Left atrial diameter and atrial fibrillation, but not elevated NT-proBNP,predict the development of pulmonary hypertension in patients with HFpEF
Yi-Xian LIU, Hui LI, Yi-Yuan XIA, Chun-Lei XIA, Xin-Liang QU,2, Peng CHU,Wen-Yin ZHOU,2, Lin-Lin ZHU,2, Li LI, Shao-Liang CHEN,2,#, Jun-Xia ZHANG,2,#
1Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China 2Laboratory of Cardiovascular Diseases, Nanjing Heart Centre, Nanjing, China
3Department of Cardiology, Guangzhou Red Cross Hospital, Jinan University, Guangzhou, China
Abstract Background The determinants of pulmonary hypertension (PH) due to heart failure with preserved ejection fraction (HFpEF) have been poorly investigated in patients with cardiovascular diseases (CVD). Methods From July 1 2017 to March 31 2019, a total of 149 consecutive HFpEF patients hospitalized with CVD were enrolled in this prospective cross-sectional study. A systolic pulmonary artery pressure (PASP) > 35 mmHg estimated by echocardiography was defined as PH-HFpEF. Logistic regression was performed to establish predictors of PH in HFpEF patients. Results Overall, the mean age of participants was 72 ± 11 years, and 74 (49.7%) patients were females.A total of 59 (39.6%) patients were diagnosed with PH-HFpEF by echocardiography. The left atrial diameter (LAD) was related to the ratio of the transmitral flow velocities/mitral annulus tissue velocities in early diastole (E/E') and the left ventricular diameter in systole (LVDs).N-Terminal pro B-type natriuretic peptide (NT-proBNP) was not found to be associated with LAD and impaired diastolic or systolic function of the left ventricle. Multivariable logistic regression showed that atrial fibrillation (AF) increased the risk of PH-HFpEF incidence 3.46-fold with a 95% confidence interval (CI) of 1.44–8.32, P = 0.005. Meanwhile, LAD ≥ 45 mm resulted in a 3.43-fold increased risk, 95% CI:1.51–7.75, P = 0.003. However, the significance levels of NT-proBNP, age and LVEF were underpowered in the regression model. Two variables, AF and LAD ≥ 45 mm, predicted the PH-HFpEF incidence (C-statistic = 0.773, 95% CI: 0.695–0.852, P < 0.001). Conclusions Two parameters associated with electrical and anatomical remodelling of the left atrium were related to the incidence of PH in HFpEF patients with CVD.
J Geriatr Cardiol 2020; 17: 400-409. doi:10.11909/j.issn.1671-5411.2020.07.002
Keywords: Atrial fibrillation; Ejection fraction; Heart failure; Left atrial diameter; Pulmonary hypertension
1 Introduction
Heart failure is an end-stage syndrome with a high mortality rate owing to deterioration caused by a variety of cardiac structural and functional abnormalities. In heart failure with preserved ejection fraction (HFpEF), systolic function assessed by the left ventricular ejection fraction (LVEF) is preserved, whereas diastolic function is compromised. The disturbed diastolic function increases the left ventricular filling pressures and the left atrial (LA) pressure, leading to pulmonary venous congestion.[1,2]Thus, pulmonary hypertension (PH) is a common complication in HFpEF patients.
PH could be estimated by echocardiography or right heart catheterization (RHC).[3]Nevertheless, RHC is invasive and cannot be performed in all the patients given its cost and procedural complications. Systolic pulmonary artery pressure (PASP) appraised by echocardiography could be comparable with that obtained by catheterization using the method described by Currie,et al.[4]Therefore, echocardiography is currently the mainstay method in the evaluation of PH.[5]In an updated classification, PH associated with left heart diseases (LHD) belongs to group 2 PH,which has been categorized into four types: PH due to heart failure with reduced ejection fraction (HFrEF), PH due to left-sided valvular heart disease, PH related to left heart inflow/outflow tract obstruction and PH due to HFpEF(PH-HFpEF).[6]
Relative to the other three types of PH associated with LHD, the determinants for PH-HFpEF were not fully understood. There are several interesting questions concerning PH development in patients with HFpEF. The first question is the role of LA on PH formation in HFpEF patients, given recent reports about stiff LA syndrome with increased risk of PH incidence after catheter ablation for atrial fibrillation(AF)[7,8]and LA appendage closure to prevent ischaemic stroke.[9]Thus, whether LA remodelling is an independent risk factor for PH-HFpEF needs to be clarified. The second question is about B-type natriuretic peptide (BNP) or N-Terminal pro B-type natriuretic peptide (NT-proBNP),the peptides secreted by the cardiac ventricles in response to volume expansion and pressure load.[10]Despite the fact that BNP confers the risk information in patients with HFpEF, it remains unknown whether bioactive BNP is an active player that contributes to PH development.[11,12]Comorbidities such as cardiovascular diseases (CVD) and metabolic disorders are highly prevalent in HFpEF patients. Whether CVD increases the risk of PH formation in HFpEF patients is elusive. It is worth mentioning that a recent study examined the clinical and echocardiographic parameters related to precapillary PH.[13]Therefore, it is worth exploring the determinants of PH formation via analysing clinical traits and NT-pro BNP and echocardiographic parameters in HFpEF patients with CVD in this prospective study.
