Ischemia/hypoxia inhibits cardiomyocyte autophagy and promotes apoptosis via the Egr-1/Bim/Beclin-1 pathway
Bo SU, Xian-Tao WANG, Yu-Han SUN, Man-Yun LONG, Jing ZHENG, Wen-Hao WU, Lang LI
Department of Cardiology, the First Affiliated Hospital of Guangxi Medical University, Nanning, China
Abstract Background Myocardial injury caused by microvascular obstruction (MVO) is characterized by persistent ischemia/hypoxia (IH) of cardiomyocytes after microembolization. Autophagy and Egr-1 were closely associated with various cardiovascular diseases, including MVO. Bim and Beclin-1 are the important genes for autophagy and apoptosis. We aimed to explore whether the Egr-1/Bim/Beclin-1 pathway is involved in regulating autophagy and apoptosis in IH-exposed cardiomyocytes. Methods Neonatal rat cardiomyocytes exposed to the IH environment in vitro were transfected with lentivirus expressing Egr-1 or Egr-1 shRNA, or further treated with 3-methyladenine(3-MA). The expressions of autophagy and apoptosis-associated genes were evaluated using RT-qPCR and Western blots assays. Autophagic vacuoles and autophagic flux were detected by transmission electron microscopy (TEM) and confocal microscope, respectively. Cell injury was assessed by lactate dehydrogenase (LDH) leakage, and apoptosis was determined by flow cytometry. Results IH exposure elevated Egr-1 and Bim expressions, and decreased Beclin-1 expression in rat cardiomyocytes. Egr-1 overexpression in IH-exposed cardiomyocytes significantly up-regulated the levels of Egr-1 and Bim, and down-regulated the level of Beclin-1. Egr-1 knockdown resulted in down-regulated expressions of Egr-1 and Bim, as well as up-regulated expression of Beclin-1. In addition, Egr-1 knockdown induced autophagy was suppressed by 3-MA treatments. TEM and autophagic flux experiments also confirmed that Egr-1 inhibited autophagy progression in IH-exposed cardiomyocytes. Egr-1 suppression protected cardiomyocytes from IH-induced injury, as evidenced by the positive correlations between Egr-1 expression and LDH leakage or apoptosis index in IH-exposed cardiomyocytes. Conclusions IH-induced cardiomyocyte autophagy and apoptosis are regulated by the Egr-1/Bim/Beclin-1 pathway, which is a potential target for treating cardiomyocyte injury caused by MVO in the IH environment.
J Geriatr Cardiol 2020; 17: 284-293. doi:10.11909/j.issn.1671-5411.2020.05.004
Keywords: Autophagy; Apoptosis; Cardiomyocyte; Egr-1; Ischemia/hypoxia
1 Introduction
The atherosclerotic plaque and thrombosis in coronary artery may lead to acute coronary syndrome (ACS) and cause microvascular obstruction (MVO).[1]The therapeutic efficacy of percutaneous coronary intervention (PCI) may seriously deteriorated in the presence of MVO or no-reflow.[2]A previous report indicated that 57% of patients demonstrated MVO even if PCI was successful.[3]In addition, some evidence has indicated that the presence of MVO or no-reflow predicted poor clinical outcomes independent of myocardial infarct size.[4]Furthermore, patients who displayed ST-segment elevation myocardial infarction (STEMI)with MVO had a three-fold higher mortality rate than those without MVO after PCI.[5]Studies have demonstrated that myocardial injury caused by MVO is characterized by persistent ischemia/hypoxia (IH) of cardiomyocytes after microembolization.[6]However, the molecular mechanisms underlying the regulation of MVO remain elusive.
Autophagy contributes to the maintenance of intracellular homeostasis and plays a significant role in cardiac physiology, and its dysregulation could lead to a variety of cardiovascular diseases.[7,8]Autophagy of cardiomyocytes can be activated under pathological stress conditions to inhibit apoptosis, and the level of apoptosis is negatively correlated with autophagy levels.[9,10]On the other hand, suppression of autophagy can result in cell death and heart dysfunction.[11,12]Therefore, targeted activation of autophagy and inhibition of apoptosis can help maintain the intracellular homeostasis.
