Long-term follow-up of antithrombotic management patterns in patients with acute coronary syndrome in China
Xiao-Ning HAN, Shu-Bin QIAO, Jun-Bo GE, Ya-Ling HAN, Ji-Yan CHEN, Zu-Yi YUAN,Bo YU, Jie JIANG,#, Yong HUO
1Department of Cardiology, Peking University First Hospital, Beijing, China
2National Center for Cardiovascular Diseases, Fuwai Hospital, Beijing, China
3Department of Cardiology, Zhongshan Hospital Affiliated to Fudan University, Guangzhou, China
4Department of Cardiology, the General Hospital of Shenyang Military, Shenyang, China
5Department of Cardiology, Guangdong General Hospital, Guangzhou, China
6Department of Cardiology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
7Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, China
Abstract Objective To describe the long-term antithrombotic management patterns (AMPs) and clinical outcomes of Chinese patients with acute coronary syndrome (ACS). Methods This was an observational, multicenter, longitudinal cohort extension study of Chinese patients who had completed the EPICOR Asia 2-year follow-up study post-hospitalization for an ACS event. Changes in AMP and clinical outcomes for up to 5 years post-ACS event were evaluated. Results Overall, 2334 patients with ACS were enrolled at 49 sites. The mean age was 61.6 years and 76.3% were men. By study end, 2093 patients completed the 3-year follow-up. At baseline (2 years post-ACS event), 72.4% of patents received one antiplatelet (AP) medication, with aspirin being the preferred one. A small proportion of patients (21.5%) was treated with two or more APs (2+ AP), and even fewer patients (6.1%) did not receive any AP medication at baseline. Upon study completion, the proportion of patients without AP therapy increased to 13.6%, while the percentage of patients on one AP and 2+ AP decreased to 69.3% and 17.1%,respectively. Numerically, a higher incidence of clinical events (composite of all-cause mortality, myocardial infarction, stroke) was observed for the 2+ AP (13.2%) subgroup than for the no AP (10.5%) and one AP (8.6%) subgroups. Furthermore, the 2+ AP subgroup exhibited the greatest number of bleeding events, outpatient visits, and hospitalization rates. Unlike myocardial infarction or stroke, bleeding events prompted an adjustment in AMP. Conclusion Most patients in China received at least one AP medication up to 5 years after an ACS event.
J Geriatr Cardiol 2020; 17: 246-255. doi:10.11909/j.issn.1671-5411.2020.05.008
Keywords: Acute coronary syndrome; Antithrombotic agents; Antithrombotic management patterns; Observational study; Real-world
1 Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide.[1]Acute coronary syndrome (ACS), a serious manifestation of CVD, is comprised of ST-segment elevation myocardial infarction (STEMI),non-ST-segment elevation myocardial infarction (NSTEMI),and unstable angina (UA).[2]While mortality rates due to ACS are decreasing in several high-income countries, currently in China, both the incidence and associated mortality rate of ACS are on the rise.[3-7]Furthermore, China is predicted to witness an almost 69% increase in ACS incidence between 2020 and 2029, which amounts to an estimated 7.8 million new events of myocardial infarction (MI) and UA.[8]A recent study showed that the treatment costs of ACS in China far exceed those in high-income economies, including Hong Kong, the Republic of Korea, and Singapore.[9]If not adequately managed, ACS will impart a substantial socioeconomic burden in China.[7]
ACS is caused by acute thrombotic occlusion of coronary arteries.[10,11]Hence, antithrombotic therapies play a critical role in the treatment and post-discharge management of ACS.[12]The American Heart Association (AHA) and the American College of Cardiology (ACC) recommend the use of dual antiplatelet therapy (DAPT) with low-dose aspirin and a P2Y12receptor antagonist, such as clopidogrel, prasugrel, or ticagrelor, for at least 1 year following an ACS event, and the long-term use of aspirin thereafter.[13]The European Society of Cardiology (ESC) notes that the duration of DAPT can be extended for up to 30 months in select patients.[14,15]
Despite widespread dissemination of ACS management guidelines, to date, few studies have investigated their implementation in China.[16,17]Furthermore, there is little information regarding clinical outcomes associated with the long-term use of antithrombotic regimens. Registry data analysis can provide real-world evidence that is representative of patients seen in clinical practice and can assist in the refinement of ACS management strategies to ultimately improve patient outcomes.[18]
The Long-tErm follow-uP of antithrombotic management patterns In acute CORonary syndrome patients (EPICOR) in Asia is an observational, longitudinal cohort study that was designed to evaluate antithrombotic drug usage in patients who were hospitalized for ACS and survived to hospital discharge.[19]The EPICOR Asia-China Extension study continued to document the long-term antithrombotic management patterns (AMPs) of Chinese patients with ACS from the EPICOR Asia study. This extension study also investigated the impact of AMPs on clinical outcomes and healthcare resource consumption. Here, we present the data for AMPs and associated outcomes in Chinese patients 2-5 years after an ACS index event.
