Vitamin D and calcium signaling in epidermal stem cells and their regeneration
Yuko Oda, Daniel D Bikle
Yuko Oda, Daniel D Bikle, Department of Medicine, University of California San Francisco, CA 94158, United States
Yuko Oda, Daniel D Bikle, Endocrine Research, Veterans Affairs Medical Center San Francisco, CA 94158, United States
Abstract
Key words: Vitamin D; Calcium; Stem cells; Epidermis; Regeneration; Wound healing
INTRODUCTION
Chronic skin wounds are estimated to affect 6.5 million patients in the United States at a cost of over $25 billion[1].A disproportionate number of these wounds are found in patients suffering from a variety of medical conditions including poor nutrition.A survey of patients with chronic leg ulcers by one of our clinical collaborators, Dr.Gasper, found vitamin D deficiency (25OHD levels less than 20 ng/mL) and decreased serum calcium (below 8.7 mg/dL) in nearly 50% of these patients (unpublished observation with permission).Moreover, chronic kidney disease but without diabetes mellitus or cardiovascular disease led to delayed healing of abdominal surgical wounds correlating inversely with 25OHD levels[2,3].
Adult stem cells (SC) residing in the skin play an important role in the regeneration of the epidermis after wound injury.Understanding the mechanisms regulating these adult SC is central for understanding epidermal regeneration during cutaneous wound healing.Skin epithelia are derived from the ectoderm and differentiate into the interfollicular epidermis (IFE), sebaceous gland (SG) and hair follicle (HF) during the embryonic developmental process.After birth, adult SC residing in the basal layer of the epidermis (eSC), isthmus (iSC) and bulge (bSC) regions of the HF regenerate the IFE, SG and HF, respectively[4-7].In the epidermis, eSCs produce transient amplifying cells, which leave the basal layer and produce differentiation marker proteins such as involucrin (IVL), keratin 1 (KRT1), loricrin (LOR) and filaggrin (FLG) in a sequential process.In the proximal portion of the HF, iSCs maintain the SG.The bSCs regenerate HFs in a cyclic manner through activation signals initiated by the dermal papilla adjacent to the bSC.
However, when the skin is injured, these stem cells and progeny from all regions of the HF, SG and IFE contribute to regeneration of the epidermis at least initially[4,7]but to a different extent.Itoet al[8]found that bSCs provide around 25% of the newly regenerated epidermal cells by using an inducibleKrt15-crePR/R26R transgene, although these cells did not persist during the healing process.Levyet al[9]demonstrated that the SCs from HF infundibulum also contribute to reepithelialization by using a Shh Cre/R26R transgene, and these cells remained in the newly formed epidermis.However, eSC in the IFE make the greatest and most persistent contribution to epidermal regeneration[4].Moreover, Langtonet al[10]showed that bSC are not required for wound re-epithelialization because epidermis lacking HF as in the paw also re-epithelize the wound but with a delayed rate.The self-renewal of these SCs and their injury induced activation as well as migration and differentiation of progeny are controlled by various signal pathways.
The vitamin D receptor (VDR) is enriched in these stem cells.Its ligand,1,25(OH)2D3, a well-known regulator of epidermal differentiation and proliferation[11]can be produced in these epidermal cells.The epidermis is the major source of vitamin D for the body.Vitamin D is produced from 7-dehydrocholesterol by irradiation with UVB from the sun.As noted keratinocytes in the epidermis metabolize vitamin D to its active ligand 1,25(OH)2D3[11].Tianet al[12]observed that topical 1,25(OH)2D3enhanced wound healing.Vitamin D signaling regulates epidermal stem cells by interaction with other signaling molecules during cutaneous wound healing.
β-catenin signaling plays an important role for the maintenance of adult SCs in skin in both the HF and IEF.The role of β-catenin in bSC function has been extensively reported[13,14], but less attention has been paid to its role in eSC function.However, a sensitive probe for β-catenin signaling in the IFE is that of anAxin 2Cre reporter that demonstrates a role for β-catenin in eSC activation[5,13].VDR appears to support activation of adult SCs through interaction with β-catenin.VDR binding to β-catenin promotes VDR transcriptional activity and facilitates cell fate determination[15,16].In the HF, VDR is essential for β-catenin signaling and bSC activation[17,18].VDR binding to βcatenin in the AF2 domain supports transcription for the bSC markerKrt15and HF differentiation genes such asPADI3andS100a3[16].Our observations also suggest that the same may be true for eSC in the epidermis as keratinocytes lackingVdrshow a blunted wound induced activation of β-catenin target gene expression at the wound edge as described below.