Early growth response 1 (Egr-1), associated with various cardiovascular diseases, is a zinc finger transcriptional protein which can be rapidly induced by various stimuli (such as ischemia, hypoxia, vascular injury, etc.).[13-15]Accumulating evidence has revealed that the pathway of Egr-1 linked with Bim and Beclin-1 is involved in regulating autophagy and apoptosis.[16,17]In our previous study, we showed that the Egr-1/Bim/Beclin-1 pathway was involved in regulating coronary microembolization-induced myocardial injury.[18]However, since myocardial injury is often associated with IH, whether the Egr-1/Bim/Beclin-1 pathway is also involved in inhibiting autophagy and promoting apoptosis in IH-exposed cardiomyocytes remain unexplored so far.
In this study, we established anin vitrocell model using primary neonatal rat cardiomyocytes that were exposed to the IH environment, which faithfully mimicked MVO-mediated myocardial injury. Through lentivirus infection mediated gene overexpression and knockdown, we explored the impacts of manipulated Egr-1 expressions on autophagy and apoptosis of IH-exposed cardiomyocytes.
2 Methods
2.1 Cell culture
The animal experiment protocols were approved by the Institutional Animal Care and Use Committee of Guangxi Medical University (Nanning, China). Neonatal cardiomyocytes were separated from the ventricles of neonatal SD rat (1-3 days old) in accordance with a standard protocol.[19]Briefly, the ventricles were digested in trypsin and collagenase II solution, and the cardiomyocytes were collected in supernatant after centrifuging. After removing fibroblasts,the unattached cells were cultivated in high-glucose Dulbecco's Modified Eagle Medium (DMEM; Gibco, USA)supplemented with 10% fetal bovine serum (FBS; Gibco,USA) and 1% penicillin-streptomycin (Solarbio, China).The medium was then replaced by fresh medium after 36 h.Low-glucose DMEM (Gibco, USA) without FBS was chosen as the culture medium to simulate an ischemic environment. The cells were then incubated in a 37°C hypoxia chamber (HERAcell VIOS 160i, Thermo Fisher Scientific,USA) saturated with 92% N2, 5% CO2, and 3% O2(v/v/v) at 37°C.
2.2 Cell treatments
The cardiomyocytes were divided into six groups: normal control group (Con), ischemia/hypoxia group (IH), IH+negative control (NC) group, IH+Egr-1 group, IH+Egr-1 shRNA group, and IH+Egr-1 shRNA+3-MA group (n= 10 rats in each group). Cardiomyocytes in IH group were exposed to IH for 9 h before the transfection and treatments, as previously described.[20]The lentiviral vectors (GeneChem,China) with Egr-1 coding sequence, Egr-1 shRNA and NC shRNA were used to manipulate the expression of Egr-1.The sequences of Egr-1 shRNAs were: 5′-TGCCAGGAGT GATGAACGCAA-3′ and 5′-TCCCAGGACTTAAAGGC TCTT-3′, while the NC shRNA sequence was: 5′-TTCTCC GAACGTGTCACGT-3′. The lentivirus at a multiplicity of infection (MOI) of 50 was used for transfection of cardiomyocytes. The medium was replaced with low-glucose DMEM without FBS after 48 h of transfection, and then cells were incubated in the hypoxia chamber. 3-methyladenine (3-MA)at the dose of 5 mmol/L was used for cell pretreatment 2 h before the lentivirus transfection.
2.3 RNA extraction and RT-qPCR
TRIzol reagent (TaKaRa, Japan) was used for extracting total RNA, and RNA concentration was quantified with the NanoDrop system (Thermo Fisher Scientific Inc., USA).Complementary DNA (cDNA) was synthesized using the TaqMan Reverse Transcription Kit (TaKaRa, Japan) following the manufacturer’s instruction. The mRNA levels of Egr-1, Bim and Beclin-1 were quantified with the SYBR Green I PCR kit (TaKaRa, Japan) through real-time quantitative polymerase chain reaction (RT-qPCR) which was implemented on the ABI PRISM 7500 system (Applied BioSystems, USA). The primers were designed and synthesized by TaKaRa Biotechnology (Dalian, China), and the sequences are listed in Table 1. The relative expression levels of targeted genes were calculated using the 2-ΔΔCtmethod,with normalization to the housekeeping gene GAPDH.
