema was induced by administration of 0.1 mL of 1%carrageenan to the right paws of the rats; 0.1 mL physiological saline was administered to the right paws of the rats in the healthy control group. The lyophilized fish mucus and diclofenac were dissolved in sterile water and administered to the rats at a dose of 25 mg/kg by gastric gavage 1 h before the administration of carrageenan.The dose of mucus was selected from the previous study conducted with rats[13]. Right paw of the rats was measured with a micrometer before the administration of carrageenan (0 h) and at 1-4 hours after the administration of carrageenan. Then, the percentage of inhibition of edema was calculated according to the method described previously[20]. At the end of the study, rats were sacrificed by cervical dislocation under xylazine (10 mg/kg, intraperitoneal,Xylazole, Provet) + ketamine (60 mg/kg, intraperitoneal, Ketalar,Pfizer) anesthesia, and the samples were collected from the right paws. Then, the samples were kept at -80 ℃ for molecular analysis and placed in 10% formalin for histopathological analysis.
2.4. RNA extraction and cDNA synthesis
Total RNA isolation was performed as previously described in our study[21]. In brief, 20 mg of hind-paw tissues in 1 mL TRIzol®were homogenized and 200 μL chloroform was added to obtain separation phase by centrifugation at 10 000 rpm for 15 min. Then, we added 500 μL isopropanol for supernatant phase collection to precipitate RNA pellet. The supernatant was discarded and the pellet washed twice in 70% cold ethanol by centrifugation at 4 ℃ at 8 000 rpm for 5 min. The RNA pellet was eluted with 50 μL of DEPC-dH2O and stored at -80 ℃ until use. The concentration and quality of RNAs were detected using NanoDrop (Colibri Microvolume Spectrometer)considering a distinct ratio of A260/A280 with 1.8-2.2 absorbance.Moreover, RNA integrity was verified by detecting an intact 28S:18S band through the running of 1 mg/10 μL RNA samples on 1%agarose gel. The contamination of genomic DNA from RNA samples was performed using DNAse-Ⅰ kit according to the manufacturer’s protocol. Briefly, 1 μg/20 μL of total RNA was exposed to 1 μL DNAse-Ⅰ enzyme incubation at 37 ℃ for 30 min. Then, the reaction was deactivated with 1 μL ETDA by incubation at 65 ℃ for 10 min. One microgram of total RNA was converted to cDNA using a commercial kit (I-Script, BioRAD) through polymerization of RNA with 1 μL oligo dT and 1 μL random hexamer primer mix and final conversion was completed by reverse transcriptase enzyme.
2.5. Gene expression by quantitative polymerase chain reaction (qPCR)
qPCR was performed to evaluate the expression profiles of the genes in the hind-paw tissues by using specific primers (Table 1)[21-25]. qPCR was established as follows: 5 μL iTaq™universal SYBR®Green, 5 pMol each primer, 1 μL cDNA, and dH2O to a final volume of 15 μL[26]. The thermal cycling conditions were set up as indicated: initial denaturation,95 ℃ for 10 min followed by 45 cycles of denaturation, annealing,and amplification at 95 ℃ for 20 s, 58 ℃ for 45 s, and 72 ℃ for 1 min,respectively. The melting curve analysis was performed as indicated:95 ℃ for 1 min, then fluorescence was detected at 1 ℃ increment from 50 ℃ to 95 ℃ by qPCR Rotor-Gene Q (Qiagen, Germany). No cDNA template was included as a negative control in each assay. To normalize the data, at least three reference genes were used, and primer efficiency was performed according to the method of primer design[27]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)was used as a reference gene to normalize qPCR data. qPCR analysis was performed for fold-change in triplicate for each gene.