The primary objective of the study was to document the long-term (2-5 years after an index ACS event) impact of AMPs in a real-life setting for patients with a history of ACS. Specifically, the study aimed to determine the number and type of antiplatelet (AP) medications used at baseline (2 years post-ACS) until 5 years post-ACS. Other objectives of this study were to evaluate: (1) the incidence of clinical events, (2) the consumption of healthcare resources (frequency and total cost of outpatient visits and hospitalizations per year), and (3) the quality of life (QoL) for the enrolled patient population 2-5 years after the ACS event.
2 Methods
2.1 Study design
The EPICOR Asia-China Extension study was an observational, longitudinal cohort study conducted across 49 sites in China (NCT01361386; Supplementary Figure S1). Participants were enrolled between December 17th, 2014, and November 26th, 2015. Participants included Chinese patients who had completed the EPICOR Asia study, a 2-year follow-up study that monitored ACS patients post hospital discharge.[19]For this study, ACS events included STEMI,NSTEMI, or UA.
2.2 Patient selection
Eligible patients were 18 years or older, had enrolled in the initial EPICOR Asia study, and had completed the 2-year follow-up.[19]Exclusion criteria included any condition/circumstance that could limit the completion of follow-up, such as the presence of comorbidities that could limit life expectancy, current participation in an interventional clinical trial, or the use of ticagrelor (or an off-label drug) for more than 12 months after their ACS event.
The EPICOR Asia-China Extension study aimed to reach a study population size of 3000 patients. This allowed for a 20%-30% expected event rate for clinical outcomes, including death, thromboembolic events, and bleeding events.[20]
2.3 Data collection
Demographic information was collected from each patient upon enrollment in the extension study. This information included physical characteristics, medical history,medication use, and response to the European Quality of Life-5 Dimensions Questionnaire (EQ-5D). Baseline values were defined as 2 years after the ACS event. The follow-up period was 2.5-5 years after the ACS event (0.5-3 years after enrollment into the EPICOR Asia-China Extension study). Patients were contacted by telephone every 6 months until 5 years after the ACS index event. The follow-up period data consisted of clinical event occurrence (including composite clinical events of all-cause mortality, MI, and stroke, and bleeding events), the prescription status for antithrombotic medication, healthcare resource utilization, and QoL assessment (EQ-5D).
2.4 Statistical analysis
All analyses were performed on the full analysis set(FAS) population, comprising all Chinese patients enrolled into the EPICOR Asia 2-year study. Descriptive statistics were used to assess differences in AMP, clinical events,healthcare resource consumption, and QoL for the study population. For continuous variables, the sample size (n),mean, median, standard deviation (SD), and minimum-maximum range are described. For categorical variables, the sample size (n), frequency, and percentage are presented. Kaplan-Meier analysis was used to evaluate the cumulative incidence of the first clinical outcome occurrence.
