Adipose-derived stem cell therapy shows promising results for secondary lymphedema
Li-Ru Hu, Jian Pan
Li-Ru Hu, State Key Laboratory of Oral Diseases, West China College of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Li-Ru Hu, Jian Pan, Department of Oral and Maxillofacial Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Abstract
Key words: Secondary lymphedema; Adipose-derived stem cells; Lymphangiogenesis; Stem cells; Cell therapy; Lymphatic regeneration
INTRODUCTION
Secondary lymphedema remains a serious global disease with primary lymphedema due to genetic defects accounting for only a subset of those afflicted[1,2].The common causes of secondary lymphedema are attributed to cancer therapy, parasite infection, and trauma[3].Most sufferers are those with cancer who require radiotherapy or lymph node dissection[4].For example, the development of breast cancer–related lymphedema results from axillary lymph node dissection, chemotherapy, radiotherapy, and postoperative complications[5].Patients with lymphedema suffer swelling, pain, and fatigue, with the dysfunction of the deformed extremities reducing the quality of life and increasing the risk of infection and lymphangiosarcoma[4].
Conservative treatments include pharmacotherapy and physiotherapy (compression and lymphatic drainage massage), whilst surgical treatments include lymphaticovenular anastomosis, lymph node transfer, fluid drainage, and liposuction[6-8].However, no effective treatments for lymphedema are available and impairment during surgical therapy can reversibly aggravate lymphedema.
In the last decade, cell-based therapies have emerged as a research hotspot due to their capacity to promote tissue regeneration.Mesenchymal stem cells (MSCs) are multipotent adult progenitor cells with favorably low immunogenicity and unique regenerative potential[9-11], making them a therapeutic option for lymphatic regeneration.MSCs include three major stem cell types, namely, bone marrow-derived MSCs (BM-MSCs), umbilical cord-MSCs (UC-MSCs), and adipose-derived stem cells (ADSCs).ADSCs have attracted increased attention due to their ease of accessibility, avoidable ethical concerns, and adequate sources.ADSCs grow stably[12]and produce various lymphangiogenic factors such as vascular endothelial growth factor C (VEGFC), making ADSC therapy a promising approach for diseases of the lymphatic system[11].
In this review, we discuss the basic information regarding the pathophysiology of lymphedema including local inflammation, fibrosis, and the deposition of adipose tissue (AT).We discuss the development of lymphedema and outline how ADSCs can improve lymphatic function.Previous studies associated with ADSC-based therapy for the treatment of lymphedema are discussed, including both animal and human studies, with a specific focus on the outcomes of ADSC therapy in the clinic.The focus of this review is to explore the efficiency and feasibility of ADSC-based therapy.In addition, the future perspectives of ADSCs in the field of lymphatic regeneration are discussed.
EMBRYONIC LYMPHANGIOGENESIS
Lymphatic endothelial cells specification
Lymphatic specification can be observed from embryonic day 9.5 (E9.5) in a subset of cells in the walls of the cardinal veins.VEGF-C and its receptor vascular endothelial growth factor receptor 3 (VEGFR3) comprise the most essential signaling pathways during initial lymphatic development, in addition to lymphatic endothelial cell (LEC) proliferation, migration, and maintenance during early embryonic growth[13-15].
Prospero homeobox protein 1 (Prox1) determines the fate of differentiation[16,17], and is a master lymphatic transcription factor during cell proliferation and the maintenance of lymphatic integrity.InProx1knockout mice, LEC budding is observed during the early stages of development, suggesting that the early expression of lymphatic vascular hyaluronan receptor - 1 (Lyve-1) together with that of Prox1 represents the first indication of lymphangiogenesis[18].Coup-TFII directs the polarized expression of Prox1 in endothelial cells within the cardinal vein[19].Notch signaling regulates normal lymphatic vessel patterning, the deletion of which leads to excessive Prox1+ LEC differentiation and lymphatic overgrowth[20].LEC precursors migrate out of the vein under the control of the VEGF-C/VEGFR-3/Prox1 axis[21].