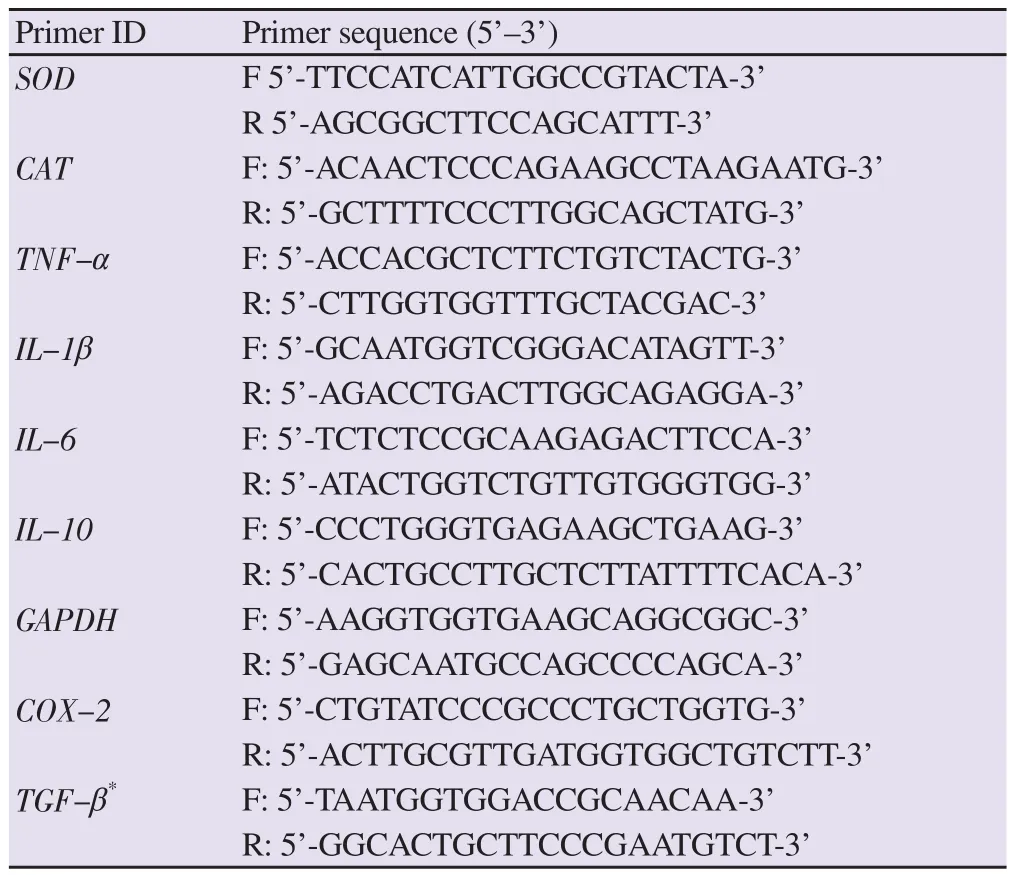
Table 1. The primers used for quantitative polymerase chain reaction[21-25].
2.6. Histopathological analysis
Tissue samples from the control and experimental groups were fixed in 10% buffered formalin for 24 h. Then, the tissues were washed in tap water. After washing, tissues were kept in 70%alcohol overnight and passed through graded alcohols (80%, 96%,and 100%). Then, the samples were cleared in benzene and methyl benzoate solutions and embedded in paraplast. Three serial sections were taken from each paraffin block using a microtome (Leica RM 2155, Germany). Hematoxylin and eosin (H&E) staining was performed on the sections for histopathological examination. The sections were evaluated under a light microscope (BX51, Olympus,Tokyo, Japan) and images were captured from the respective regions with a digital camera integrated with the microscope (DP74,Olympus, Tokyo, Japan). For the specimens, histopathological examination was determined as a 0-5 score grade based on the edema and inflammatory cell infiltrate detected in epidermis, dermis,and hypodermis. The severity of inflammation was determined as follows: 0 = no, 1 = mild, 2 = mild/moderate, 3 = moderate, 4 =moderate/severe, and 5 = severe as previously described[28]. An inflammatory score was evaluated semi-quantitatively.
2.7. Statistical analysis
The quantitative qPCR (Ct) data were calculated as fold changes.The mean Ct values from the healthy control samples were used as the reference points, and the mean Ct values of other groups were used to calculate the fold changes from those reference points based on the 2-ΔΔCtmethod[29]. The normalized data were evaluated using analysis of variance and Tukey’s post hoc test. P < 0.05 was considered as significant difference. Graphs were generated using GraphPad Software, Inc 8.0.