AMPs were also assessed in patients who were stratified according to the following criteria: ACS final diagnosis(STEMI, NSTEMI, or UA), type of surgery for ACS index event, and AMP at baseline (2 years after the ACS index event). The type of ACS surgery was categorized as: (1)invasive, which included patients who underwent percutaneous coronary intervention (PCI); or (2) coronary artery bypass grafting (CABG); or (3) non-invasive. The AMP subgroup was applied to the FAS, ACS final diagnosis, and surgery subgroups.
2.5 Ethics approval
This study was performed in accordance with ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonization (ICH),Good Clinical Practice (GCP), and applicable legislation on non-interventional studies. Patients provided written informed consent prior to enrollment.
3 Results
3.1 Patient disposition
In total, 2334 patients were enrolled into this extension study, of whom, 2093 (89.7%) completed a 5-year followup after the ACS index event (Figure 1). The remaining 241(10.3%) patients discontinued participation from the study,due to loss of follow-up (140 patients, 58.1%), death (65 patients, 27.0%), and voluntary discontinuation (27 patients,11.2%). From the initial EPICOR Asia study, three patients did not complete the 2-year follow-up, and two subjects continuously used ticagrelor for over 12 months. These five patients were still included in the FAS. For all enrolled patients, the mean duration of follow-up was 4.98 years post-ACS.
3.2 Demographics and clinical characteristics
Patient demographic and baseline characteristics are summarized in Table 1. The mean age was 61.6 ± 10.7 years and 76.3% were men. Most study participants (76.3%) resided in urban areas and 2283 (97.8%) patients had medical insurance coverage. The principal ACS diagnosis in the study population was STEMI (49.7%, 1160/2334), followed by UA (35.6%, 831/2334) and NSTEMI (14.7%, 343/2334).Most patients in the study had undergone an invasive operation (CABG or PCI) upon experiencing the ACS index event (79.1%, 1846/2334).
3.3 AMP trends 2 years post-ACS
AMPs for the FAS population and subgroups at study entry are summarized in Table 2. At baseline, 1690 patients(72.4%) were treated with one AP, with most receiving aspirin (68.4%, 1597/2334). Fewer patients were treated with 2+ APs at baseline (21.5%, 501/2334). Aspirin and clopidogrel were the most frequently prescribed combination in the 2+ AP subgroup (21.2%, 495/2334). The remaining 143(6.1%) patients were not receiving AP therapy at baseline.

Figure 1. EPICOR Asia-China Extension study: patient disposition. * Five patients failed to fulfill the inclusion and exclusion criteria.Three patients did not complete the 2-year follow-up for the EPICOR Asia study. Two patients continuously used ticagrelor for over 12 months after their ACS index event. Despite these deviations, these patients were included in the full analysis set. ACS: acute coronary syndrome.

Table 1. Demographic characteristics of participants enrolled into the EPICOR Asia-China Extension study.
When examining AMPs within the ACS subgroups at baseline, more patients in the NSTEMI subgroup (28.9%,99/343) were treated with 2+ AP than those in the STEMI(20.7%, 240/1,160) or UA (19.5%, 162/831) subgroup. Furthermore, a greater proportion of patients who had undergone invasive ACS surgery were treated with 2+ AP (22.9%,423/1846) at baseline than that of patients who had undergone non-invasive ACS surgery (16.0%, 78/488).
3.4 Long-term AMP changes post-ACS
AMPs for FAS patients were assessed every 6 months during the 2-5 years follow-up period. AMPs were also broken down by ACS diagnosis and operation subgroup(Figure 2). Overall, there was an increase in the proportion of patients whose AMP changed from baseline during the study (Supplementary Figure S2A). Closer examination of AMP subgroups revealed that the percentage of FAS patients receiving no AP therapy increased during the study(Figure 2A). By study completion (5 years post-ACS event),the proportion of patients with no AP therapy was 13.6%(308/2263). A corresponding decrease in the number of FAS patients in both the one AP and 2+ AP subgroups was noted and, by study end, the percentage of patients in these two subgroups was reduced to 69.3% (1569/2263) and 17.1% (386/2263), respectively. In addition, the percentage of patients remaining in the one AP and 2+ AP subgroups reflected this decline (Supplementary Figure S2B).