2 Methods
2.1 Study design and population
This is a prospective, single-centre, cross-sectional, hospital-based study designed to assess the predictors for PH-HFpEF incidence in Chinese CVD patients. Subjects were consecutively enrolled from Nanjing First Hospital, a public tertiary care university hospital in Nanjing, China.Patients were recruited in this study if they were (1) ≥ 18 years old; (2) with CVD, defined as with at least one of the diagnosis of hypertension, coronary heart diseases and type 2 diabetes; and (3) left ventricular ejection fraction (LVEF)≥ 50% by echocardiography. The exclusion criteria were elaborated in a previous report of HFpEF study.[14,15]The definition of type 2 diabetes was fasting blood glucose ≥ 7.0 mmol/L, random blood glucose ≥ 11.1 mmol/L and HbA1c> 6.5% or the use of hypoglycaemic medications. The definition of coronary heart disease was stenosis of main coronary arteries ≥ 50% detected by percutaneous coronary angiography or coronary computed tomography angiography.The definition of hypertension was office blood pressure ≥140 mmHg systolic and/or ≥ 90 mmHg diastolic or receiving pharmacological treatment. The definition of HFpEF was based on criteria as previously described:[14,15](1) patients have at least one symptom or sign of dyspnoea, fatigue, rales or ankle swelling; (2) LVEF ≥ 50%; (3)NT-proBNP ≥ 280 pg/mL; and (4) LAD > 40 mm, E/E' ≥13,E'/A' < 1 or concurrently with AF. AF was diagnosed if patients had paroxysmal (≥ 2 episodes recurrent AF, terminates spontaneously within 7 days), persistent (sustained beyond 7 days, or lasting less than 7 days but necessitating pharmacologic or electrical cardioversion) or longstanding persistent AF (continuous AF of greater than 1-year duration). The study protocol and informed consent were approved by the Institutional Review Board of Nanjing First Hospital. Written informed consent for participation was obtained from all enrolled patients.
2.2 Echocardiography and PH definition
The collection and processing of echocardiographic data have been previously described.[14]Briefly, transthoracic echocardiography (TTE) was performed according to the international accepted guidelines[16]by board-certified cardiologists trained in echocardiography. To allow comparisons among individuals with different body sizes, chamber measurements of patients were indexed by body surface area (BSA) as recommended guideline. All measurements were performed by averaging three beats in patients with sinus rhythm or five beats in patients with AF. LAD was evaluated by LA anteroposterior (AP) measurement in the parasternal long-axis view using M-mode or 2-dimensional echocardiography. A typical case of echocardiographic measurement of LAD was shown in Figure 1A. To appraise left ventricular (LV) structure changes, LV diameter in diastole (LVDd) or in systole (LVDs) were examined. To assess the systolic function of LV, LVEF (biplane Simpson assessment), stroke volume (SV), and fractional shortening(FS) were measured. To evaluate the diastolic function of LV, peak transmitral flow velocities of early (E) and late (A)in diastole were obtained by colour flow Doppler mode.Furthermore, myocardial tissue velocities of early (E') and late (A') diastole were obtained by tissue Doppler mode.The ratios of E/A, E'/A' and E/E' were calculated accordingly.