2.8. Ethical statement
All the protocols were approved by the Local Ethics Committee of Animal Research Studies at Kastamonu University (No: 2018/24).
3. Results
3.1. Effect of fish mucus on carrageenan-induced rat paw edema
The effects of fish mucus on carrageenan-induced paw edema in rats are presented in Table 2. All fish mucus treatments inhibited carrageenan-induced paw edema. Administration of mucus of S.aurata, S. fontinalis, O. mykiss, and D. labrax inhibited carrageenaninduced paw edema at ratios of 29.37%, 27.78%, 24.60%, and 1.59% at 1 h and 74.86%, 57.92%, 52.46%, and 74.32% at 4 h,respectively. The degrees of inhibition in groups treated with mucus of O. mykiss, S. fontinalis, and S. aurata at 1 h and groups treated with mucus of D. labrax and S. aurata at 4 h were similar to that in the diclofenac group.
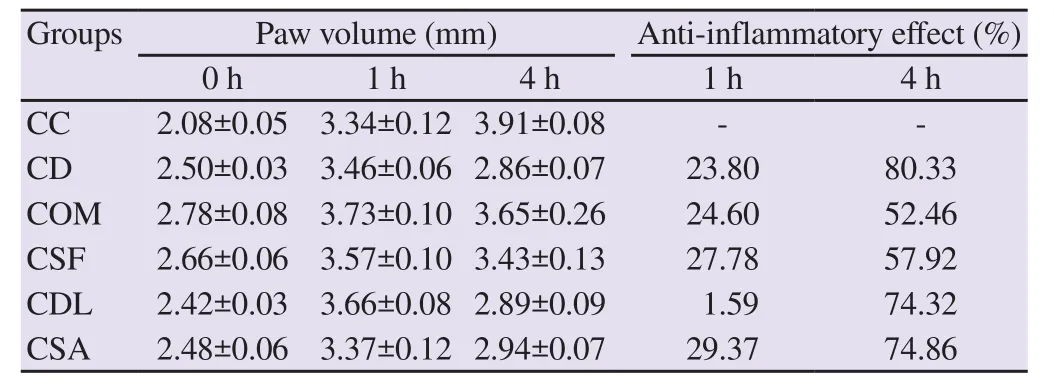
Table 2. Effects of fish mucus and diclofenac on carrageenan-induced paw edema in rats (mean ± SEM).
3.2. Molecular findings
3.2.1. Gene expression of pro-inflammatory and antiinflammatory cytokines
The changes in TNF-α, IL-1β, IL-6, and IL-10 mRNA expression levels are presented in Figure 1. The administration of carrageenan significantly increased IL-1βand IL-6 mRNA expressions in paw tissue compared to healthy control and diclofenac groups (P < 0.05 and P < 0.001, respectively). The administration of O. mykiss and S.aurata mucus reduced the carrageenan-induced increase in IL-1βmRNA expression (P < 0.01). In addition, O. mykiss, S. fontinalis,and S. aurata mucus significantly decreased IL-6 mRNA expression(P < 0.001) at a degree similar to that observed when diclofenac was administered. TNF-αand IL-10 mRNA expression levels were stable in all groups, and there was no significant difference between the groups (P > 0.05).
3.2.2. Gene expression of COX-2 and TGF-βmRNA
The changes in COX-2 and TGF-βmRNA expression levels are summarized in Figure 2. There was no statistically significant difference between the groups in terms of COX-2 and TGF-βmRNA expression levels (P > 0.05). Carrageenan-induced increase in COX-2 mRNA expression, which was not statistically significant,was reduced by administering mucus of O. mykiss, S. fontinalis, S.aurata, and D. labrax. The decreased TGF-βmRNA expression by carrageenan administration was increased in the group treated with fish mucus with no significant difference.
