Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration
Ji-Zhong Yang, Li-Hong Qiu, Shao-Heng Xiong, Juan-Li Dang, Xiang-Ke Rong, Meng-Meng Hou, Kai Wang, Zhou Yu, Cheng-Gang Yi
Ji-Zhong Yang, Li-Hong Qiu, Shao-Heng Xiong, Juan-Li Dang, Xiang-Ke Rong, Meng-Meng Hou, Kai Wang, Zhou Yu, Cheng-Gang Yi, Department of Plastic Surgery, Xijing Hospital, Fourth Military Medical University, Xi'an 710032, Shaanxi Province, China
Abstract
Key words: Extracellular matrix; Decellularized adipose matrix; Decellularized adipose tissue; Adipose-derived extracellular matrix; Adipose tissue extracellular matrix; Adipose matrix; Stem cells; Soft tissue regeneration; Decellularization methods
INTRODUCTION
Soft tissue defects caused by trauma, tumors, and aging are often seen in clinical work, and tissue regeneration is undoubtedly one of the biggest challenges.Stem cell therapy has always played an important role in the field of regenerative medicine[1-3].Stem cells achieve tissue metabolism and regeneration of post-traumatic defects through two unique attributes: (1) The ability to self-renew in the process of symmetric division; and (2) The ability to multidirectionally differentiate in the process of asymmetric division[4].Although stem cells play an important role in soft tissue regeneration, risks and challenges also exist.Stem cells often require extensive expansionin vitro, which increases the risk of shortened telomeres, impaired function, and contamination[5].It is common for stem cells to fail to stabilize in the recipient region after implantation, leading to a poor survival.
Therefore, from the application perspective of tissue regeneration, what stem cells need more is a natural biomaterial scaffold.It can provide stem cells with a microenvironment for growth and support for their colonization, adhesion, proliferation, and differentiation[6-10].The dynamic and specific microenvironment of stem cell proliferation and differentiation is called a niche.The main component of the niche is the extracellular matrix (ECM), which can dynamically regulate the behavior of stem cells and provide extracellular clues for stem cell recognition[6,11].The ECM is composed of various collagens, glycoproteins, and growth factors and seems to be a static network structure, but it is actually in a process of continuous remodeling with dynamic interaction with stem cells[12,13].Generally, stem cell proliferation and differentiation are accompanied by changes in the ECM structure.For example, stem cells bind to matrix protein residues to change local conformation[14,15], or stem cell remodeling reveals hidden binding sites of the ECM to promote self-adhesion and proliferation[16,17].
Despite the advances in bionic technology and the rapid development of polymer materials science, there is still a huge challenge to fully simulate the biological properties of the ECM.Most artificial scaffolds fail to meet the requirements of biologically active vectors due to their lack of the ability to induce stem cell differentiation and the potential for dynamic interaction with cells[18-20].Therefore, the acellular matrix of the target tissue/organ is an ideal bioactive scaffold.A cell-free, natural ECM scaffold can be obtained through a previously developed protocol.It is characterized by a rich biomolecular and unique three-dimensional (3D) structure that can play a key role even if the acellular matrix differs from the anatomical region of the donor site[21].
At present, there are many studies on the use of xenogeneic and allogeneic acellular matrix for different hosts[22,23].The risk of immunogenic residues limits the application of xenogeneic tissues[24,25].Human allogeneic tissues may be the most desirable source of the ECM.Adipose tissue comes from a wide variety of sources, and lipoaspirate is largely discarded every year as medical waste.With the rapid development of adipose tissue engineering, many researchers have tried to develop better acellular solutions to obtain decellularized adipose matrix (DAM)[26-28].DAM continues to integrate with surrounding soft tissues and plays an important role in the entire regeneration process of the recipient area[29].
Currently, there are many protocols for the preparation of DAM.Different preparation methods have different effects on key components of DAM, and further affect the growth of stem cells and regeneration of soft tissues[30-34].This review outlines the importance of DAM to provide an inductive microenvironment for stem cells in tissue regeneration.In particular, considering the DAM for tissue engineering purposes, the different decellularization methods used are fully described (Figure 1).In addition, the problems that still need to be addressed with regard to DAM are also described, as well as possible future developments of these emerging bioscaffold materials.
LITERATURE SEARCH
A literature search was conducted using the PubMed Advanced Search Builder.An advanced search was performed using “decellularized adipose tissue OR adiposederived matrix OR acellular adipose matrix OR decellularized adipose matrix” as the title elements, and identified 236 studies.After further analysis and evaluation on whether the title and abstract involve fat-derived ECM and whether the article is written in English, a total of 75 studies were included.