2.4 Western blot
Protein was extracted from the cardiomyocytes with radioimmunoprecipitation assay (RIPA) buffer (Solarbio,China). Bicinchoninic acid (BCA; Beyotime, China) was used to detect the concentration of protein. 10% or 12%sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE) was used to isolate the protein (20 μg) which was subsequently transferred to polyvinylidene difluoride(PVDF) membranes (Merck Millipore, USA). The membranes were incubated for 1 h at room temperature (RT)with 5% nonfat milk plus tris-buffered saline with 0.1%Tween-20 (TBS-T), and then incubated overnight at 4 °C with the primary antibodies against LC3B, P62, Egr-1, Beclin-1, Bim, Cleaved-caspase 3 and GAPDH (CST, USA).After washed three times in TBS-T, secondary antibody(CST, USA) was used to incubate with the membrane for 1 h at room temperature. The protein bands were measured by an imaging system (FLUORCHEMFC3, ProteinSimple, USA)with an enhanced chemiluminescence kit (Thermo Scientific, USA). Densitometric analysis was conducted using the ImageJ software (National Institutes of Health, USA). The alteration levels in protein expression were normalized to GAPDH.
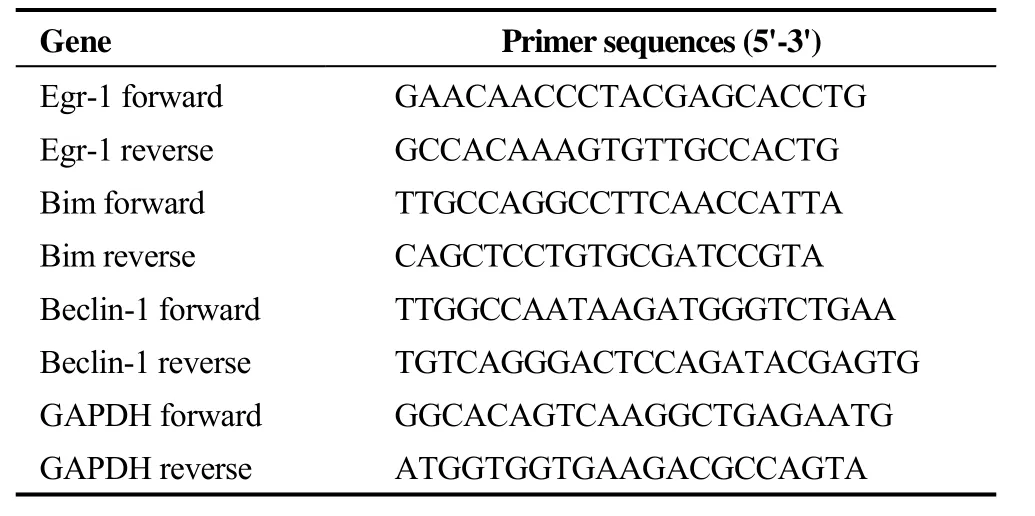
Table 1. The sequences of PCR primers used in this study.
2.5 Transmission electron microscopy (TEM)
The autophagosome and autolysosome were observed using TEM. Cardiomyocytes cultured in 6-well plates were digested and centrifuged. After being fixed with 2.5% glutaraldehyde immediately, cells were transferred to 1% osmium tetroxide. The cells were sequentially dehydrated, embedded, sectioned, mounted and stained. The specimens were observed using a TEM (H-7650, Hitachi, Tokyo, Japan).
2.6 mRFP-GFP-LC3 adenovirus infection
For virus infection of cardiomyocytes, the adenoviruses expressing tandem fluorescent-tagged LC3 (mRFP-GFPLC3; GeneChem, China) were added to primary cardiomyocytes cultured in confocal dishes directly. The transfection medium was replaced by fresh culture medium after two hours, and then the cells were incubated in low-glucose DMEM without FBS under the hypoxia condition for subsequent experiments. The fluorescent dots represented autophagy progression were measured using a confocal microscope (NIKON, Tokyo, Japan). Autophagic flux was evaluated by quantifying GFP, RFP, and merged points (dots/cell).
2.7 Cytotoxicity assay
Primary cardiomyocytes cultured in 6-well plates were respectively transfected with lentivirus as previously described after 24 h of plating. The lactate dehydrogenase(LDH) leakage assay was implemented using the cytotoxicity detection kit (Jiancheng Bioengineering Institute, China)to measure cell injury following the manufacturer’s instruction. The absorbance was measured at 450 nm.