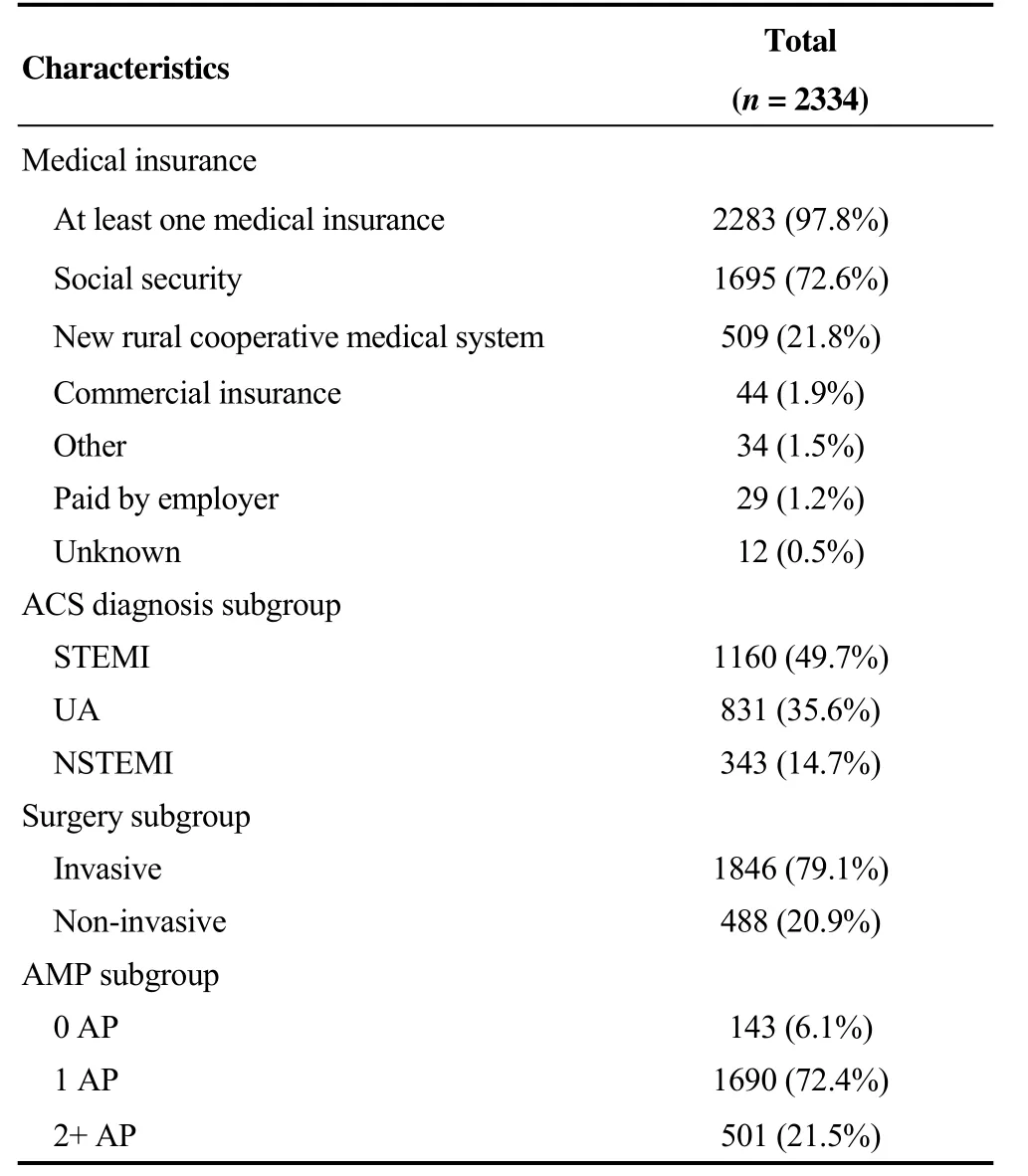
Table 1. Cont.