OVERVIEW OF ECM/DAM
The ECM is a 3D complex network structure composed of various collagens and glycosaminoglycans (GAGs), and provides effective biological information for the growth and differentiation of stem cells, and enables cell-cell and cell-ECM dynamic interaction through the establishment of a natural ecological microenvironment[35].Stem cells continue to reshape the microenvironment created by the ECM, while the reshaped the ECM also constantly changes the behavior of stem cells[13].This can keep the growth of stem cells in equilibrium with the degradation of the ECM and play a continuous and stable role in the entire tissue regeneration process[36].At present, the ECM of various tissues including the skin[37], cartilage[38], bone[39], tendon[40,41], skeletal muscle[42,43], blood vessels[44,45], nerves[35,46], cornea[47], heart valves[48,49], myocardium[50,51], lung[52,53], liver[54,55], kidney[56,57], small intestine[58], and bladder[59]has been widely used in clinical or preclinical research in various fields.
In recent decades, the DAM extracted from a large amount of waste adipose tissue has aroused interest among researchers because of its abundant sources and excellent potential in soft tissue regeneration[60].A large amount of adipose tissue can be obtained by using the developed method of degreasing and decellularization[26].The DAM, which provides a natural microenvironment for the growth of stem cells [especially adipose-derived stem cells (ASCs)], has the following characteristics.First, the complex structure is composed of collagens I[22,61,62], IV[26,61,63,64], and VI[65], laminin[22,26,61,62,66,67], fibronectin[34,68], elastin[28], GAGs[22,28,62,63,69], and other biologically active macromolecules.Fibrillar collagen and glycoproteins provide structural stretch resistance and resilience[70], and play an important role in the entire dynamic remodeling process of stem cells.Second, the structure contains growth factors such as vascular endothelial growth factor (VEGF)[22,63,69,71], basic fibroblast growth factor (bFGF)[22,63,71], and transforming growth factor (TGF)-β[23], which are associated with specific ECM domains or proteins and play an irreplaceable role in the entire process of soft tissue regeneration[72,73].
In addition, there are different names about DAM, including decellularized adipose tissue[26,74-77], adipose-derived matrix[23,24], and acellular adipose matrix[78].For the convenience of explanation, this article collectively names DAM from adipose tissue of different sources (including human, pig, mouse,etc.).
DIFFERENT PREPARATIONS OF DAM
There have been many studies on DAM (Table 1).Different preparation methods result in the retention or loss of DAM key components to varying degrees, and affect the growth of stem cells and regeneration status of soft tissues[65,79].The goal of DAM preparation is to remove all immunogenic components (such as nucleic acids and fragments) from all cells, while retaining the biologically active components of the ECM (including collagens, proteins, growth factors, and GAGs) and suitable 3D structure and mechanical properties, to provide host stem cell growth and differentiation microenvironment after transplantation[80].However, all current decellularization schemes will inevitably cause different degrees of damage to the structure and composition of DAM[60].

Table 1 Different studies of decellularization and sterilization methods for preparation of DAM

SDS: Sodium dodecyl sulfate; EDTA: Ethylenediaminetetraacetic acid; SC-CO2: Supercritical carbon dioxide; Gu: guanidine.
The current effective decellularization protocol is achieved by a combination of physical, chemical, and enzymatic methods (Table 2).Usually, the first step is to destroy the cell membrane components by physical means (freezethaw cycle[26]and homogenization[28,81]).Second, chemical methods include the use of detergents[33]/nondetergents[82]to dissolve cytoplasmic and nuclear components and alcohols (such as isopropanol[26,83,84]) to remove lipid residues.Finally, cell residues and degraded nucleic acid fragments are removed by enzymatic methods[28,68,81](including DNase and RNase).The above steps can be combined with continuous mechanical stirring and shaking, to shorten the action time of reagents, improve efficiency, and reduce structural damage[62,85,86].In addition, in order to avoid the immune response caused by the residues of chemical and enzymatic substances, thorough washing at each step is essential[26].Flynnet al[26]was the first to prepare complete DAM through the abovementioned comprehensive method.After 5 d of nondetergent solution, DAM was finally obtained with a high retention rate (30%-45% of the original amount).After a series of characterizations, components such as collagens I and IV and laminin of DAM, which are important for adipogenesis and stem cell proliferation, are retained[26].This method has been widely used and improved by many researchers subsequently.