Calcium signaling also is important for cutaneous wound repair through interaction with vitamin D signaling.VDR profoundly affects calcium signaling within the cell including the induction of the calcium sensing receptor (CaSR) and a number of the pathways regulated by both calcium and vitamin D that are involved in epidermal differentiation.CaSR is a seven transmembrane domain, G protein coupled receptor first identified in parathyroid cells[19], that we cloned from keratinocytes[20].CaSR is essential for the keratinocyte response to calcium[21,22], but like the VDR its role in wound healing has received little attention.Thein vivorole of CaSR in calcium signaling in epidermis is demonstrated by aCasrnull mice, in which the transmembrane domain and intracellularCasris deleted fromKrt14expressing keratinocytes[23].The expression of the CaSR is increased by 1,25(OH)2D3, causing the keratinocyte to be more sensitive to calcium actions[24,25].Moreover, to our surprise, CaSR deletion reduces VDR expression[23].
Calcium signaling mediates epidermal remodeling after wound injury through adherens junction (AJ) signaling.In skin epithelia, the core molecular components of the AJ are cadherins and their binding partners β-catenin, α-catenin, and p120-catenin.The AJ signaling plays an essential role in epidermal differentiation via its role in activating phospholipase (PLC)γ, that in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol trisphosphate (IP3) and diacylglycerol (DAG), signaling molecules critical for the differentiation process through their release of calcium from intracellular stores (IP3) and activation of several protein kinase Cs[26].Formation of the E-cadherin/catenin complex is regulated by both VDR and calcium[26].The Ecadherin/catenin complex also links to the underlying cytoskeletonviaα-catenin that helps form an epithelial sheet in enabling the cell migration required to re-epithelialize the wound in addition to promoting its subsequent differentiation to regenerate the epidermis[27].
In this review, we focus on the roles of vitamin D signaling and calcium through their receptors (VDR, CaSR) in the epidermis for the control of adult SCs and progeny during the epidermal response to wound injury, where a defective response leads to poor wound healing.
VITAMIN D AND CALCIUM SIGNALING IN INJURY INDUCED ACTIVATION OF EPIDERMAL SC THROUGH INTERACTION WITH β-CATENIN SIGNALING
Studies of the role of VDR in skin have previously focused on hair cycling as alopecia (hair loss) is a striking phenotype in globalVdrknockout (KO) mice[28], in which a gradual decrease in bSC has been reported accompanied with impaired β-catenin signaling[18].Delayed wound healing is reported in these mice, but it was primarily attributed to altered dermal fibroblast function not to alterations in epidermal function[29].However, our studies showed that VDR has an essential role in epidermal SCs and progeny during cutaneous wound healing.Wound closure is delayed and wound re-epithelialization is retarded inVdrconditional knockout (cKO) mice in whichVdris specifically removed fromKrt14expressing epidermal SCs and progeny[30,31].The number of SCs residing in the IFE as well as the HF is decreased[30]demonstrating impaired self-renewal of these SCs.In addition,VdrcKO results in blunted SC proliferation and in impaired β-catenin signaling[30]that plays an important role in the epidermal response to wounding.These results are observed only whenVdrcKO mice are maintained on a low dietary calcium.Therefore, we explored the cooperative role of calcium signaling with vitamin D signaling by generating conditional double knockout mice (cDKO), in which bothVdrandCasrare deleted fromKrt14expressing epidermal SCs and progeny[32].Delayed wound closure and retarded wound re-epithelialization is also observed in these cDKO mice ingesting normal calcium diets.Injury activated SC proliferation is impaired at the wounding edges.In addition, injury induced induction of β-catenin target genes was also blunted in cDKO wounds[32].These responses are similar to skin wounds inKrt14specificβ-cateninKO mice.These results show thatVdrandCasrare essential for injury-induced SC activation at least in part via stimulation of β-catenin signaling.
VITAMIN D AND CALCIUM SIGNALING IN ADHERENS JUNCTION FORMATION ESSENTIAL FOR EPIDERMAL REMODELING
Our transcriptomic study also revealed that AJ signaling is a top canonical pathway altered in the epidermis of mice that lack bothVdrandCasr(cDKO) inKrt14expressing keratinocytes[32].The expression levels of E-cadherin and the levels of the epithelial specific desmosome component desmoglein 1 are decreased in wounds of cDKO mice[32].In addition, the expression of the IFE early differentiation marker KRT1, middle differentiation marker IVL, and late differentiation marker LOR in the epidermis did not extend across the wound and remained disorganized in the shortened epithelial tongues at the remodeling stage of wound healing ofVdrcKO[30].The reduction of E-cadherin and differentiation markers are also observed inCasrcKO mice, in whichCasris removed from epidermal SC and progeny[33], in association with a delay in wound closure and reduced wound re-epithelialization.Similarly, cell migration is impaired in anin vitrowound scratch model whenVDR[30],CaSR[33]or bothVDRandCaSR[32]are silenced in cultured human keratinocytes.Therefore, vitamin D and calcium signaling are critical for keratinocyte migration and Ecadherin/catenin mediated epidermal differentiation, each essential for epidermal regeneration during wound healing.