Tricuspid regurgitant (TR) jet velocity and maximal TR velocity were measured using continuous wave Doppler.Parasternal long- and short-axis or RV-modified apical four-chamber views were applied to measure maximal TR jet velocity. PASP was calculated from the maximal right ventricular (RV) – right atrial pressure (RAP) gradient using the modified Bernoulli equation.[5]PASP = 4 × (maximal TR jet velocity)2+ RAP. In patients with AF, the third beat after two consecutive relatively equal RR intervals was used as described in the ‘index-beat method’.[17]RAP was estimated in the subcostal view according to the inferior vena cava (IVC) size and collapsibility as follows: 3 mmHg if IVC < 15 mm and collapse ≥ 50%, 5 mmHg if IVC between 15 and 21 mm and collapse ≥ 50%, 15 mmHg if IVC ≥ 21 mm and collapse between 10% and 50%, 20 mmHg if IVC≥ 21 mm and collapse < 10%, and 10 mmHg in all other cases. PH was defined as echocardiographic PASP > 35 mmHg.[18]
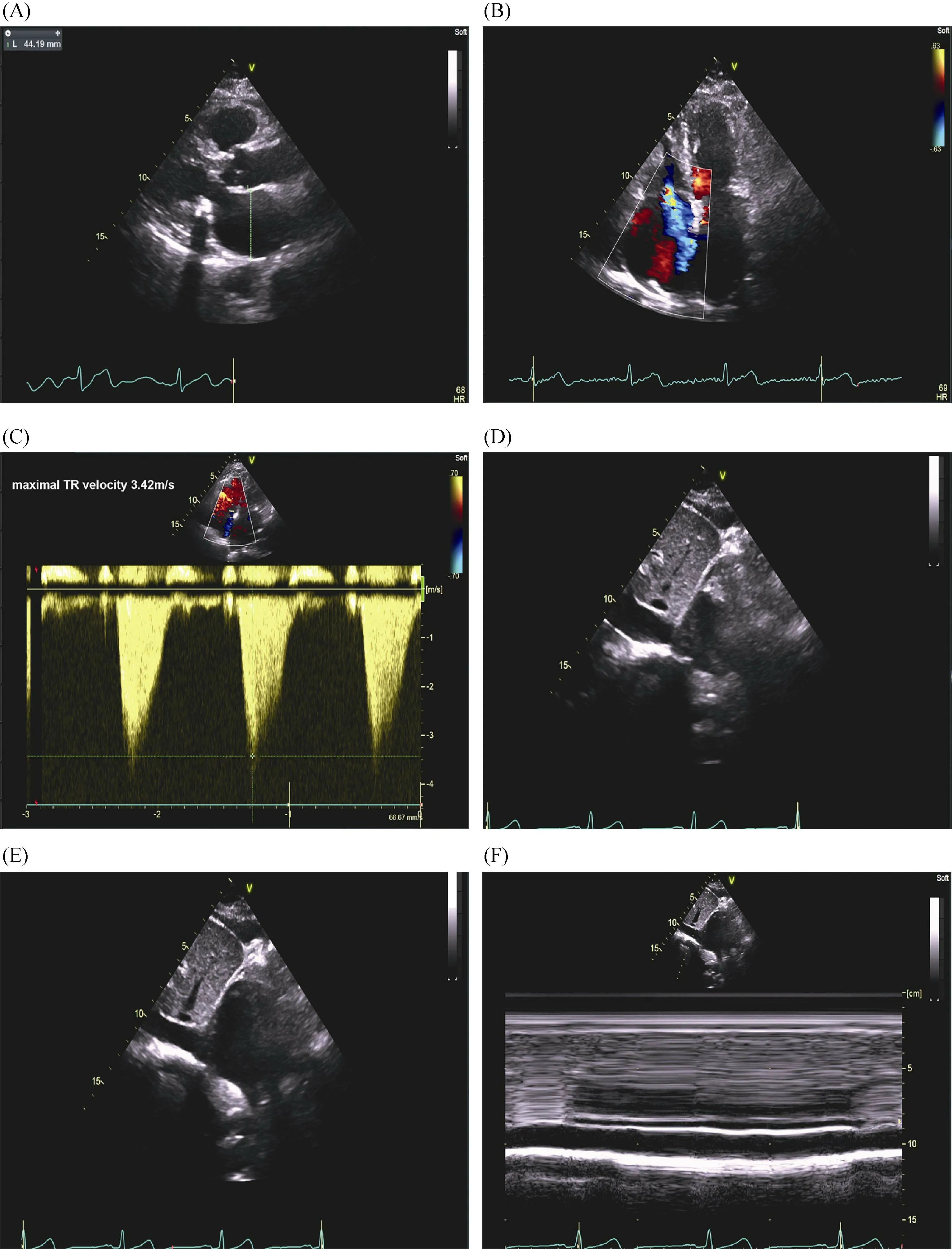
Figure 1. A representative case of echocardiographic measurement of systolic PASP. A 76-year-old woman with history of hypertension, diabetes and paroxysmal atrial fibrillation was admitted for heart failure with preserved ejection fraction. Her NT-proBNP was 661.9 pg/mL. (A): Left atrial diameter was evaluated by left atrial anteroposterior measurement in the parasternal long-axis view using 2-dimensional echocardiography; (B): right ventricular modified apical four-chamber views showed severe tricuspid regurgitation; (C):maximal tricuspid regurgitant velocity measured by continuous wave Doppler was 3.42 m/s; (D-F): in the subcostal view, the RAP was estimated at 10 mmHg given that the inferior vena cava was between 15 and 21 mm and collapsed between 10% and 50%. Using the modified Bernoulli equation: PASP = 4 × (maximal tricuspid regurgitant jet velocity)2 +RAP; therefore, PASP was estimated to be 57 mmHg. PASP:pulmonary artery pressure; RAP: right atrial pressure.