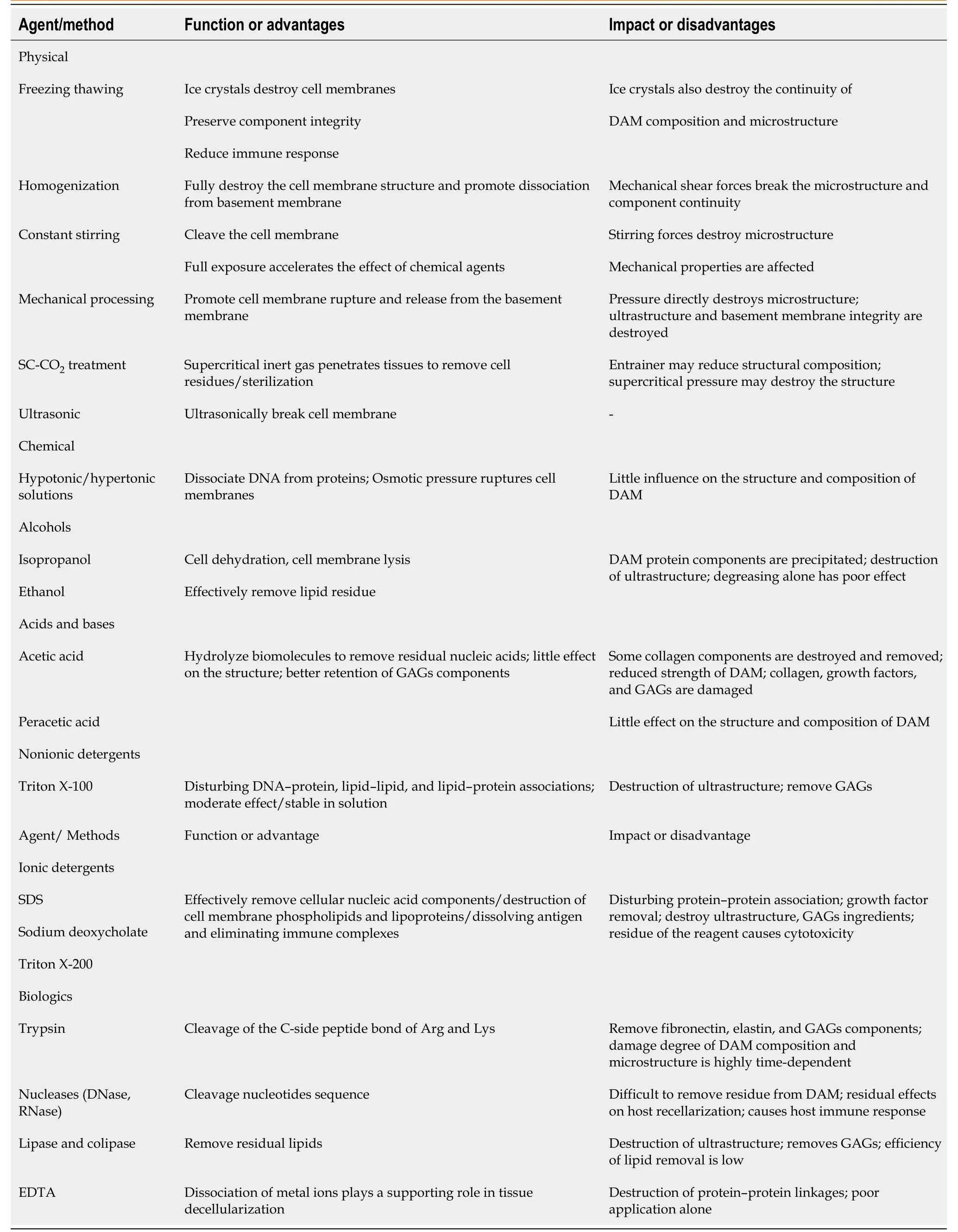
Table 2 Comparison of each physical, chemical, and biological treatments in the adipose tissue decellularization protocols
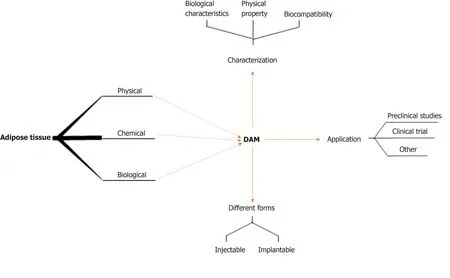
Figure 1 Preparation, characterization, different forms, and applications of decellularized adipose matrix.DAM: Decellularized adipose matrix.
