Association of frailty with all-cause mortality and bleeding among elderly patients with acute myocardial infarction: a systematic review and meta-analysis
Prapaipan Putthapiban, Wasawat Vutthikraivit, Pattara Rattanawong, Weera Sukhumthammarat,Napatt Kanjanahattakij, Jakrin Kewcharoen, Aman Amanullah
1Department of Internal Medicine, Einstein Medical Center, Philadelphia, PA, USA
2Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, Tx, USA
3University of Hawaii Internal Medicine Residency Program, Honolulu, HI, USA
4Institute of Heart and Vascular Health, Einstein Medical Center, Philadelphia, PA, USA
Abstract Background Frailty is a multidimensional syndrome that reflects the physiological reserve of elderly. It is related to unfavorable outcomes in various cardiovascular conditions. We conducted a systematic review and meta-analysis of the association of frailty with all-cause mortality and bleeding after acute myocardial infarction (AMI) in the elderly. Methods We comprehensively searched the databases of MEDLINE and EMBASE from inception to March 2019. The studies that reported mortality and bleeding in AMI patients who were evaluated and classified by frailty status were included. Data from each study were combined using the random-effects, generic inverse variance method of DerSimonian and Laird to calculate hazard ratio (HR), and 95% confidence interval (CI). Results Twenty-one studies from 2011 to 2019 were included in this meta-analysis involving 143,301 subjects (mean age 75.33-year-old, 60.0% male). Frailty status was evaluated using different methods such as Fried Frailty Index. Frailty was statistically associated with increased early mortality in nine studies(pooled HR = 2.07, 95% CI: 1.67-2.56, P < 0.001, I2 = 41.2%) and late mortality in 11 studies (pooled HR = 2.30, 95% CI: 1.70-3.11, P <0.001, I2 = 65.8%). Moreover, frailty was also statistically associated with higher bleeding in 7 studies (pooled HR = 1.34, 95% CI: 1.12-1.59, P < 0.001, I2 = 4.7%). Conclusion Frailty is strongly and independently associated with bleeding, early and late mortality in elderly with AMI. Frailty assessment should be considered as an additional risk factor and used to guide toward personalized treatment strategies.
J Geriatr Cardiol 2020; 17: 270-278. doi:10.11909/j.issn.1671-5411.2020.05.006
Keywords: Acute myocardial infarction; Bleeding; Frailty; Mortality
1 Introduction
Frailty is a complex clinical phenotype that reflects an age-associated decline in reserve, responding to physiological stressors.[1]Common manifestations of frailty are slowness, reduced activity, low energy level, and unintentional weight loss.[2]Prevalence of Frailty in elderly adult ranging from 4% to 59.1% and more common in female.[3]It varied depending on the study population, age group as well as an assessment strategy.[4]Prevalence of frailty found to be higher with older age;[5]hence, frailty becomes more compelling with an aging society.
There have been over 20 tools developed to assess frailty.[6]At least one of five core domains of frailty phenotype, including slowness, weakness, low physical activity,exhaustion, and shrinking is evaluated to determine frailty status.[2]For example, Fried Frailty Index was developed from the Cardiovascular Health Study, consisting of unintentional weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity. Frailty was defined if 3 or more mentioned criteria present[1]and was found to be independently predictive of hospitalization and death.
Frailty has been associated with increased mortality in several cardiovascular diseases.[2]Cacciatore,et al.[7]showed that chronic heart failure patients who were frail had a lower 10-year survival (6%vs. 31%). Anand,et al.[8]studied the relationship between frailty and outcomes following transcatheter aortic valve implantation. Interestingly, frailty was associated with increase both early and late mortality, HR =2.35 (95% CI: 1.78-3.09,P< 0.001) and HR = 1.63 (95%CI: 1.34-1.97,P< 0.001), respectively. Some studies showed a connection between less aggressive management of acute coronary syndrome in frail patients.[4,9,10]
Dual antiplatelet is a cornerstone in AMI treatment according to 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction (STEMI)[11]and 2014 AHA/ACC guideline for the management of patients with non-ST elevation acute coronary syndrome.[12]Bleeding is one of the major complications following AMI management.Comparing risk and benefit is often time delicate, especially in the elderly population. Thus, we conducted a systematic review and meta-analysis to assess the associated of frailty with all-cause mortality and bleeding in elderly AMI patients.