POTENTIAL MECHANISMS FOR VITAMIN D AND CALCIUM SIGNALING TO REGULATE WOUND RE-EPITHELIALIZATION
Compensatory and/or interacting aspects of vitamin D and calcium signaling on wound healing exist.We examined two processes; (1) β-catenin signaling, that induces epidermal proliferation to produce the cells that subsequently regenerate the epidermis[4,5], and (2) the E-cadherin/catenin complex that is critical for keratinocyte differentiation as well as migration during wound induced re-epithelialization[34].Our working model summarizes our findings illustrating the reduction in proliferation due to defects in the nuclear actions of β-catenin and decreased re-epithelialization caused by failure of migration, differentiation and formation of the AJ in the epidermis of mice in which vitamin D and calcium signaling are disrupted (Figure 1).Potential molecular mechanisms by which vitamin D and calcium interact to control migration and proliferation of keratinocytes during wound induced epidermal regeneration are shown (Figure 2).First, VDR induces genes including CaSR and β-catenin regulating differentiation genes in the epidermis and HFs[11].VDR may facilitate β-catenin binding to its response elements in target genes such as cyclin D1 to promote cell proliferation[16].VDR also is required for calcium, mediated by the CaSR, to form Ecadherin/catenin complex to stimulate AJ signaling[22].This is due in part to activation of the Src/Fyn kinases that phosphorylate the catenins facilitating their incorporation into the E-cadherin/catenin complex.This complex provides a reservoir of β-catenin in the membrane.The shift of β-catenin from the nucleus to the membrane is crucial to allow differentiation, in part by reducing its proliferative function in the nucleus.
The E-cadherin/catenin complex includes α-catenin, that links the complex to the cytoskeleton subsequently enabling cells to migrate.The complex also includes the enzymes phosphatidyl inositol 3 kinase (PI3K) and phosphatidyl inositol 4-phosphate 5-kinase 1α (PIP5K1α)[34,35].These enzymes sequentially phosphorylate of PIP and PIP2 to PIP3 to activate PLC-γ1 and other signaling molecules such as Akt.PLC-γ1 cleaves PIP2 to form IP3 and DAG; IP3 releases intracellular [Ca]i from intracellular stores, which is essential for the acute response to wounding, and DAG which along with calcium activates PKCα.PKC regulates the activity of AP-1 transcription factors involved in differentiation.Moreover, PKCα phosphorylates desmoplakin, a component of desmosomes, that loosens their intercellular adhesion to enable keratinocyte migration across the wounds[36].
Efficient wound repair is critical for life by restoring the integrity of the skin to prevent the invasion by infectious organisms and other harmful materials and the loss of body fluids.Understanding the mechanisms by which the SC populations in the skin respond to wounding should lead to better therapies to promote more efficient healing.Examining the role of vitamin D and calcium signaling in this process is an important step in this direction.Moreover, our studies address the more general question of tissue regeneration, both pathologic as in chronic wounds and physiologic as in normal wound repair in the skin and other tissues.The roles of β-catenin and Ecadherin signaling in SC as they are regulated by vitamin D and calcium during the response to wounding and epidermal remodeling are central to the wounding response.Further study of the shift in the transcriptional profile during wounding as affected by deletion ofVdrand/orCasris likely to reveal a better understanding of the molecular mechanisms by which VDR and CaSR sequentially regulate the different aspects of the wounding response with the potential that these results will lead to new approaches to treatment of chronic wounds.
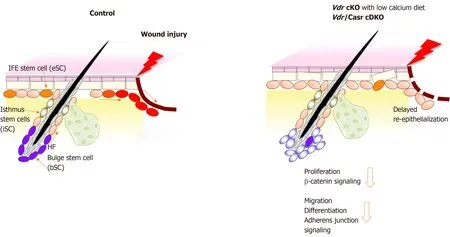
Figure 1 Schematic model showing that deficiency in both vitamin D receptor and calcium sensing receptor prevents proliferation and migration of keratinocytes thus delaying wound re-epithelialization of the wounded epidermis.
These studies have clinical significance as vitamin D deficiency is linked with poor wound healing[2,3].Improved wound healing of patients with diabetic foot ulcers with vitamin D supplementation compared to placebo is supported by a randomized clinical trial of oral vitamin D supplementation[37].Likewise, calcium alginate dressings are superior to other wound care products[38]indicating the clinical role of calcium signaling in wound repair.Like vitamin D signaling, calcium signaling is expected to play an important role for activation, migration and differentiation of the SCs regenerating the epidermis.