2.3 Biochemical analyses
Patients’ fasting blood samples were collected on the morning of the next day within 24 h after admission for routine measurements of haematology, clinical chemistry,biomarkers of heart failure and myocardial injuries. The serum NT pro-BNP assay was described at length previously.[14]
2.4 Statistics
We performed baseline descriptive statistics according to incidence of PH-HFpEF. Continuous values are expressed as the mean ± SD for normal distribution or medians and 25thto 75thinterquartile ranges (IQRs) for non-normal distribution. Categorical variables are expressed as numbers and percentages. Statistical significance was a two-sided significance level of 0.05. Numerical variables were analysed by Student’sttest for normal data and Wilcoxon rank-sum scores for non-normally distributed data; categorical variables were analysed by the Chi-square test or Fisher’s exact test when necessary. Pearson correlation with the Sidak test was used to explore the collinearity of covariates.
Variables that were significantly associated with PHHFpEF in univariate analysis (P< 0.05) were included in‘forward conditional’ fashion in multivariate logistic regression usingP< 0.1 as an entry threshold and PH-HFpEF as the dependent variable. Continuous data were dichotomized according to the cut-off value of the receiver operating characteristic curve (ROC). The cut-off values of age, NTpro BNP, LAD and LVEF for predicting PH-HFpEF were 72 years, 800 pg/mL, 45 mm and 64%, respectively. LAD and AF were fitted for model discrimination to predict patients to be diagnosed with HFpEF using C-statistics. Model calibration (agreement between observed and expected cases)was tested using the Hosmer-Lemeshow goodness-of-fit test.All statistical analyses were performed with Stata 12 (StataCorp, College Station, Texas, USA).
3 Results
Following the pre-specified inclusion and exclusion criteria, a final cohort 149 patients with CVD and HFpEF were enrolled in Nanjing First Hospital from July 1 2017 to March 31 2019. Among them, 59 (39.6%) patients were adjudicated as PH-HFpEF by echocardiography (Figure 2).Figure 1B-F shows a typical case of echocardiographic measurement of PASP for a patient who was classified as a PH-HFpEF patient. Overall, the mean age of participants was 72 ± 11 years, and 74 (49.7%) patients were females.

Figure 2. Flow chart of patient inclusion and exclusion in our study. Graphic displays how PH-HFpEF and non-PH-HFpEF groups were derived. Abbreviations: CVD: cardiovascular diseases; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; PH: pulmonary hypertension.
3.1 Baseline characteristics of PH-HFpEF vs. non-PHHFpEF patients
Clinical characteristics of the PH-HFpEF group (n= 59)and the non-PH-HFpEF control (n= 90) are summarized in Table 1. Between the PH-HFpEF and non-PH-HFpEF groups, those with PH were older (75 ± 9vs. 70 ± 11 years,P= 0.009), and the prevalence of AF was higher (57.6%vs.15.6%,P< 0.001). However, there was no statistically significant difference between PH-HFpEF and non-PH-HFpEF patients regarding sex and lifestyle exposures (alcohol habit and smoking). The prevalence rates of coronary heart diseases, diabetes, hypertension, and stroke were similar between groups.