Physical treatment
The physical treatment method has had the following improvements.First, the number of cycles is increased based on three freezethaw cycles[67,68,78,87-89]or the freezing temperature is reduced from -80 to -196 °C (liquid nitrogen)[67,78,88,89]or adding ultrasonic treatment[25,87]during the freeze-thaw process.According to the research, within a certain range (1-5 times), increasing the number of freezethaw cycles will not have much effect on the microstructure of DAM, and cell debris and residues cannot be removed only by freezethaw treatment.With the increase in the number of freezethaw cycles (6-18 times), the microstructure of DAM is damaged[78].Second, Choiet al[28,81]changed the freezethaw treatment to homogenization.The homogenization can quickly and fully damage the cell membrane structure, but the longterm effect of mechanical shear force destroys the microstructure of DAM and results in the loss of specific components (such as laminin)[28,81].Subsequently, Kimet al[90]reduced the time of homogenization, to protect the integrity of DAM structure and composition[23,24].Third, Younget al[62]replaced freezethaw and homogenization with continuous stirring or mechanical pressing, with the aim of accelerating chemical and enzymatic surface contact in the later stages to shorten reaction time[31,86].Fourth, Wanget al[91]tried to use the advanced technology of supercritical carbon dioxide (SC-CO2), and only used ethanol as an entrainer to decellularize and degrease adipose tissue to obtain DAM.Finally, Patiet al[92]abandoned the physical processing steps and directly used chemical and enzymatic methods to obtain DAM[33,34,64].
Chemical treatment
Chemical methods also have different application modifications.Alcohol, acid/base, or ionic/nonionic detergents affect the structure and composition of DAM to varying degrees.Hypertonic saline dissociates DNA from proteins in a gentle way to achieve decellularization and has little effect on the microstructure and composition of DAM[69,71,89,93].Although the types and concentrations of alcohols are not the same, there seems to be no significant difference in lipid removal[25].Alcohols such as isopropanol,n-propanol, and ethanol are superior to lipase in removing lipids from tissues.They can remove lipids in a short period of time, but at the same time, they can also denature the protein components of DAM (such as collagen and LN) and destroy the ultrastructure[22].Therefore, caution should be exercised when using them.Acidic reagents can hydrolyze the biomolecules of tissues, acetic acid may cause damage to certain collagen components and reduce the structural stability of DAM[88], while peracetic acid is a commonly used disinfectant and can also be used as a decellularizing agent because it can gently remove residual nucleic acids.It has little effect on the composition and structure of DAM[22,31,92,94].In general, ionic detergents (including SDS and sodium deoxycholate) are more effective in removing cellular components than nonionic detergents (such as Triton X-100), but they also damage the ultrastructure of DAM, and more GAGs and growth factor components are also removed[95].The comprehensive application of multiple chemical agents may aggravate the loss of DAM components (such as GAGs and collagen) and destruction of the structure (including mechanical properties)[60].
Biological treatment
Nuclease, trypsin, lipase, and EDTA are widely used as biological reagents.The removal of cell debris and residual lipids or degradation of nucleic acid fragments is their main functions.It is also difficult to use enzymes alone to completely remove cell residues.In addition, the residues of enzyme reagents may further affect the growth and differentiation of stem cells, and even cause an adverse immune response in the host.Nucleases (DNase, RNase,etc.) cleave nucleotide sequences after cell membrane rupture and help remove nucleic acid residues[83,84,96-99].Trypsin and a chelator (such as EDTA) are often used in combination.Trypsin can efficiently remove cell residues and destroy collagen, elastin, GAGs, and other components, and the damage to the structure and components of DAM also increases with the time of action (time dependent)[83,84,93,96-99]; EDTA helps DAM proteins dissociate from cells.These two reagents have a poor effect when used alone, and only when combined can they play a synergistic role, and EDTA can reduce trypsin digestion time and reduce tissue damage[22,67,89].Lipase and co-lipase are often used in combination to remove residual lipids[32,62,85].In addition, Poonet al[23]used guanidine alone or combined with hydrochloric acid to remove lipid residues, and the growth factors detected in DAM were well retained[24,100].