2.8 Flow cytometry
The Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) double staining kit (BD Biosciences,USA) was used to measure cardiomyocytes apoptosis by flow cytometry. Briefly, IH-treated cardiomyocytes were collected, washed with ice-cold phosphate buffered saline(PBS), and resuspended in 500 μL binding buffer. Cells were then incubated with Annexin V-FITC and PI (5 μL each) for 15 min at RT in the dark. Data were acquired with a flow cytometer (FACSCalibur, BD Biosciences, USA)within one hour, and analyzed with the FlowJo software(BD Biosciences, USA).
2.9 Statistical analysis
Data are expressed as mean ± SD and analyzed by one-way analysis of variance (ANOVA). All statistical parameters were calculated using SPSS 22.0 software (SPSS Inc., USA). Differences withP <0.05 were considered statistically significant.
3 Results
3.1 Manipulating Egr-1 expression in cardiomyocytes affected mRNA levels of Bim and Beclin-1 under IH condition
Manipulation of Egr-1 expression was achieved through infection of cells with the lentivirus expressing Egr-1 or Egr-1 shRNA. The RT-qPCR results indicated that Egr-1 overexpression during IH significantly up-regulated the levels of Egr-1 mRNA and Bim mRNA, and down-regulated the level of Beclin-1 mRNA. Conversely, the levels of Egr-1 mRNA and Bim mRNA decreased, while Beclin-1 mRNA level increased upon shRNA-mediated knockdown of Egr-1(Figure 1). Collectively, these results revealed that Egr-1 is involved in regulating the transcriptional expression of Bim and Beclin-1 following IH in rat cardiomyocytes.
3.2 Egr-1 was involved in regulating the expression of autophagy associated proteins in IH-exposed cardiomyocytes
The expressions of LC3II, P62 and Beclin-1 were closely associated with autophagy progression. Therefore, we detected the protein levels of these molecules by western blot assays. The results demonstrated that the expressions of LC3II and Beclin-1 proteins were significantly increased after shRNA-mediated Egr-1 knockdown, while the expressions of P62 and Bim proteins were significantly decreased.Conversely, the expressions of LC3II and Beclin-1 proteins were decreased after Egr-1 overexpression, while the expressions of P62 and Bim proteins were increased dramatically. As expected, the expressions of LC3II and Beclin-1 proteins were obviously decreased upon treatments with the autophagy inhibitor 3-MA in Egr-1 knockdown cardiomyocytes (Figure 2). Taken together, these results indicated that Egr-1 was involved in autophagy inhibition in IH-exposed cardiomyocytes, which was associated with the Egr-1/Bim/Beclin-1 pathway.

Figure 1. Alteration of Egr-1 expression impacted the mRNA levels of Bim and Beclin-1 in cardiomyocytes under IH condition.The expressions of Egr-1 (A), Bim (B) and Beclin-1 (C) mRNA levels were determined by RT-qPCR. n ≥ 3 for each group; *P < 0.05 and**P < 0.01 compared with the control group; ΔP < 0.05 and ΔΔP < 0.01 compared with the IH group; ∇P < 0.05 and ∇∇P < 0.01 compared with the IH+shRNA group. Con: control; IH: ischemia/hypoxia; NC: negative control; shRNA: Egr-1 shRNA; 3-MA: 3-methyladenine.
Figure 2. Egr-1 inhibited the expression of autophagy associated proteins in IH-exposed cardiomyocytes. Representative western blot images show the target bands of Egr-1/Bim/Beclin-1 (A) and LC3/P62 (E) in six groups. The expressions of Egr-1 (B), Bim (C), Beclin-1(D), LC3II (F) and P62 (G) were quantitated by normalization to GAPDH. N ≥ 3 for each group; *P < 0.05 and **P < 0.01, compared with the control group; ΔP < 0.05 and ΔΔP < 0.01 compared with the IH group; ∇P < 0.05 and ∇∇P < 0.01 compared with the IH+shRNA group.Con: control; IH: ischemia/hypoxia; NC: negative control; shRNA: Egr-1 shRNA; 3-MA: 3-methyladenine.
3.3 Egr-1 inhibited autophagy progression in IH-exposed rat cardiomyocytes
The formation of typical double membrane structure means the emergence of autophagic vacuoles in cells, which can be measured by TEM. When the intracellular components are surrounded by double membrane-bound autophagic vesicles, they will form autolysosomes with lysosomes and subsequently degrade, resulting in swelling of intracellular mitochondria. Our TEM results suggested that the amounts of autophagosomes were increased after shRNAmediated knockdown of Egr-1 in cardiomyocytes. Conversely, Egr-1 overexpression led to mitochondrial swelling and autophagosomes reduction (Figure 3).