Table 2. AMPs at baseline for the study group.
The increasing number of patients who received no AP therapy during the study was noted for the three ACS events,as well as for the two subgroups that underwent surgery(Figure 2B and 2C). The rate of AMP change from baseline to study completion was highest in patients with NSTEMI(23.8%, 78/328), followed by those with STEMI (19.4%,219/1128) and UA (17.2%, 139/807) (Supplementary Figure S2C). However, both the invasive and non-invasive surgery subgroups demonstrated a similar rate of AMP change from enrollment to study completion, at 19.2% (345/1796)and 19.5% (91/467), respectively (Supplementary Figure S2C).
3.5 AMP medication and clinical events
Clinical event occurrence over the study duration was assessed in the FAS population and in subgroups, according to AMP at baseline. Among the 2334 patients in the study,9.7% of patients (227/2334) exhibited 286 composite clinical events of all-cause mortality, MI, and stroke. The number of FAS patients who experienced composite clinical events was numerically higher in the 2+ AP subgroup(13.2%, 66/501) than in the no AP (10.5%, 15/143) and one AP subgroups (8.6%, 146/1690). The cumulative incidence for all predefined clinical events is summarized in Table 3.
3.6 Mortality
By study end, there were 65 deaths (2.8%, 65/2334) in the FAS population. The cumulative incidence rate for allcause mortality was slightly higher in the no AP subgroup(4.2%, 6/143) than in the one AP (2.7%, 46/1690) and 2+AP (2.6%, 13/501) subgroups (Table 3).
3.7 MI and stroke
In the FAS population, 105 (4.5%) patients experienced 120 MI events and 88 (3.8%) patients experienced 101 stroke events. The number of patients who experienced MI was higher in the 2+ AP subgroup (7.8%, 39/501) than in one AP (3.6%, 61/1,690) and no AP (3.5%, 5/143) subgroups. Stroke incidence was lowest in the patients who were not receiving AP therapy. However, in general, the number of events was low and the differences between the AMP subgroups was minimal (Table 3).
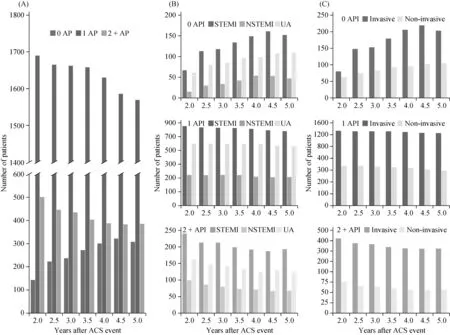
Figure 2. Long-term AMP trends in the EPICOR Asia-China Extension study. The number of (A) FAS, (B) ACS event, and (C) surgery subgroup patients for no AP, one AP and two or more (2+) APs during the study. ACS: acute coronary syndrome; AMP: antithrombotic management pattern; AP: antiplatelet; FAS: full analysis set; STEMI: ST-segment elevation myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; UA: unstable angina.