Lymphatic sprouting growth
Mature lymphatic structures are composed of capillaries, pre-collectors, and collecting vessels, and rely on the formation of lymph sacs and lymphatic plexuses.At approximately E10.5, Prox1 + LECs begin to migrate.At E11.5, VEGF-C/VEGFR-3 signaling stimulates lymph sac morphogenesis, and its hyperactivation leads to the overgrowth of lymph sacs[22].
Lymphatic maturation
Lymph sacs and lymphatic plexuses undergo further remodeling to form a functional lymphatic vessel network from E15.5 to early post-birth stages[23].The formation of lymphatic valves, the recruitment of mural granulosa cells, and the deposition of the basement membrane are signs of maturity for collecting vessels.The transition of the LEC junctions from zippers to buttons characterizes the process of lymphatic capillary maturation[24].
PATHOLOGICAL CHANGES DURING LYMPHEDEMA
The pathophysiology of lymphedema remains poorly understood due to the lack of suitable animal models[25,26].Rodent tails and hindlimb models fail to accurately recapitulate latent onset in the human body, which ranges from 3 mo to 3 years[27,28].However, a positive feedback loop is widely accepted during lymphedema development, involving local inflammation, the fibrosis of lymphatic vessels, and the deposition of adipose fat[29,30].Lymphedema is therefore chronic, potentially progressive, and irreversible.
Local chronic inflammation and pathological lymphangiogenesis
In animal models of lymphedema, the infiltration of lymphocytes and macrophages is observed.Lymphatic function can be improved through immunosuppressive drug targeting at T cells, including tacrolimus[31]and atorvastatin[27].In contrast to acute inflammation, active T cells play an important role in the progressive development of lymphedema including neolymphatic vessel formation and fibrosis[4,32].CD4+ cells play a key role in impaired lymphangiogenesis and lymphatic dysfunction, whilst the depletion of CD8+ cells has minimal effects[33].CD4knockout mice with acquired lymphedema exhibit lower levels of swelling and improved lymphatic function.The number of CD4+ cells is also positively associated with the severity of edema[4].However, CD4+ T cells have different roles when cooperating with macrophages.Ogataet al[27]found that the addition of CD4+ T cells had no effect on tube formation.However, when CD4+ T cells were co-cultured with macrophages, new lymphatic tubes were observed.Macrophages are essential during lymphangiogenesis.Recent studies show that RAMP1 signaling accelerates lymphedema by inhibiting the recruitment of macrophages[34].
T cell-derived cytokines including interleukin (IL)-4, IL-13, IL-17, interferon gamma (IFN-γ), and transforming growth factor (TGF)-β1 negatively regulate lymphangiogenesis.In vitro, IL-4, IL-13, and IL-17 have been shown to inhibit LEC proliferation through the downregulation of LEC genes[35].IL-4, IL-13, IL-17, and TGFβ1 participate in the development of fibrosis-related diseases[4].IFN-γ and IL-17 activate macrophages through VEGF-C production during lymphangiogenesis in different disease models.IFN-γ and IL-17 can activate macrophages and enhance VEGF-C production during lymphangiogenesis of different disease models[27].
A chronic inflammatory environment initiates diverse lymphangiogenesis processes[32].Lymphatic vessels do not reform spontaneously, and the remodeling of pre-existing vessels occurs, leading to the dilation of vessels and decreased contractile frequencies[36,37].It remains unclear how VEGF-C/VEGFR-3 signaling regulates lymphedema-induced lymphangiogenesis.
Pathological changes of adipose tissue
A stable lymphatic system is critical for homeostasis, immunity, and lipid reabsorption[33].Adipocytes are the main parenchymal cells in AT that contribute to energy storage and pathogen defense.Adipocytes are sensitive to pathological changes such as inflammation[36].The accumulation of lymphatic fluid that contains free fatty acids can lead to fat deposition by activated adipocytes, upregulating fat differentiation genes[24,35,38].Enhanced lipid storage leads to the hypertrophy and hyperplasia of adipocytes.In AT samples from lymphedema patients, a decrease in elastic fibers and an increase in collagen fibers are observed[30].Active adipogenesis and fibrosis alter the physiological structure of AT.