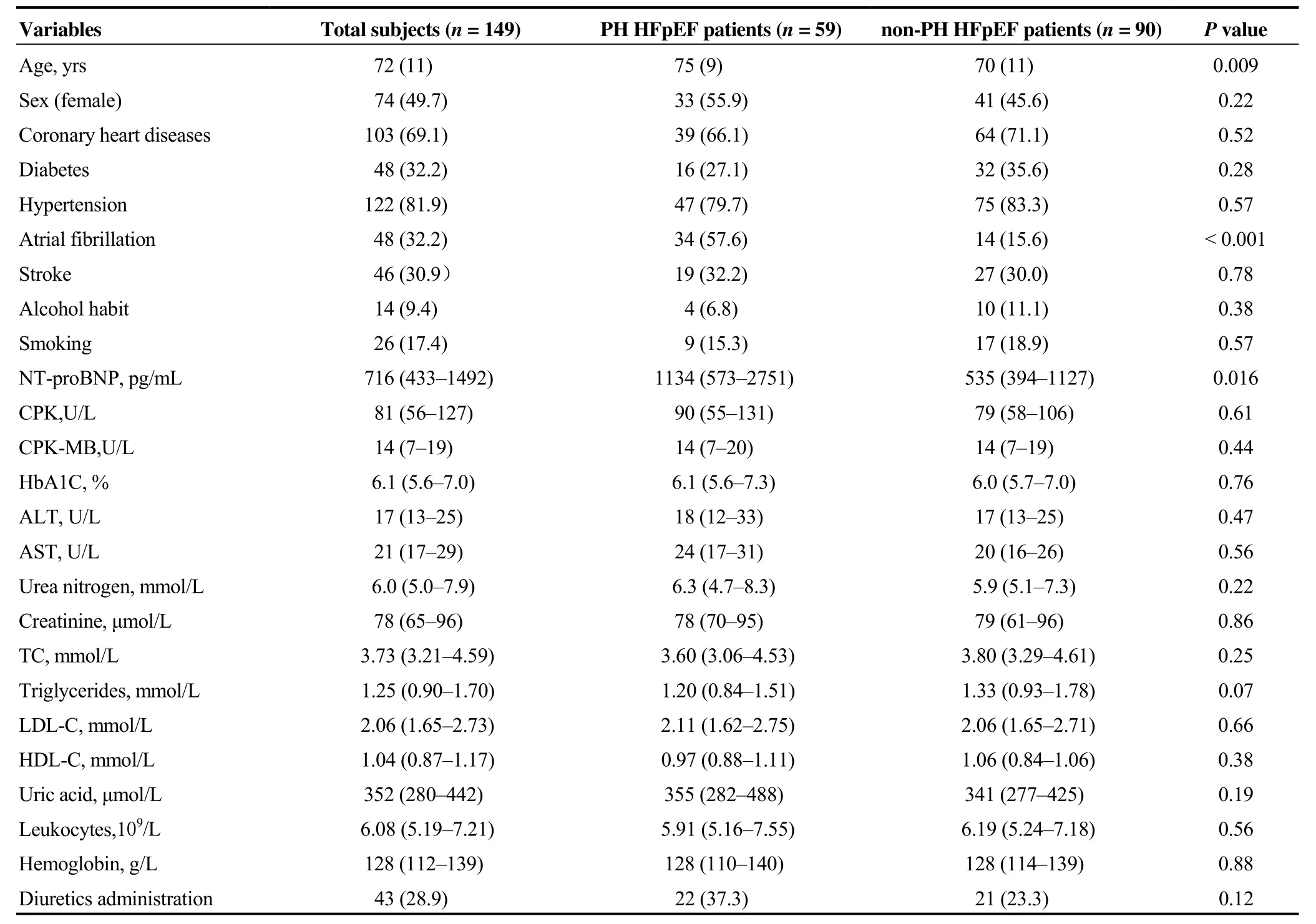
Table 1. Baseline characteristics of PH-HFpEF vs. non PH-HFpEF patients.
The cardiac biomarker level of NT-proBNP was higher in PH-HFpEF patients compared to non-PH-HFpEF patients(1134vs. 535 pg/mL,P= 0.016), whereas no significant differences were noted regarding creatine phosphokinase(CPK), MB isoenzyme of CPK (CPK-MB), glycosylated haemoglobin, renal function, liver function, lipids, leukocytes and haemoglobin levels. And the diuretics were not frequently used in PH-HFpEF group.
3.2 Echocardiographic changes in patients with PHHFpEF vs. non-PH-HFpEF patients
We further studied echocardiographic changes in HFpEF patients with or without PH. PH-HFpEF patients had larger LAD, 47vs. 42 mm,P< 0.001. Peak E velocity significantly increased in PH-HFpEF patients, 81vs. 66 cm/s,P=0.003, resulting in a promoted E/A ratio, 0.83vs. 0.71,P=0.002. Peak A' velocity significantly reduced in PH-HFpEF patients, 8vs. 9 cm/s,P= 0.002, leading to a higher E'/A'ratio, 0.67vs. 0.60,P= 0.008. However, the trend of increased E/E' ratio in PH-HFpEF patients was not statistically significant when compared to that in non-PH-HFpEF patients, 14.8vs. 11.9,P= 0.08, Table 2.