3.2.3. Gene expression of CAT and SOD mRNA
The changes in CAT and SOD mRNA expression levels are presented in Figure 3. CAT mRNA expression was significantly higher in the healthy control and diclofenac groups than that in the carrageenan, S. fontinalis, O. mykiss, and S. aurata groups. There was no difference in CAT mRNA expression between the carrageenan and fish mucus groups (P > 0.05). SOD mRNA expression was higher in the diclofenac group than carrageenan, S. fontinalis and S. aurata treated groups (P < 0.001). Moreover, SOD mRNA expression was higher in the O. mykiss group than that in the carrageenan and S.aurata group (P < 0.05).
3.3. Histopathological findings
Histopathological evaluation of healthy control and experimental groups is summarized in Table 3 semi-quantitatively. In all experimental groups, epidermis showed normal histological structure while inflammatory lesions were detected in dermis and hypodermis.We observed that the degree of edema and leukocyte infiltration was lower in fish mucus groups than in the carrageenan group. Moreover,the diclofenac treated group alleviated edema and leukocyte infiltration more significantly than fish mucus treated groups.Although the majority of leukocyte infiltrations in inflammation were neutrophils, we also observed a few lymphocytes. Interestingly,the degree of leukocyte infiltration was lower in S. aurata mucus treated group compared to other fish mucus groups (Figure 4).
4. Discussion
The study was conducted with the most farmed fish species in Turkey including brook trout, rainbow trout, gilthead sea bream, and European sea bass[30]. The effect of mucus of four fish species on the inflammation was evaluated by assessing the development of edema and performing molecular and histopathological evaluations. It was found that all mucus treatment groups showed varying degrees of anti-inflammatory activities.
The fish mucus could be used for its pharmacological effects considering the fatty acid profiles of fillet extract and mucus extract.In addition, obtaining mucus without slaughtering fish provides economic benefits[18]. Oral administration of Channa striatus(Haruan) extract for 6 months to women who have undergone cesarean section was safe and did not have any side effects on the hematological and biochemical parameters[31].
In this study, all mucus samples decreased the degree of carrageenan-induced paw edema. The degrees of edema inhibition in groups treated with mucus of S. fontinalis, O. mykiss, and S. aurata at 1 h and in groups treated with mucus of D. labrax and S. aurata at 4 h were similar to the degree of edema inhibition in the group thatwas administered diclofenac. The inhibition at 4 h (52.46%-74.86%)was considerably higher than those at 1 h (1.59%-29.37%) after all mucus administrations. It is known that carrageenan-induced inflammation has 2 phases. The early phase (up to 2 hours) involves the release of histamine, serotonin, and bradykinin, and the delayed phase (2-5 hours) involves neutrophil infiltration and prostaglandin formation[6]. The fact that all fish mucus maximally inhibited edema formation at 4 h, similar to the effect of diclofenac, may be attributed to the inhibition of neutrophil infiltration owing to the anti-inflammatory effect. Haruan extracts obtained from different fish species significantly inhibited PGD2-induced paw edema but did not have any effect on bradykinin- and histamine-induced paw edema[32], which is supportive of this result.

Table 3. Histopathological evaluation of experimental groups.
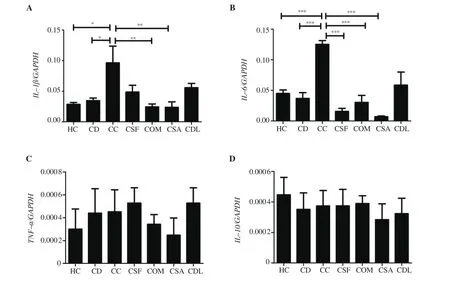
Figure 1. The expressions of (A) IL-1β, (B) IL-6, (C) TNF-α, and (D) IL-10 mRNA. Data was expressed as mean ± standard error of means. *P < 0.05, **P <0.01, ***P < 0.001 represent significant difference.