ADSCs are multipotent cells with the potential to differentiate into multilineage cells including osteocytes, myocytes, chondrocytes, adipocytes, astrocytes, and endotheliocytesin vitroandin vivo[39].However, significantly fewer stem cells exist in pathologic AT compared to normal AT.The differentiation potential of ADSCs to adipocytes can compensate for inadequate lipid storage capacity[40].Lymphedema leads to the consumption of anti-inflammatory macrophages, which play an important role in the prevention of inflammation and tissue repair.Conversely, inflammatory macrophages are prevalent in hypertrophic AT[34].
ADSC-BASED THERAPIES
We searched PubMed, ClinicalTrials.gov, and EMBASE for published articles on lymphedema.“ADSCs”, “stem cell”, “cell therapy”, and “lymphedema” were used as the main search terms.All relevant studies performed from November 2009 to 2019 were selected.In total, five articles reported animal experiments (Table 1) and four reported human experiments (Table 2).In all studies, the location of lymphedema, cell origin, injection methods, evaluation methods, and the results were analyzed.
Animal studies
Mice are the only used animal models for lymphedema, which occurs in either the hindlimbs or tails as a result of circumferential incision.Irradiation is used as an auxiliary method with circumferential incision[41,42].ADSCs are harvested from the AT of the same species from both intraabdominal and inguinal regions.Hwanget al[43]used ADSCs isolated from the human body, and complications related to rejection reactions were not observed.VEGF-C hydrogel sheets when applied to the injection site can reduce edema 3 to 4 d post-treatment.The reduction in the circumference and volume at the edema site following the injection of ADSCs occurs within 2-4 wk of treatment.
The dose of cells injected varied from 1 × 104to 2 × 106, and all showed improved lymphatic function.Yoshidaet al[42]divided mice into groups injected with 1 × 104, 1 × 105, and 1 × 106ADSCs.The number of lymphatic vessels significantly increased at 2 wk in a dose dependent manner.Increased LYVE-1 expression with a treatment dose of 1 × 106cells was significantly higher than that with treatment doses of 1 × 105and 1 × 104cells.Likewise, a higher dose of 1 × 105showed significantly greater LYVE-1 expression than the dose of 1 × 104.Stem cells were subcutaneously injected at the site of lymphedema.Shimizuet al[44]showed that ADSCs stimulate lymphangiogenesis by secreting VEGF-C and through the recruitment of lymphatic endothelial progenitor cells.Ackermannet al[45]reported that ADSC therapy promotes lymphangiogenesis and lymphedema but to lower levels than platelet-rich plasma (PRP).
Human studies
Peña Quiánet al[46]provided a case report on a patient with edematous lower limbs resulting from recurrent lymphangitis.Autologous ADSCs (1-2.2 × 109) were injected and a greater number of new lymphatic ramifications and lymph nodes were observed at 6 mo post-treatmentvialymphoscintigraphy.Toyserkaniet al[47]used autologous ADSCs in a patient suffering breast cancer–related lymphedema with deformed upper limbs.Fat grafting was performed at the same time.A total of eight axilla injections were performed at a total dosage of 4.0 × 107cells.The time of follow-up was 4 mo, and positive outcomes were observed.The reduction of arm volume along with the decrease in heaviness and tension led to a lower requirement for compression therapy.In 2017, Toyserkaniet al[48]enrolled ten patients to explore the feasibility and safety of ADSC therapy.Patients received the same treatment at a slightly higher dose of 5 × 107cells.However, volume reduction was not significant after 6 mo of follow-up.Up to 50% of the patients reported an alleviation of their discomfort and had a lower requirement for conservative management.In 2019, Toyserkani et al[49]performed lymphoscintigraphic evaluations after one year of follow-up.No changes in arm volume and only mild transient complications related to liposuction were noted.