The accumulation of LC3 was resulted from the increase in autophagosome formation or the reduction in the infused autophagosome-lysosomes. Therefore, we transfected mRFPGFP-LC3 adenovirus to IH-exposed rat cardiomyocytes with various treatments, and further detected the formations of autolysosomes (red) and autophagosomes (yellow) by confocal microscope. The autophagic flux experiments demonstrated that the intracellular dots increased obviously after viral transfection of Egr-1 shRNA, indicating the enhancement of autophagic flux. On the contrary, Egr-1 overexpression, as well as additional 3-MA treatments in the group with Egr-1 knockdown, markedly reduced the autophagic flux in IH-exposed rat cardiomyocytes (Figure 4).
3.4 Egr-1 suppression protected cardiomyocytes from IH-induced injury
The levels of LDH and apoptosis can reflect cardiomyocytes injury. We found that IH exposure increased the LDH levels in rat cardiomyocytes, which was significantly attenuated by additional transfection of Egr-1 shRNA (Figure 5A). In addition, IH exposure also increased the level of Cleaved-caspase 3, a key apoptosis protein, and this alteration was significantly augmented by Egr-1 overexpression and reversed by Egr-1 knockdown (Figure 5B and 5C).Furthermore, Annexin V-FITC/PI staining-based flow cytometry assay also showed that cardiomyocytes apoptosis was increased during IH exposure and further augmented by Egr-1 over-expression, but was markedly attenuated after Egr-1 silencing (Figure 5D and 5E). Therefore, injury of cardiomyocytes caused by the IH environment can be inhibited by Egr-1 suppression, as evidenced by reduced LDH levels and apoptosis rates in cardiomyocytes.

Figure 3. Autophagic vacuoles were reduced after Egr-1 over-expression and increased following Egr-1 knockdown in IH-exposed cardiomyocytes. Autophagic vacuoles were measured by TEM, and the representative images are shown with red arrows indicating autophagolysosomes or autophagosomes. Magnification 30,000 ×. Con: control; IH: ischemia/hypoxia; NC: negative control; shRNA: Egr-1 shRNA; 3-MA: 3-methyladenine.

Figure 4. Autophagic flux in IH-exposed rat cardiomyocytes with manipulated Egr-1 expression measured by confocal microscope.The representative images show the fluorescence of GFP and mRFP in cardiomyocytes, and quantifications on the numbers of the autolysosomes (white) and autophagosomes (black) per cells in 6 groups. Magnification 400 ×. N ≥ 3 for each group; *P < 0.05 compared with the control group; ΔP < 0.05 compared with the IH group; ∇P < 0.05 compared with the IH+shRNA group. Con: control; IH: ischemia/hypoxia;NC: negative control; shRNA: Egr-1 shRNA; 3-MA: 3-methyladenine.
4 Discussion
MVO frequently occurs and is considered as a serious complication during ACS and PCI. Many studies have shown that the presence of MVO is strongly associated with poor prognosis, including increases in mortality and pathological ventricular hypertrophy.[21,22]It is indicated that myocardial injury caused by MVO is characterized by persistent IH of cardiomyocytes after microembolization.[6]In this study, we identified an important pathway involving the molecules Egr-1, Bim and Beclin-1 in regulating autophagy and apoptosis ofin vitroIH-exposed rat neonatal cardiomyocytes (Figure 6). Our data suggest that targeted downregulation of Egr-1 expression can promote autophagy and inhibit apoptosis in cardiomyocytes.

Figure 5. Egr-1 suppression protected cardiomyocytes from IH-induced injury. (A): The levels of LDH leakage from cardiomyocytes in the indicated six groups were quantitated; (B & C): the levels of Cleaved-caspase 3 protein expression in the indicated 6 groups were detected by western blots; (D & E): the apoptosis levels of cardiomyocytes in the indicated six groups were measured by flow cytometry. N ≥ 3 for each group; *P < 0.05 and **P < 0.01 compared with the control group; ΔP < 0.05 and ΔΔP < 0.01 compared with the IH group; ∇P < 0.05 and ∇∇P < 0.01 compared with the IH+shRNA group. Con: control; IH: ischemia/hypoxia; NC: negative control; shRNA: Egr-1 shRNA;3-MA: 3-methyladenine.