The reduction of LV systolic function was observed between PH-HFpEF and non-PH-HFpEF patients. As shown in Table 2, the median LVEF in the PH-HFpEFvs. non-PHHFpEF groups was 63%vs. 64%,P= 0.047. Congruously,enlargement of LVDs, 33vs. 31 mm,P= 0.022, was noted between PH-HFpEF and non-PH-HFpEF patients. However,FS and SV were not significantly reduced in PH-HFpEF patients, Table 2.
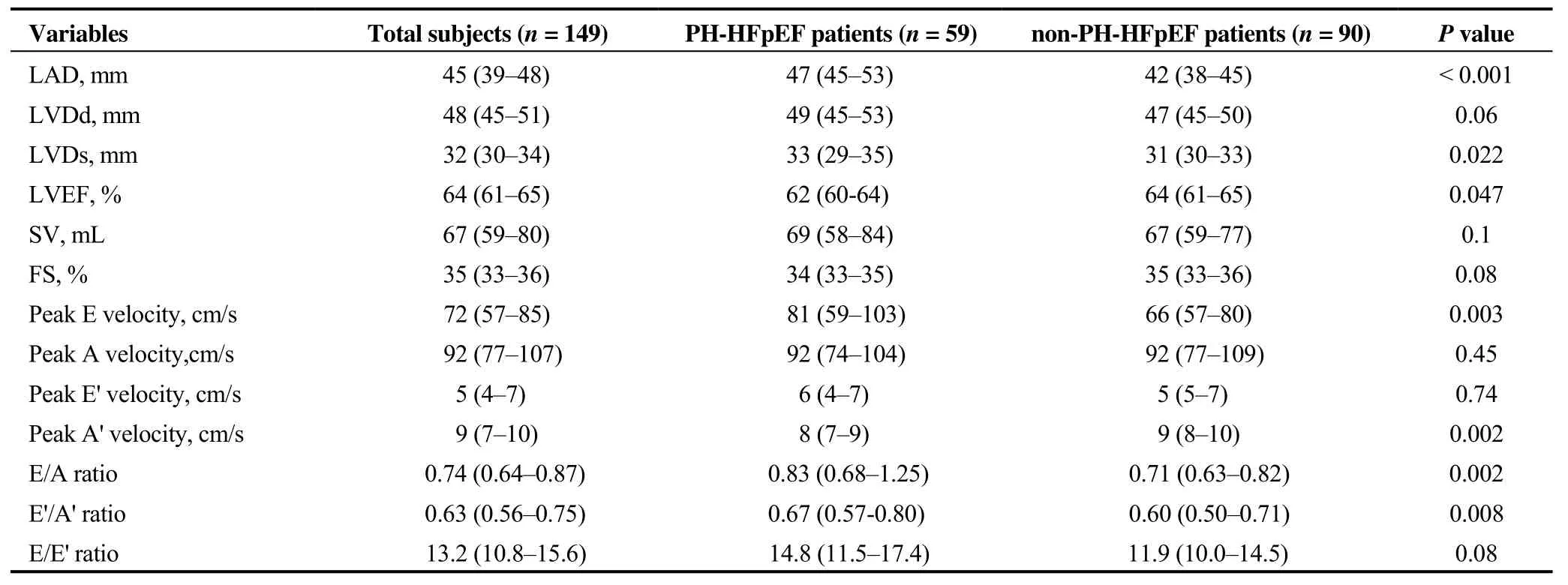
Table 2. Echocardiographic characteristics of PH-HFpEF vs. non-PH-HFpEF patients.

Figure 3. Matrix of correlation for echocardiographic predictors of LA enlargement. (A): LAD was associated with E/E' ratio (r =0.28, P = 0.02) but not with E'/A'or E/A ratio. E'/A' and E/A ratios were correlated in HFpEF patients. LAD was related to LVDs, r = 0.42, P< 0.001. (B): LVDs were negatively related to LVEF and FS. LVEF and FS were closely correlated. A: transmitral flow velocitiesin late diastole; A': mitral annulus tissue velocities in late diastole; E: transmitral flow velocities in earlydiastole; E': mitral annulus tissue velocities in early diastole; FS: fractional shortening; HFpEF: heart failure with preserved ejection fraction; LA: left atrium; LAD: left atrial diameter;LVDs: left ventricular diameter in systole; LVEF: left ventricular ejection fraction.