CONCLUSION
In summary, we have discussed the role of vitamin D and calcium signaling in epidermal SCs and progeny essential for normal wound re-epithelialization in the epidermis.We propose that vitamin D and calcium promote wound reepithelialization, through both β-catenin and AJ signaling.
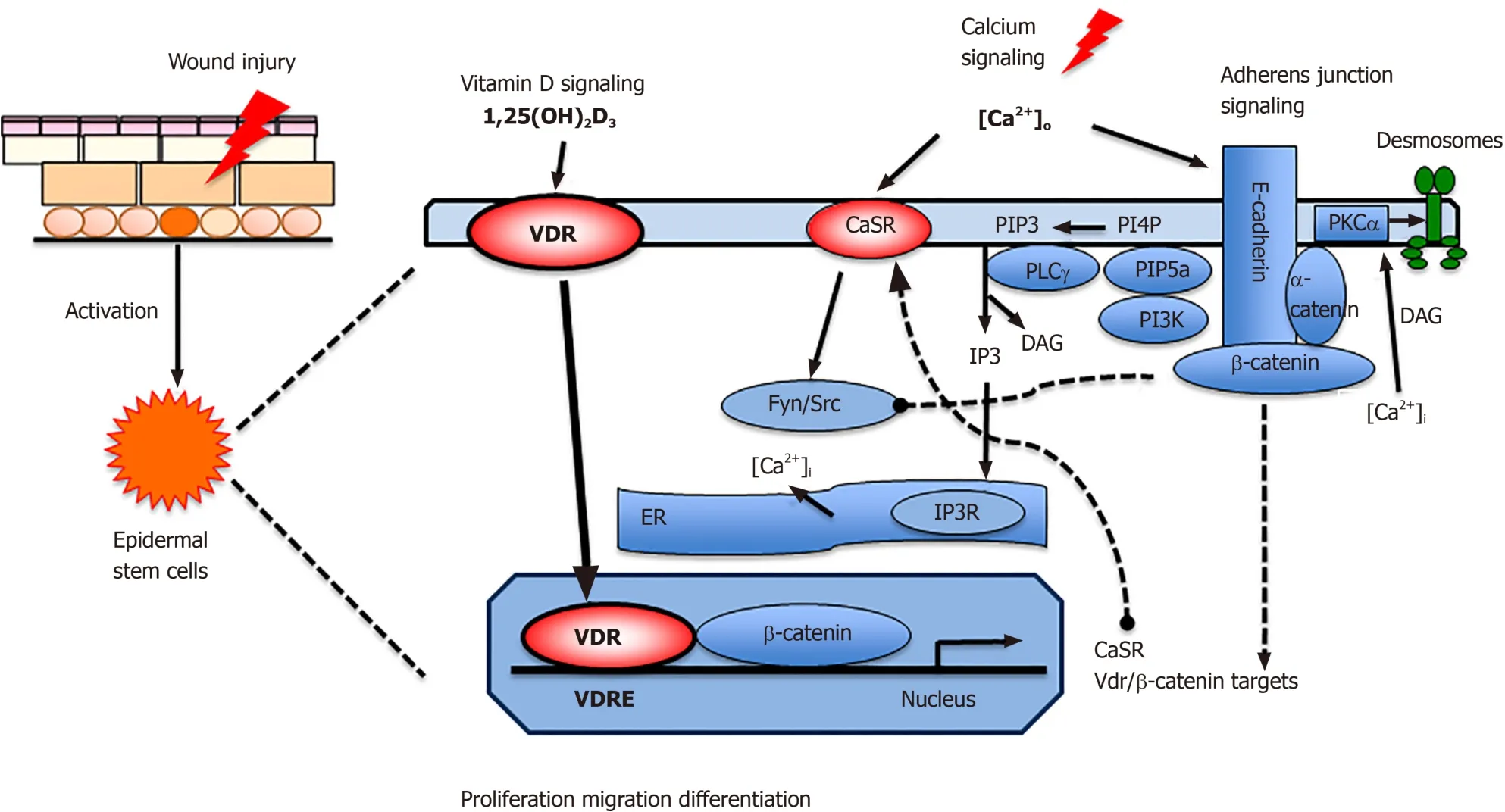
Figure 2 Proposed model in which vitamin D and calcium signaling mutually regulate β-catenin and AJ signaling essential for wound reepithelialization.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Potential of transposon-mediated cellular reprogramming towards cell-based therapies
- Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface
- Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species
- Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