Different sterilizations of DAM
After preliminary preparation, it is important to sterilize the prepared DAM when conductingin vivoorin vitroexperiments.This mainly removes bacteria and viruses.At present, the methods for sterilizing biological scaffolds mainly include alcohols, acids, ethylene oxide, UV irradiation, and SC-CO2.The prepared DAM is usually stored in a 1% penicillin and streptomycin solution at 4°C[96-98,101,102], and then sterilized with 70% ethanol solution.Some researchers use 100% ethanol alone to sterilize biological scaffolds[25].Four percent ethanol solution and 0.1% peracetic acid are often used in combination for sterilization, with significant effect[61,92,103], and they also have little effect on the structure and composition of DAM.Wanget al[79]used ethylene oxide for sterilization, but the effect on the microstructure of DAM is unclear.However, there is no doubt that residual reagents after ethylene oxide treatment may cause adverse host reactions and affect the function of the biological scaffold after implantation.In addition, Younget al[62,85]used UV radiation for sterilization.During the sterilization process, the collagen component of DAM may be partially denatured, which may accelerate degradation of the stent material in the body[62,85].More research is needed.As an innovative method, SC-CO2is applied to the decellularization of adipose tissue, and it sterilizes biological materials[91].The specific impact on biological materials needs further comparative research.
CHARACTERIZATION OF DAM
Just as researchers have developed different preparation schemes, there is currently no uniform standard procedure for characterizing DAM materials.It is impractical to remove all cellular residues, but quantitative analysis of residual cellular components (such as phospholipids and double-stranded DNA) is possible.At present, characterization of DAM generally includes: Simple evaluation of the general effects of decellularization and degreasing of materials using simple histological staining and electron microscopy (EM); and DNA quantification, biochemical analysis, and mechanical stress testing to further evaluate various aspects of DAM.This section provides a brief summary.
DAM biological characteristics test
For detection of cell residues, the first approach is histological staining and biochemical analysis.Simple histological staining including hematoxylin and eosin and oil red O staining to roughly check whether the nuclear and lipid components are removed[26,28,31,62,104].Immunohistochemical staining includes DAPI and Hoechst staining to determine the presence of visible nucleic acid and cellular component residues.This is followed by further biochemical tests, including DNA quantification and reverse transcription-polymerase chain reaction analysis.Gilbertet al[105]have suggested the criteria for acellular matrix: DNA content < 50 ng/mg dry weight double-stranded DNA and DNA fragment length < 200 bp.This standard may be one of the most important for the application of biological materials, because hindering the further growth and differentiation of stem cells and causing adverse host reactions may be directly related to DNA residues.
DAM structure and physical property detection
In terms of detecting the structure and composition of DAM, the microstructure and structural stability of DAM are first detected by scanning electron microscopy (SEM) and mechanical stress testing[26,92].As mentioned above, the effect of microstructure on stem cells may be crucial, where stiffness is a key indicator[106].The process of stem cells responding to their environment after sensing external forces is called mechanical transduction.All types of stem cells have the ability to sense the structure and stiffness of DAM[11].Cell morphology, skeleton, and migration can interact with DAM in the short term.The more important effects of proliferation and differentiation are longterm[106].The porosity and 3D microstructure of DAM were observed by SEM[26].Mechanical stress tests include Young modulus, storage modulus, and loss modulus, which are used to comprehensively evaluate the mechanical integrity, elasticity and rheological properties of materials[103].The compression mechanical test of DAM was carried out with a universal testing machine.The sample was compressed to 50% of the initial height at a low constant rate.The compressive modulus was calculated using a linear region of stress-strain curve[103].Perea-Gilet al[107]used the atomic force microscopy to determine the mechanical properties of decellularized myocardium tissue samples, such as stiffness and Young's modulus.This is followed by further analysis of its composition by staining and biochemical analysis.Masson trichrome staining can quickly and easily detect gross collagen components.Immunohistochemical staining can detect components such as types I, IV, and VI collagen, laminin, fibronectin, and elastin in more detail[26,62].However, there is currently no effective detection for the quality of these proteins in DAM.The specific contents of DAM (such as TGF-β and VEGF) and GAGs can be accurately detected and analyzed by ELISA[33].