3.3 Echocardiographic predictors for LA enlargement
Then, we explored the relationship between LAD and LV diastolic dysfunction in HFpEF patients. LAD was significantly associated with E/E1 ratio (r= 0.28,P= 0.02).The E1/A1 and E/A ratios were correlated in HFpEF patients, whereas the associations of LAD with E1/A1 and E/A ratio were not significant, Figure 3A.
The LV systolic function of HFpEF was preserved compared to HFrEF, whereas it was impaired compared to normal controls.[19]Therefore, we also explored the relationship of LAD with LV systolic function in HFpEF patients. LAD was closely related to LVDs,r= 0.42,P< 0.001. LVDs were negatively related to LVEF and FS, Figure 3B.
Considering the existence of collinearities of echocardiographic parameters and the high incidence of AF in this cohort, we chose LAD and LVEF as candidate echocardiographic covariates for multivariable logistic regression.
3.4 Relationship of NT-proBNP with LA and LV changes in HFpEF patients with or without PH
Next, we evaluated the relationship of elevated NTproBNP with LA and LV changes in HFpEF patients with or without PH. The correlations of NT-proBNP with LAD and E'/A' ratio assumed opposite trends between PH-HFpEF and non-PH-HFpEF patients, even though not statistically significant, indicating the possibly indiscrete pathophysiology of PH-HFpEF and non-PH-HFpEF patients, Figure 4A& B. A correlation was not found between NT-proBNP with E/E' (Figure 4C), which was incongruous with a previous small sample size study.[20]Similarly, neither LVEF nor LVDs were observed to statistically correlate to NTproBNP, Figure 4D&E.

Figure 4. Relationship of NT-proBNP with LA and LV changes in HFpEF patients with or without PH. The correlation of NTproBNP with LAD (A) and E'/A' ratio (B) took an opposite trend between PH-HFpEF and non-PH-HFpEF patients, though the trend was not statistically significant. A correlation was not found between NT-proBNP and E/E' ratio (C). Neither LVEF (D) nor LVDs (E) was observed to statistically correlate to NT-proBNP. A': mitral annulus tissue velocities in late diastole; E: transmitral flow velocities in early diastole; E':mitral annulus tissue velocities in early diastole; FS: fractional shortening; HFpEF: heart failure with preserved ejection fraction; LA: left atrium; LAD: left atrial diameter; LV: left ventricle; LVDs: left ventricular diameter in systole; LVEF: left ventricular ejection fraction;NT-proBNP: N-terminal pro B-type natriuretic peptide.

Table 3. Association of clinical, NT-proBNP and echocardiographic traits with incident PH-HFpEF.

Figure 5. ROC curve to predict PH-HFpEF. AF and LAD ≥45 mm predicted good PH development in HFpEF patients. The C-statistic was 0.773 (95% CI: 0.695 to 0.852), P < 0.001. AF:atrial fibrillation; HFpEF: heart failure with preserved ejection fraction; LAD: left atrial diameter; PH: pulmonary hypertension;ROC: receiver operating characteristic.
3.5 Multivariable logistic regression for predicting PH-HFpEF
After univariate analysis, age, AF, NT-proBNP, LAD and LVEF, which were found to be associated with PH development in HFpEF patients, were included in the multivariable regression analysis. Multivariable logistic regression showed that AF increased the risk for PH-HFpEF incidence 3.46-fold, with a 95% CI: 1.44–8.32,P= 0.005.Meanwhile, LAD ≥ 45 mm had a 3.43-fold increased risk,95% CI: 1.51–7.75,P= 0.003, as shown in Table 3. Using AF and LAD ≥ 45 mm could predict PH incidence in Chinese CVD patients with HFpEF, and the C-statistic was 0.773 (95% CI: 0.695-0.852),P< 0.001, Figure 5. The Hosmer- Leme show test showed good fitness of the model(χ2= 0.049,P= 0.976).
4 Discussion
PH, which occurs frequently in HFpEF patients, is associated with increased mortality in this population.[21]However, data on the combined values of clinical predictors and echocardiographic parameters on incident PH-HFpEF are scarce. In this study, we examined the clinical, biochemical and echocardiographic predictors of PH development in HFpEF patients hospitalized with CVD. We found that two simple variables indicating electric and anatomic remodelling of LA, as shown by AF and LAD, were associated with PH formation in HFpEF patients.
Risk factors are a variety of exposures that contribute to the susceptibility to the incidence of diseases. They can be demographic, environmental, genetic or related-disease states.It was demonstrated that the prevalence of hypertension and diabetes mellitus increased in patients with pulmonary venous hypertension compared to patients with pulmonary arterial hypertension.[22]However, in our study, AF was associated with an increased risk of PH-HFpEF and should be deemed as a risk factor for it, whereas the correlations of CVD, stroke, alcohol habit and smoking with PH-HFpEF incidence were not found.