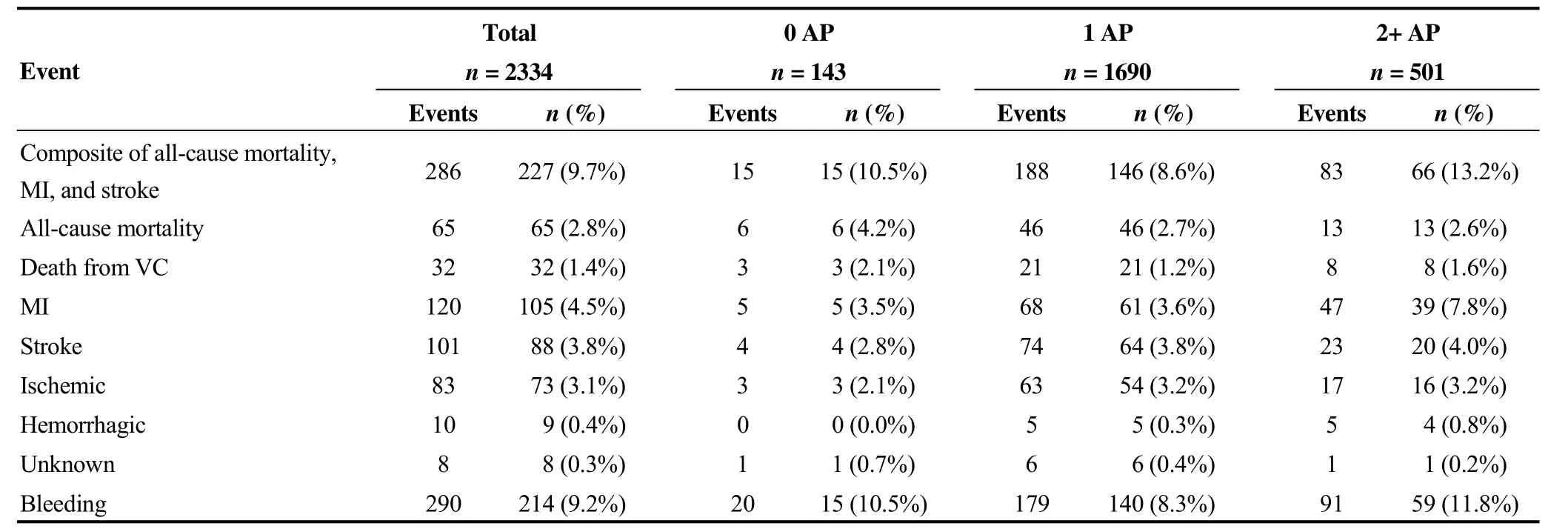
Table 3. Cumulative incidence of all predefined clinical events for the entire study.
3.8 Bleeding
The risk of bleeding is a frequent and serious complication in the management of ACS.[21]In this study, 214 (9.2%)patients in the FAS population reported 290 bleeding events(Table 3). The highest proportion of patients experiencing a bleeding event was observed in the 2+ AP subgroup (11.8%,59/501), followed by 10.5% (15/143) and 8.3% (140/1690)in the no AP and one AP subgroups, respectively. However,20.0% (4/20) of the bleeding events in the no AP subgroup were classified as major life-threatening and other major bleeding events (Supplementary Table S1). Serious bleeding events were observed at a lower frequency for the one AP and 2+ AP subgroups, at 6.7% (12/179) and 3.3% (3/91),respectively (Supplementary Table S1). Further details for bleeding events in this study are presented in Supplementary Table S1.
3.9 Changes in AMP due to clinical events
AMP dose changes, due to clinical event occurrence,were noted during the extension study. Generally, with the exception of one event in the stroke group, MI and stroke events experienced by patients in this study did not result in an AMP change (Table 4). However, bleeding events prompted an AMP dose change (47.0%, 109/232). Bleeding-induced AMP change was greatest in the 2+ AP subgroup(56.2%, 41/73), followed by the one AP (45.4%, 64/141)and no AP (22.2%, 4/18) subgroups. Changes in AMPs due to clinical events are summarized in Table 4.

Table 4. Changes in AMP due to clinical events for the entire study.
3.10 Outpatient visits, hospitalization, and QoL in AMP subgroups
Table 5 summarizes the frequency and cost of outpatient visits over the study duration. In total, 2063 patients (88.4%,2063/2334) had outpatient visits, and the median (range)number of visits per year was 6.0 (0.3-116.7) visits. Of the AP subgroups, most patients in the 2+ AP subgroup (93.4%,468/501) had outpatient visits, followed by participants in the one AP (87.9%, 1485/1690) and no AP (76.9%, 110/143)subgroups. Similarly, the mean frequency of outpatient visits was highest in the 2+ AP subgroup (9.73), followed by the one AP (8.41) and no AP (6.90) subgroups. The median(range) cost of outpatient treatment per year for participants in the entire study was 4687.5 (20.0-202,789.5) RMB (Table 5). Medication costs accounted for most of the total cost of outpatient visits per year.