DAM biocompatibility testing
In terms of biocompatibility, coculture of DAM with mesenchymal stem cells (mainly ASCs) to detect the adhesion and proliferation of stem cells on the material is required[30,69,82].Flynnet al[26]verified the fat regeneration potential of the acellular matrix by detecting expression of adipogenic genes such asPPARγandC/EBPα.They also found that the GAPDH activity of DAM differed when prepared from adipose tissue with different body mass index (BMI; BMI is inversely proportional to GAPDH activity)[26].LIVE/DEAD analysis was performed by staining living and dead cells using a combination of Calcein and EthD-1[28,62]; Kokaiet al[33]used calcein AM to further stain the cocultured material, and then used laser confocal imaging to show different colors to infer the depth of the stem cell infiltration of the scaffold material.SEM at different times shows the dynamic interaction process of stem cells and materials at the microscopic level.At the same time, the authors exposed ASCs to the adipose matrix for 21 d, and then used boron-dipyrromethene staining, followed by confocal imaging to observe the increase in lipid content.After transplanting DAM with/without stem cells into the subcutaneous tissue of animals, hematoxylin and eosin, Masson, and perilipin A immunofluorescence staining were used to observe adipose tissue regenerationin vivo[33].
DIFFERENT FORMS OF DAM
After degreasing and decellularizing, DAM can be processed into different shapes of biological scaffolds and used with or without stem cells.It can be roughly divided into injectable and implantable types according to different usage methods.
The main advantages of injectable DAM are convenience and noninvasiveness, including powders and gels.DAM powder is digested into gel with pepsin, and then the pH is adjusted to the normal range with sodium hydroxide solution.During use and storage, the temperature should be controlled below 10 °C to prevent curing[62].DAM (powder or gel) is usually absorbed to varying degrees after implantation.Some researchers have tried to use polymer crosslinking, which slows down the rate of stent degradation and enhances angiogenesis and fat induction[86].
The advantage of implantable DAM is that the structural integrity is preserved, including foam and sheets.Foam-like DAM is lyophilized into porous foam by dissolving with α-amylase, which has a milder effect than pepsin.Another type of bead foam is that the DAM solution is rapidly frozen after electrospray technology, and then freeze-dried at low temperature.Chemical crosslinking is avoided, andin vivoexperiments have confirmed that foamed DAM has fat-forming ability and biocompatibility[83].The DAM is cast in a superficial mold, and a sheet-like DAM is obtained after freeze-drying.Experiments have shown good mechanical integrity and multicellular compatibility[93].
DAM can also be combined with other artificial composite materials, such as methylcellulose (MC)[100], methacrylated glycol chitosan (MGC)[84], methacrylate chondroitin sulfate (MCS)[84], and polycaprolactone (PCL)[92], to be used as stem cell growth scaffolds.It has been shownin vitrothat composites can effectively enhance host stem cell invasion and angiogenesis[32].The use of PCL/DAM composites as bioink for 3D printing has boomed in recent years.This open porous structured scaffold has been verifiedin vitroto have better oxygen and nutrient exchange capacity than ordinary DAM gels[92,103].
PRECLINICAL STUDIES ON APPLICATIONS OF DAM
At present, as a biological scaffold for tissue engineering, DAM is used alone[33]or in combination with stem cells[69]in various fields, including adipose tissue engineering, wound healing, nerve repair, cartilage and bone tissue engineering, andin vitrobiomimetic system research.
Adipose tissue engineering
DAM is the most widely studied as a filler for soft tissue defects.Stem cells are seeded on DAM and injected or transplanted into subcutaneous tissue, which provides a natural microenvironment for the growth of stem cells to further promote adipogenesis and angiogenesis.After coculture of DAM and ASCs, DAM can express the adipogenesis markers PPARγ and C/EBPα (major regulators of adipogenesis and differentiation) at high levels without exogenous adipogenesis induction compared to ordinary monolayer cultures such as Triplicate tissue culture polystyrene and Cell Aggregate[26].Expression of these two genes plays a cross-regulatory role in the entire adipogenic differentiation and plays an important role in maintaining the transformation of adipocytes to mature phenotypes.After ASCs/stromal cells were seeded in DAM microcarriers and then cultured in a low-shear fine-tuning culture system for adipogenic culture, expression of the adipogenic genesPPARγ,C/EBPα, andLPLwas higher than that of ordinary gelatin microcarriers[30].This indicates that DAM plays an important role in mediating adipogenic differentiation of ASCs.After implanting DAM loaded with ASCs into the subcutaneous tissue of rats or nude mice[69], the implanted area showed significant recellularization and angiogenesis[32,108].This shows that DAM plays an important role in supporting stem cell infiltration and tissue remodeling.Hanet al[98]used ASCs for seeding on DAM bioscaffolds, and then implanted them into the subcutaneous tissue of rats.Cell tracking technology was used to verify that the new adipose tissue originated from the host[98], and ASC-seeded DAM contributed to fat formation by promoting neovascularization and modulating the inflammatory response.In addition, research on the combination of DAM and artificial composites is also developing.For example, light crosslinked MGC/MCS and DAM form a composite biological scaffold.In vitrostudies showed that DAM can also enhance the viability, retention, and lipid accumulation of ASCs.MCS composites containing 5 wt% DAM were transplanted into the subcutaneous tissue of rats.After 12 wk, it was observed that DAM seeded with ASCs significantly increased regeneration of adipocytes[83,84].ASCs were seeded in 3D printed PCL/DAM composite bioscaffolds and then implanted into the subcutaneous tissue of nude mice.The results after 12 wk showed that there were a reasonable number of mature adipocytes and functional blood vessels in the DAM area[92].