Table 1 Adipose-derived stem cell-based therapy for secondary lymphedema in animals
CONCLUSION
Stem cells can differentiate into multilineage cells that exist in nearly all tissues and organs.In theory, damaged tissues and organs can recover after stem cell implantation.Secondary lymphedema affects millions with sufferers experiencing persistent, uncomfortable, and dysfunctional extremities.However, existing therapies including conservative and surgical methods fail to improve lymphatic function.
ADSCs can be isolated from ATs by mildly invasive procedures.A prominent characteristic of ADSCs is their low immunogenicity, due to the low levels of expression of major histocompatibility complex (MHC) and costimulatory molecules[50].ADSCs produce immunomodulatory cytokines including TGF-β that block IFN-γ-induced MHC expression[51].The downregulation of MHC can avoid immune surveillance, producing immune-privileged cells ADSCs[52].Complications relating to immune rejection are therefore sparse.ADSCs remain stable over long passages and can differentiate with low rates of apoptosis.As such, ADSC-based therapy may play an important role in secondary lymphedema.ADSCs can differentiate into progenitor cells for lymphangiogenesis and secrete VEGF-C.Both animal and human studies show positive outcomes after the injection of ADSCs with minimal complications.ADSC-based therapy is therefore promising for the treatment of secondary lymphedema.
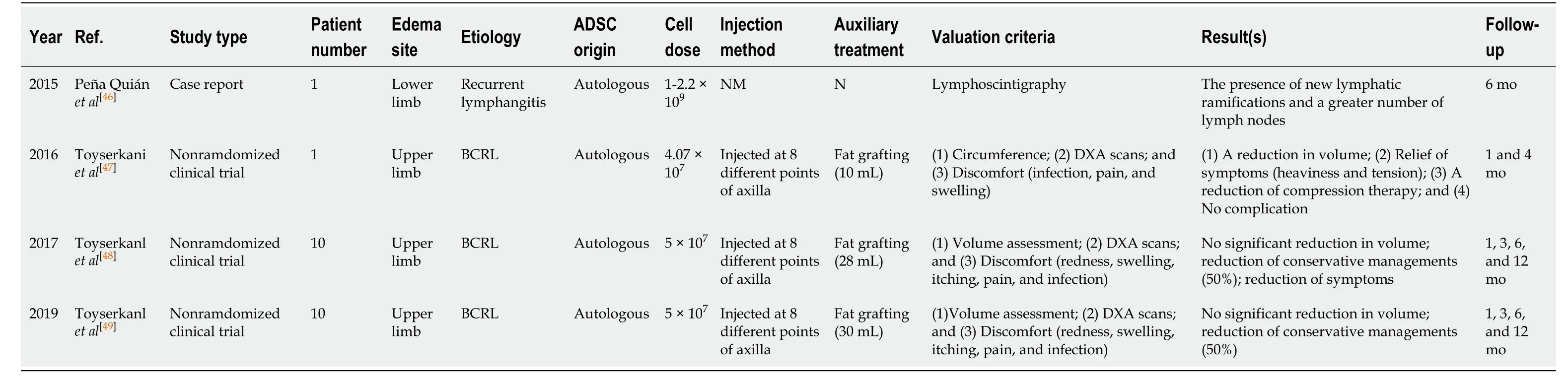
Table 2 Adipose-derived stem cells-based therapy for secondary lymphedema in human
However, some issues remain for ADSC-based therapy.Suitable animal models are required as pathological changes in acute inflammation differ from those of chronic inflammation, producing variable therapeutic outcomes.Second, a larger number of clinical trials with larger samples and longer follow-up periods are required.In addition, the safety of ADSC-based therapy should be assessed.In lung cancer models[53], ADSCs interact with LLC1 cells through their ability to secrete IL-6 and enhance malignant characteristics in vitro and in vivo.We believe that ADSC-based therapy is therefore key to the future treatment of secondary lymphedema.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Potential of transposon-mediated cellular reprogramming towards cell-based therapies
- Approaches to promoting bone marrow mesenchymal stem cell osteogenesis on orthopedic implant surface
- Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species
- Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration
- Vitamin D and calcium signaling in epidermal stem cells and their regeneration
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