The frequency, type, and cost of hospitalizations documented over the study period are summarized in Table 6.Less than half of the patients were hospitalized over the course of the study (43.8%, 1023/2334). Most hospitalizations were non-emergency in nature (81.3%, 1500/1845), of which 44.8% (838/1871) were for cardiovascular events.The median (range) cost of hospitalization was 4458.0(66.4-221,854.3) RMB (Table 6).
When hospitalization rates for the study population were analyzed according to AMP at baseline, it was noted that more than half of the patients in the 2+ AP subgroup were hospitalized during the follow-up period (54.5%, 273/501).Conversely, a smaller proportion of patients in the one AP and no AP subgroups were hospitalized during the study, at 41.0% (693/1,690) and 39.9% (57/143), respectively.Analysis of patient QoL by AMP at baseline and during the follow-up did not reveal any differences in patient-reported outcomes.

Table 5. Frequency and cost of outpatient visits in the AMP subgroups for the study.

Table 6. Frequency and cost of hospitalization in the AMP subgroups for the study.
4 Discussion
The findings from this China-specific extension of the observational EPICOR Asia study revealed that a majority of patients received at least one AP during the 2-5 years after an ACS index event. Aspirin was found to be the preferred antithrombotic therapy. Current AHA, ACC, and ESC guidelines recommend DAPT for at least one year following ACS, as well as an indefinite use of aspirin thereafter.[13-15]Data from this study indicate that the long-term management of ACS in China are largely aligned with current guidelines. However, our study did record a proportion of ACS patients who did not continue AP therapy 2-5 years after the ACS index event. This observation highlights a need for further research on the recommended long-term management of ACS in China.
This EPICOR Asia-China Extension study also examined the proportion of patients with ACS continuing with 2+AP after an ACS index event. Notably, the proportion of Chinese patients 2 years post-ACS that remained on 2+ AP was lower than that of Latin American and European patients with ACS (EPICOR study) at the end of their 2-year follow-up (21.5%, 501/2334vs.56.5%, 4859/8593).[22]A parallel EPICOR study from India also revealed a considerably greater proportion of patients with ACS remaining on DAPT 2 years after ACS (55.6%, 1342/2414), which indicates that this disparity is not a consequence of China’s economic status.[23]
Randomized trials, mostly in patients with stents, and meta-analyses have established that prolonged DAPT reduces the incidence of non-fatal ischemic events at the cost of increasing hemorrhagic episodes.[24,25]The larger Latin American and European EPICOR study, with a 2-year follow-up after ACS, noted that nearly 85% of coronary events and cardiovascular deaths occurred in patients receiving DAPT.[22]Conversely, the Spanish EPICOR arm, which utilized a similar 2-year follow-up period, noted a slight but insignificant decrease in the number of coronary events with DAPT compared to that with single AP therapy, at 6.3%(43/678) and 10.2% (6/59), respectively.[26]The frequency of mortalities in the Spanish EPICOR study also demonstrated a similar trend, with DAPT at 5.5% (37/678) and single AP therapy at 6.8% (4/59).[26]
In the present study, a numerically higher frequency of clinical events (composite of all-cause mortality, MI, and stroke) was observed for the 2+ AP subgroup than for the no AP and one AP subgroups. However, our study was unable to reach the target population size of 3000 patients. This shortage of patients may have resulted in an underestimation of clinical outcomes due to long-term AMP usage. Furthermore, an accurate extrapolation of results from the EPICOR Asia-China Extension study to a global context is limited by the lack of similar observational studies with longer follow-up times.