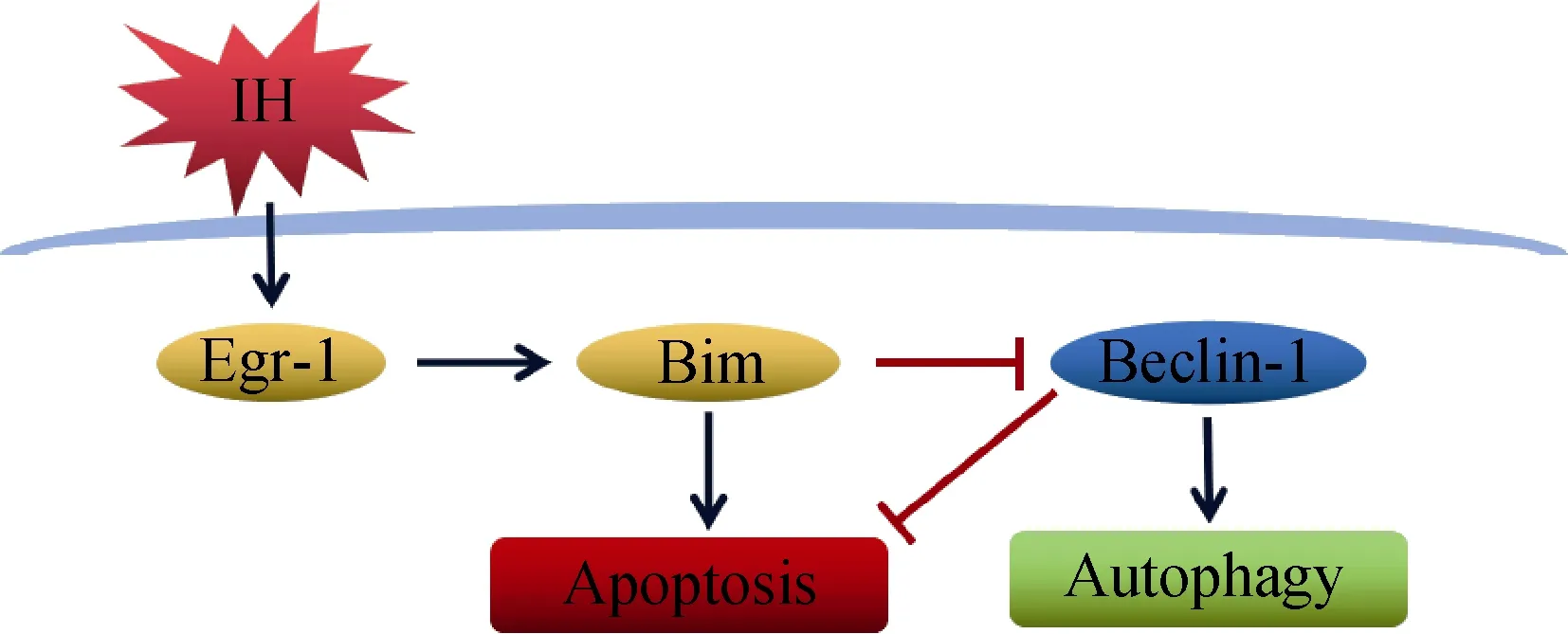
Figure 6. The molecular mechanism of inhibiting autophagy and promoting apoptosis through the Egr-1/Bim/Beclin-1 pathway in IH-exposed cardiomyocyte. IH inhibited autophagy and promoted apoptosis in rat cardiomyocte by the signaling pathway, which up-regulated the expression of Egr-1, and then activated Bim and suppressed Beclin-1. Based on the results in this study, IH-induced cardiomyocyte autophagy and apoptosis may be associated with the Egr-1/Bim/Beclin-1 pathway. IH: ischemia/hypoxia; →: promotion; ⊥: suppression.
Continuous IH can result in cardiomyocytes apoptosis or necrosis.[23]The apoptosis and necrosis in cardiomyocytes caused by IH may lead to left ventricular remodeling and cardiac dysfunction.[24]In addition, autophagy plays significant physiological roles in cardiac physiology and homeostasis, and maintains normal function in response to stress,such as ischemia and heart failure.[25,26]On the contrary,dysregulated autophagy is associated with a variety of cardiovascular diseases.[27]We observed that IH stimulation significantly decreased LC3II expression, and increased P62 and Egr-1 expressions, indicating a negative correlation between Egr-1 expression and autophagy induction in cardiomyocytes. Indeed, further experiments confirmed that under the IH condition, Egr-1 overexpression suppressed autophagy while Egr-1 knockdown enhanced autophagy, as evidenced by western blot assays, TEM and autophagic flux results.