The atrium assumes reservoir, conduit and active emptying functions during cardiac cycles.[23]In patients with pulmonary arterial hypertension, right atrial dysfunction independently predicts mortality and hospitalization.[24]LA enlargement in HFpEF patients impairs atrial compliance and reduces atrial pump function, especially during exercise stress.[25–28]Impaired LA global systolic function correlates with increased pulmonary vascular resistance and reduced pulmonary arterial compliance, as measured by cardiac catheterization in HFpEF and HFrEF patients.[29]Nevertheless, clinical predictors were not included when evaluating the association of LA size or function with PH-HFpEF occurrence. In this study, we found that enlarged LAD (LAD≥ 45 mm) predicted the incidence of PH in HFpEF patients,even in the presence of clinical predictors.
In our study, we found that both LV diastolic and systolic dysfunctions were related to LAD but not related to the elevated PASP in HFpEF patients. The E/E' ratio is the most established echocardiographic predictor of elevated LV filling pressures and diastolic dysfunction. E/E' ratio > 15 was likely to increase the LV diastolic pressure[30]and possibly raised pulmonary venous pressure, even though these findings were not reaffirmed in another investigation.[31]However, our study did not discern the association of the E/E' ratio with elevated PASP in HFpEF patients. Our results once again underscored the importance of LA remodelling, other than LV diastolic dysfunction in PH formation in HFpEF patients.
Biomarkers of heart failure have incremental predictive or diagnostic value over traditional risk factors or tests.NT-pro BNP or BNP was found to have comparable prognostic impact among HFpEF and HFrEF patients in a CHART-2 study.[32]However, others found that the BNP level was an independent predictor of adverse events solely in HFrEF patients, but not in HFpEF patients.[33]Moreover,in a previous study, a correlation was found between NT-proBNP and E/E'.[20]Therefore, we explored the relationship of NT-proBNP level with echocardiographic parameters. In our study, however, NT-proBNP was neither associated with LA and LV echocardiographic parameters nor predicted PH incidence in HFpEF patients. Therefore,the predictive or diagnostic value of NT-proBNP in HFpEF patients needs to be thoroughly investigated in the future.
There are some limitations of our study. First, Dopplerbased estimations of PASP do not agree perfectly with invasive measurements using RHC.[34]Second, we used LAD instead of LA volume as the LA size to predict PH development in HFpEF patients. Nevertheless, in clinical reality other than for research purposes, LAD is the most widely adopted LA size index. The conclusion drawn in our study is easily generalized in clinical practice. Third, there was an obviously opposite trend of correlation of NT-proBNP and LAD between PH-HFpEF and non-PH-HFpEF patients,even though the statistical significance was not reached. If a correlation truly exists, it might explain why, in the multivariate analysis, the significance of NT-proBNP was underpowered when LAD was adopted as a covariate in predicting PH-HFpEF. Furthermore, if a correlation truly exists,studies with larger sample sizes are warranted to confirm whether NT-proBNP is an independent risk factor for LA remodelling in PH-HFpEF patients.
In conclusion, there are prominent differences of AF incidence and LAD among PH-HFpEFvs. non-PH-HFpEF patients. Our data show that electrical and anatomical remodelling of LA plays an important role in PH development in HFpEF patients with CVD.
Acknowledgement
We thank Xiao-Fei GAO (Nanjing First Hospital) for the consultation of statistics. This research is funded by the National Natural Science Foundation of China (Grant No.81700398, No. 81970309 and No. 81770441), the Natural Science Foundation of Guangdong Province No. 2016A030 313430 and Nanjing Municipal Healthcare Grant YKK16127.The authors declare they have no conflicts of interest.
 Journal of Geriatric Cardiology2020年7期
Journal of Geriatric Cardiology2020年7期
- Journal of Geriatric Cardiology的其它文章
- The prognostic role of high-sensitivity C-reactive protein in patients with acute myocardial infarction
- Associations of soybean products intake with blood pressure changes and hypertension incidence: the China-PAR project
- Modified subintimal plaque modification improving future recanalization of chronic total occlusion percutaneous coronary intervention
- Quality of life, physical performance and nutritional status in older patients hospitalized in a cardiology department
- Remote monitoring of implantable cardioverters defibrillators: a comparison of acceptance between octogenarians and younger patients
- Plasma big endothelin-1 is an effective predictor for ventricular arrythmias and end-stage events in primary prevention implantable cardioverterdefibrillator indication patients