Bleeding is the most common adverse event associated with DAPT.[24,25]Results from this study have demonstrated that bleeding events were slightly higher in the 2+ AP subgroup than in the other AP subgroups. Despite the higher rate of DAPT continuation in other nations and regions, the overall bleeding rates seen in other EPICOR trials were far lower than those in our study. The Russian and Spanish EPICOR studies noted 4.7% (23/493) and 4.0% (27/678) of patients receiving DAPT exhibited bleeding complications,respectively, at the end of the 2-year follow-up.[26,27]While it is high likely that the increased rates of bleeding seen in this study were because of the longer length of follow-up, a few studies have noted that Asian patients are more susceptible to bleeding risks during antithrombotic therapy and the management of their ACS.[28,29]
Findings from this study also indicate that ACS presents a long-term financial and societal burden, not only for patients with ACS, but also for the Chinese healthcare system.Although approximately 90% of patients in this study reported receiving the recommended treatment for management of ACS 2-5 years after the index event, the majority still visited a hospital for outpatient consultation. Indeed,almost half of the patients were still hospitalized for cardiovascular reasons (44.8%). Medications comprised the largest proportion of costs from outpatient visits. Most patients(74.7%) in the study were earning a pre-tax household income of ≤ 65,000 RMB per year. For these patients, medication costs alone would account for 7% of their expenditure. Furthermore, given that approximately 50% of patients in the study were retired, other intangible costs such as the burden on family and caregivers, as well as nursing home costs, would likely be substantial.
The strengths of this study are its observational and non-interventional nature, which results in an patient overview that is reflective of real-life clinical practice. Moreover,current data detailing the impact of AMPs on healthcare resources beyond 2 years after an ACS event are limited in Chinese patients. This extension study, therefore, builds on findings from the EPICOR Asia study by detailing longterm AMPs utilization in China, the impact of clinical outcomes, and the utilization of healthcare resources up to 5 years after the ACS index event.
As with any observational study, the EPICOR Asia-China Extension study has several limitations. The sample size was small as it included patients who survived hospitalization as a result of ACS, but who were already enrolled in the initial EPICOR Asia study. The likely exclusion of older and moribund patients in the initial and extension EPICOR Asia-China studies could have accounted for the perceived differences in clinical and non-clinical events when compared to that in other registries.[19]Furthermore,the non-interventional nature of this study may have influenced the accuracy of data collection and analysis. For example, patient-reported follow-up procedures and clinical events could have precipitated an under-reporting of bleeding events or prompted patients to continue their medication for longer than they might do otherwise. Finally, given the descriptive nature of this study, an inference on the observed differences between evaluated subgroups could not be performed. A larger study should be conducted to examine whether long-term AP usage is associated with the occurrence of clinical events in Chinese patients with ACS.
In conclusion, data collected from the China-specific extension of the EPICOR Asia study provides an insight into long-term AMPs in patients with ACS. Most patients received one AP (principally aspirin) between 2-5 years after their ACS index event. Results from this study also highlight the long-term financial and societal burden of ACS to both patients and the healthcare system in China. In summary, most patients with ACS in China are receiving guideline-recommended therapy. However, further education is required to ensure that all patients receive at least one AP in the long term. Further studies in a larger population of patients are needed to determine any association between AMPs and long-term clinical outcomes in patients who have been discharged from a hospital following an ACS event.
Acknowledgments
We would like to thank the investigators and study teams,as well as the patients and their families for their participation in this study. We also want to thank the hospitals that participated in the study (Supplementary Table S2). Editorial assistance was provided by Isuru Wijesoma from MediTech Media (Singapore), which was funded by Astra-Zeneca in accordance with Good Publication Practice(GPP3) guidelines.
 Journal of Geriatric Cardiology2020年5期
Journal of Geriatric Cardiology2020年5期
- Journal of Geriatric Cardiology的其它文章
- Relationship between high sensitivity C-reactive protein and angiographic severity of coronary artery disease
- Association between serum uric acid level and endothelial dysfunction in elderly individuals with untreated mild hypertension
- Association of frailty with all-cause mortality and bleeding among elderly patients with acute myocardial infarction: a systematic review and meta-analysis
- Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients
- Ischemia/hypoxia inhibits cardiomyocyte autophagy and promotes apoptosis via the Egr-1/Bim/Beclin-1 pathway
- What is the cause of the neck hematoma? A rare complication of percutaneous coronary intervention of acute coronary syndrome: a case report