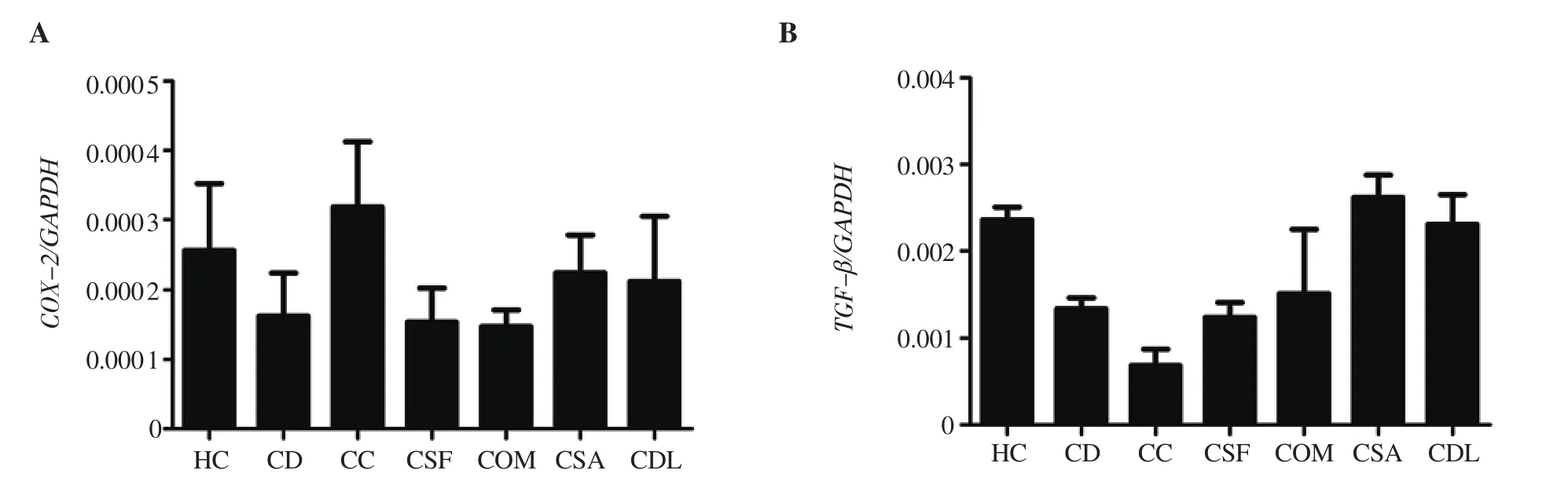
Figure 2. The expressions of (A) COX-2 and (B) TGF-β mRNA. Data was expressed as mean ± standard error of means.
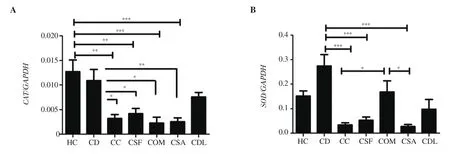
Figure 3. The expressions of (A) CAT and (B) SOD mRNA. Data was expressed as mean ± standard error of means. *P < 0.05, **P < 0.01, ***P < 0.001 represent significant difference.
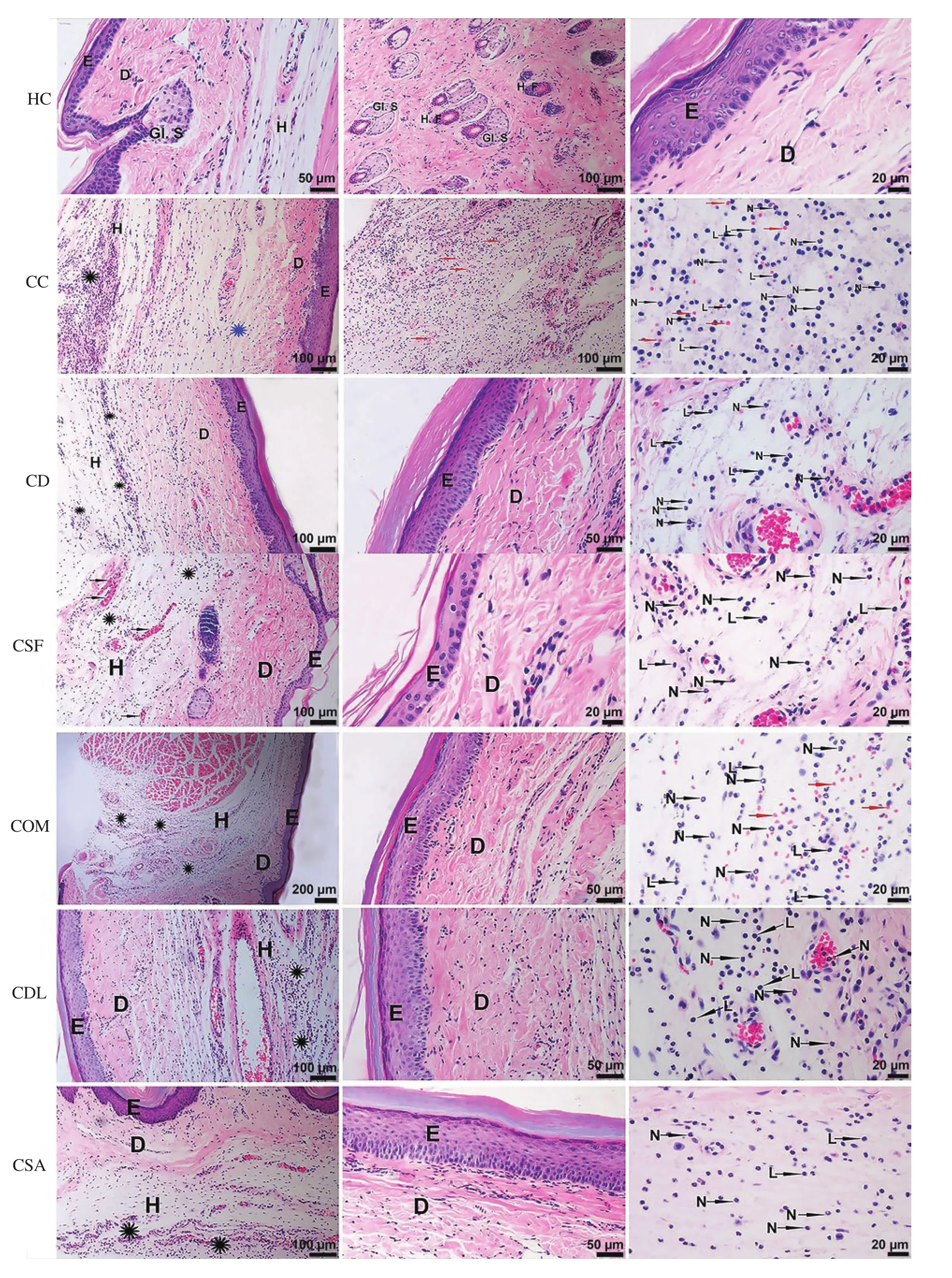
Figure 4. Histopathological results of all exprimental groups by hematoxylin and eosin staining. E: Epidermis. D: Dermis. H: Hypodermis. Black asterisk:Inflammatory leukocyte infiltration in the hypodermis. Blue asterisk: Collagen degeneration in the dermis. Red arrow: Erythrocyte in the interstitium(hemorrhage). L: Lymphocyte. N: Neutrophil. Gl. S: Glandula sebacea. H.F: Hair follicle.
According to the histopathological evaluation, fish mucus treatment reduced leukocyte infiltration and carrageenan-induced paw edema.The effect of diclofenac on edema and leukocyte infiltration was higher than all mucus groups. Among all fish mucus groups, S.aurata showed the most effective anti-inflammatory activities.It has been reported that Haruan extracts reduced edema and inflammatory cell infiltration[33,34]as well as the myeloperoxidase level, which is a marker of leukocyte infiltration[35]in a rabbit model of osteoarthritis and a rat model of chronic dermatitis. Haruan mucus increased endothelial cell proliferation and angiogenesis and accelerated wound healing[36]. Although the mechanism of the antiinflammatory action of Haruan extract is not entirely known, its anti-inflammatory effect may be attributed to its high amino acid(glycine, arginine, alanine, and proline) and fatty acid (arachidonic acid, palmitic acid, stearic acid, and linoleic acid) content[33,34,37].Although there is no information on the amino acid content of fish mucus, it has been reported that fish mucus is rich in fatty acids such as arachidonic acid, palmitic acid, stearic acid, and linoleic acid,similar to Haruan extract[18,37]. Haruan extract has anti-inflammatory effects despite its high arachidonic acid content, which is a precursor of the inflammation mediators for PG and leukotrienes (LT)B4. This effect could be associated with N-arachidonoyl glycine (lipoaminoacid), which has anti-inflammatory effects, and linoleic acid, which inhibits LTB4[33,35]. In this study, fish mucus reduced edema and leukocyte infiltration, which may be associated with its fatty acid and amino acid content.