Egr-1 is an immediate-early gene in cardiovascular disease, which can regulate many downstream genes that are involved in cardiomyocytes injury via various pathways in adverse conditions.[28,29]Evidence showed that Egr-1 can target Bim to promote apoptosis.[16]Bim, as one of BH3-only proteins, is the pivotal regulatory protein in apoptosis,which can suppress pro-survival proteins or motivate proapoptotic proteins to initiate apoptosis pathway in adverse stimuli.[30,31]Moreover,in vitrostudies revealed that hypoxia with low glucose can distinctly promote the expression of Bimand induce cardiomyocytes apoptosis.[32]In addition, Bim is also an important protein that regulates autophagy. Some studies have reported that Bim negatively regulates autophagy through the cytotoxic effects, and the role of inhibiting autophagy is independent of its proapoptotic function.[17]The effects of Bim on autophagy appear to depend on Beclin-1, and Bim interacts with Beclin-1 and inhibits autophagy.[33]Beclin-1 is a vital autophagic protein which can regulate both autophagosome formation and processing. The function of cardiomyocytes was dramatically influenced by the alteration of Beclin-1 expression, which positively or negatively associates with the autophagy levels.[34]We revealed a negative correlation between the expression levels of Egr-1 and Beclin-1 in IH-exposed cardiomyocytes. The expressions of Bim and Beclin-1 at both mRNA and protein levels changed significantly following overexpression or suppression of Egr-1 according to our results of RT-qPCR and western blots. These results indicated that Egr-1 is involved in regulating the expression of Bim and Beclin-1, and the Egr-1/Bim/Beclin-1 pathway can be activated by IH.
It is worth noting that the significantly decreased autophagy caused by 3-MA treatment in Egr-1 knockdown cells was not associated with further Egr-1 downregulation,compared with the Egr-1 shRNA alone group. Therefore,the Egr-1/Bim/Beclin-1 pathway seems to be independent of the molecular mechanism of 3-MA, which inhibits autophagy by blocking autophagosome formation via the inhibition of type III Phosphatidylinositol 3-kinases.[35]In addition,further 3-MA treatment in Egr-1 knockdown cells resulted in increased apoptosis index, which supports the antagonistic relationship between autophagy and apoptosis. Indeed,autophagy activation is likely to inhibit apoptosis to improve cellular conditions.[36]According to the results of the expressions of the key autophagic proteins and autophagic flux experiments, administration of Egr-1 shRNA can activate autophagy in rat cardiomyocytes. Meanwhile, it can attenuate cleaved caspase3 expression and diminish the leak of LDH after exposure to IH. Therefore, suppression of Egr-1 can promote autophagy and protect cardiomyocytes during IH.
In summary, IH-induced cardiomyocyte autophagy and apoptosis are associated with the Egr-1/Bim/Beclin-1 pathway. Egr-1 inhibits cardiomyocyte autophagy and promotes apoptosis at least partially through the Bim/Beclin-1 pathway. Suppression of Egr-1 expression protects cardiomyocytes from injury through activating autophagy and reducing apoptosis. These findings suggest that the Egr-1/Bim/Beclin-1 pathway is a feasible intervention target for prevention and therapy of cardiomyocyte injury caused by MVO in the IH environment.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant No. 81770346) and the Innovation Project of Guangxi Graduate Education (Grant No. YCBZ2019040).
 Journal of Geriatric Cardiology2020年5期
Journal of Geriatric Cardiology2020年5期
- Journal of Geriatric Cardiology的其它文章
- Long-term follow-up of antithrombotic management patterns in patients with acute coronary syndrome in China
- Relationship between high sensitivity C-reactive protein and angiographic severity of coronary artery disease
- Association between serum uric acid level and endothelial dysfunction in elderly individuals with untreated mild hypertension
- Association of frailty with all-cause mortality and bleeding among elderly patients with acute myocardial infarction: a systematic review and meta-analysis
- Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients
- What is the cause of the neck hematoma? A rare complication of percutaneous coronary intervention of acute coronary syndrome: a case report