Wound healing
Clinically, deep burns or large skin trauma are usually treated by flap transfer surgery.Patients often have infections, fluid loss, and electrolyte disorders[109-112].Leeet al[113]used DAM sheet scaffold dressing to treat full-thickness skin wounds on the back of rats.The results showed that the wound healing rate, epithelial formation rate, and microvascular density were significantly higher than those of ordinary wound dressings[113].Wooet al[114]applied a double-layer dressing (the upper layer was made of titanium dioxide and chitosan film by electrospinning, and the lower layer was DAM) to a full-thickness wound in rats.It was showed that it can accelerate the induction of fresh granulation tissue regeneration and reduce epidermal scar formation.These results indicate that the components of DAM (such as collagen, laminin, fibronectin, and GAGs) and various growth factors (such as VEGF and bFGF) can promote regeneration of the ECM in the wound area, further recruit adipose stem cells, fibroblasts, and epithelial cells to accelerate tissue reconstruction and vascular regeneration[114].
Nerve repair
Regeneration is difficult after nerve tissue damage.Linet al[89]used DAM containing ASCs in a rat cavernous nerve injury model, and showed the best recovery of erectile function in rats with DAM seeded with stem cells, but the results did not reach statistical significance due to large differences.However, we also saw substantial recovery of erectile function and histological improvement associated with DAM seeded with ASCs, which has potential for clinical application in the future[89].
Cartilage and bone tissue engineering
Cartilage is difficult to repair due to its nonvascular nature and long-term wear and tear.Cartilage-derived ECM has been used in research on cartilage regeneration[115,116].The cartilage decellularized matrix seeded with ASCs can completely repair articular cartilage defects with hyaline cartilage.At the same time, the contents of GAGs and type II collagen and biomechanical properties have been proven to be comparable to those of natural cartilage[115].Adipose-derived mesenchymal stem cells are also used for cartilage regeneration, which differentiates into chondrocytes and can produce important proteins required for articular cartilage (such as the mucus glycoprotein Lubricin)[117-119].This alternative treatment has proven to be effective.However, due to limited resources, the prospect of clinical application is limited.Choiet al[81]have prepared an ECM/stem cell composite, which formed cartilage-like tissue after being cultured in cartilage induction medium for 45 d, and at the same time, the expression of cartilage-specific GAGs and type II collagen increased.This shows that DAM containing endogenous active factors can support cartilage differentiation of human ASCs and help with cartilage-specific glycoprotein and collagen synthesis[81], which has potential clinical value in the synthesis of cartilage-like tissue.
Bone has significant capacity of regeneration, but patients with large-scale bone defects need surgical autogenous bone transplantation, which causes damage and infection of the donor site[120-122].Artificial composite scaffolds are poorly biocompatible and cannot support vascular regeneration and bone tissue growth[122], while the source of acellular bone tissue is insufficient to meet clinical needs[123,124].Mohiuddinet al[125]used DAM hydrogels to treat C57BL/6 mice for critical size repair of femoral defects.The results showed that hydrogel can enhance expression of type I collagen and osteopontin, while the hydrogel-treated group significantly enhanced bone regenerationin vivo[125].
Bionic research in vitro
The composition and structure of DAM show that it can mimic the natural microenvironment of stem cells and even tumor cells in the body.Research shows that when seeding on DAM or chemically modified DAM[30,31,33,64,74,84,92,96,126], ASC[76,81,84,89,93,85,82,98,104,127], smooth muscle cells[90], umbilical vein endothelial cells[90], chondrocytes[90], and neuroblasts[25]can maintain high viability and excellent proliferation, indicating that DAM may become the ideal 3D culture system for the large-scale expansion of stem cellsin vitro.Dunneet al[93]used DAM as a 3D cell culture system and established a human breast cancerin vitrobionic system to study the growth of breast cancer cell lines (MCF-7, BT474, and SKBR3) and the sensitivity of anticancer drugs (lapatinib and doxorubicin).This restored the original characteristics of breast cancer cell growthin vivo, and expression of adhesion molecules in tumorsin vivo.This is undoubtedly beneficial to the screening and research of antitumor drugs[93].