In this study, the administration of carrageenan significantly increased the IL-1βand IL-6 mRNA expression in paw tissue.The administration of O. mykiss and S. aurata mucus decreased the carrageenan-induced increase in IL-1βmRNA expression while administration of O. mykiss, S. fontinalis, and S. aurata mucus reduced in IL-6 mRNA expression, similar to the effect of diclofenac. There was no difference between the groups in terms of TNF-αand IL-10 mRNA expression levels. The previous studies reported that carrageenan increased the TNF-α, IL-1β, and IL-6 levels[6,20]. TNF-α, IL-1β, and IL-6 play an important role in mediating inflammatory responses. The first cells that migrate to the inflammation area as a response to macrophages and mast cells during the development of inflammation are neutrophils.Lymphocytes and other inflammatory cells are activated in the inflammation process, leading to the release of growth factors,cytokines, and chemokines[38]. One of the possible reasons that mucus administration reduced IL-1βand IL-6 mRNA expression levels could be the inhibition of leukocyte infiltration. There was no difference between the groups in terms of COX-2 and TGF-βmRNA expression levels. In our study, the administration of all fish mucus decreased the carrageenan-induced increase in COX-2 mRNA expression with no statistically significant difference. In a rat model with scalpel incision on the paw, S. fontinalis mucus decreased the increase in COX-2 mRNA expression, but O. mykiss mucus did not have any effect[13].
The results of the present study also showed there was no difference between the carrageenan group and all mucus groups in terms of the CAT mRNA expression levels. Higher SOD mRNA expression was observed in the diclofenac and O. mykiss treated groups than in the carrageenan group. In the study of Cetin et al., S. fontinalis mucus decreased the myeloperoxidase and nitric oxide levels and increased the tGSH and SOD levels, whereas O. mykiss mucus did not have any effect on these parameters[13]. The haruan extract reduced the malondialdehyde and peroxide levels associated with chronic cadmium hepatotoxicity in rats[39]. Haruan extract and mucus have antioxidant properties[36,37]. Conversely, the contents of fish mucus depend on the fish species as well as endogenous and exogenous factors[17]. The difference between the antioxidant effects of mucus obtained from different fish species may also be linked to these factors.
In conclusion, all fish mucus showed anti-inflammatory effects on the acute paw edema by alleviation of edema and the reduction in inflammatory cell infiltration. Mucus from different fishes,which may possess anti-inflammatory activities can be used for the anti-inflammatory treatment in clinical practice. However,in this study, the evaluation of mRNA expressions of cytokines,antioxidant markers, and COX-2 was insufficient to explain the antiinflammatory effects of mucus obtained from four fish species on the acute rat paw edema. Therefore, further research is necessary to determine the mechanism of the anti-inflammatory action of fish mucus.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Funding
This study was supported by the Coordination Unit of Scientific Research Projects, University of Kastamonu (Project No. KUBAP01/2018-72).
Authors’ contributions
MH, OC, KU, and AYS contributed to conception, design, analysis,and acquisition, drafted the manuscript, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. MO, ET and GA contributed to analysis and were accountable for all aspects of work ensuring integrity and accuracy.
 Asian Pacific Journal of Tropical Biomedicine2020年10期
Asian Pacific Journal of Tropical Biomedicine2020年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-diabetic properties and bioactive compounds of Teucrium polium L.
- Chemical characterization, docking studies, anti-arthritic activity and acute oral toxicity of Convolvulus arvensis L. leaves
- Anti-MMP-2 and MMP-9 activity of Salsola komarovii Iljin extract and its solvent fractions
- Rice bran hydrolysates induce immunomodulatory effects by suppression of chemotaxis, and modulation of cytokine release and cell-mediated cytotoxicity