CLINICAL TRIAL ON APPLICATION OF DAM
Most studies on DAM were preclinical studies combined with stem cells.Recent research has shown that stem-cell-free DAM can also promote adipose tissue regeneration.For the first time, Kokaiet al[33]applied DAM alone to a clinical trial.DAM prepared from cadaveric human adipose tissue was applied to the subcutaneous tissue of nude mice and the subcutaneous wrist dorsum of humans.After 24 wkin vivo, the material retention rate was 44% ± 16%, and the regeneration of adipocytes could be clearly observed by immunofluorescence assays, such as perilipin A.Clinical trials evaluated biocompatibility, volume retention, and soft tissue regeneration over a 16-wk period.There were wrist pain, redness, swelling, and itching at the initial stage during the observation period, which may have been related to the initial inflammation.At 16 wk, the average graft retention was about 47%.No inflammation or necrosis was observed in pathological observation, and adipose tissue was formed around the dilated vessels.Although the study had many limitations, the role of DAM in promoting adipose regeneration was verified[33].
OTHER APPLICATIONS
As an important endocrine organ, adipose tissue is closely related to many metabolic diseases such as diabetes mellitus (DM).The specific mechanism of how the ECM is involved in regulating adipocyte metabolism is unclear.The relationship between DAM and DM has been the focus of research.The ECM of adipose tissue is closely related to metabolic diseases.Factors such as hypoxia, inflammation, and fibrosis of the ECM are related to insulin resistance and DM[128-132].After collecting visceral and subcutaneous adipose tissue, Bakeret al[99]used metabolic assays to measure glucose uptake, lipolysis, and adipogenesis in adipocytes in normal cell culture and 3D DAM culture.The results show that DAM with diabetes can cause metabolic dysfunction in adipocytes of non-DM patients; nondiabetic DAM can rescue metabolic dysfunction in adipocytes of DM patients.This indicates that the ECM is involved in regulating glucose uptake and lipolysis as a target for manipulating adipose tissue metabolism[99].
Autologous fat transplantation is used in the clinic to treat vocal cord paralysis.Although biocompatible, its unpredictable absorption rate is also a limitation[133,134].Kimet al[100]used the MC/DAM composite hydrogel for rabbit vocal cord paralysis studies, and showed that the composite hydrogel group had no early absorption after 8 wk, and the physiological symmetry of vocal cord vibration returned to normal levels.The composite hydrogel overcomes the shortcoming of the indefinite absorption rate of autologous fat transplantation.Its good biocompatibility and positive functional recovery make it possible for clinical use as stable vocal cord enhancement laryngoplasty[100].
CONCLUSION
In the past 10 years, the preparation of DAM has been improved by different decellularized techniques.The material retains the main collagen components and most structural proteins and growth factors.This biologically active system can recruit host stem cells and mimic the growth microenvironment to promote the regeneration of soft tissues.Furthermore, DAM can be processed into different forms for different applications.For DAM, preliminary progress has been made in soft tissue regeneration and metabolic diseases.The combination of DAM with stem cells or growth factors has important value in preclinical studies such as wound healing, nerve repair, cartilage and bone tissue engineering, and bionic system.It is believed that with further exploration and research on DAM, it will play a major role in the field of stem cells and soft tissue regeneration.
However, residues of chemical and enzymatic reagents in the current preparation methods are still problems to be resolved.At the same time, the microstructural destruction and component loss (such as collagen and protein) caused by decellularized reagents and inefficient acellular technology are problems that require improvement.Will it be possible to develop a high-efficiency and high-retention decellularization technology based on physical methods to obtain a more complete DAM in the future? Considering this, a deep understanding of the cascade interaction in tissue regeneration, which is induced by DAM structural proteins and infiltrating host stem cells, is required.
ACKNOWLEDGMENTS
The authors thank each of their colleagues at the Institute of Plastic Surgery, Xijing Hospital, Fourth Military Medical University for their full cooperation and support.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Potential of transposon-mediated cellular reprogramming towards cell-based therapies
- Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface
- Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species
- Vitamin D and calcium signaling in epidermal stem cells and their regeneration
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