2 Methods
2.1 Search strategy
Two investigators (PP and WS) independently searched for published studies indexed in MEDLINE and EMBASE databases from inception to March 2019 using a search strategy that included the term for “frailty (and its synonyms)” and “myocardial infarction”. Only English language publications were included. A manual search for additional pertinent studies and review articles using references from retrieved articles was also completed.
2.2 Inclusion criteria
The eligibility criteria included the following: (1) prospective cohort study reporting the incidence of frailty in AMI patients. No restriction was placed on frailty criteria.(2) The primary outcome was all-cause mortality after AMI,either reported in short (< 1 year) or long term (> 1 year).The secondary outcome was significant bleeding, which defined as intracranial hemorrhage, retroperitoneal hemorrhage, hemoglobin drop ≥ 4 g/dL or any bleeding requiring blood transfusions. Studies that report either primary or secondary outcome were included. (3) Calculation of relative risk, hazard ratio, odds ratio, incidence ratio, or standardized incidence ration with 95% CI or provision of sufficient raw data for these calculations were provided.
2.3 Data extraction
A standardized data collection form was used to obtain the following information from each study: title of study,name of first author, year of study, year of publication,country, number of participants, demographic data of participants, criteria used to identify frailty, type of AMI, outcome of interest (either all-cause mortality or bleeding), and average duration of follow-up.
To ascertain the accuracy, four investigators (NK, WV,PR, JK) independently performed this data extraction process. Any data discrepancy was resolved by referring back to the original articles. The Newcastle-Ottawa quality assessment scale was used to evaluate each study in three domains:recruitment and selection of the participants, similarity and comparability between the groups, and ascertainment of the outcome of the interest among cohort studies.[13]
2.4 Data synthesis and analysis
We performed a meta-analysis of the included studies using a random-effects model given a wide number of frailty criteria used. The extracted studies were excluded from the analysis if they did not present an outcome in each intervention group or did not have enough information required for continuous data comparison. We pooled the point estimates from each study using the genetic inverse-variance method of Der Simonian and Laird.[14]The heterogeneity of effect size estimates across these studies was quantified using theI2statistic and Q statistic. For Q statistic,substantial heterogeneity was defined asP< 0.10. TheI2statistic rages in value from 0 to 100% (I2< 25%, low heterogeneity;I2= 25%-50%, moderate heterogeneity; andI2>50%, substantial heterogeneity).[15]A sensitivity analysis was performed to assess the influence of the individual studies on the overall results by omitting one study at a time.Publication bias was assessed using a funnel plot.[16]Pooled HR, sensitivity analysis, funnel plot and forest plot were performed using the Stata SE 15.1 software from StatCorp LP. Egger test was also performed using the Stata SE 15.1 software from StatCorp LP.
3 Results
3.1 Description of included studies
Our search strategy yielded 535 potentially relevant articles (368 articles from EMBASE and 167 articles from MEDLINE). After exclusion of 152 duplicates, 354 underwent title and abstract review. Three hundred and six articles were excluded at this stage since they were not cohort studies, and they were not conducted in patients with AMI,leaving 48 articles for full-length article review. Twenty-one articles were excluded since no report of frailty related to exceptional outcomes. Four articles were excluded due to duplicated population, and three articles were excluded because of no primary data. Therefore, twenty prospective cohort studies of AMI patients were in clouded in this meta-analysis. Figure 1 outlines the search and literature review process. The summary of the clinical characteristic of included studies is provided in Table 1. The Newcastle-Ottawa scales of the included studies are described in the supplement Table 1S.
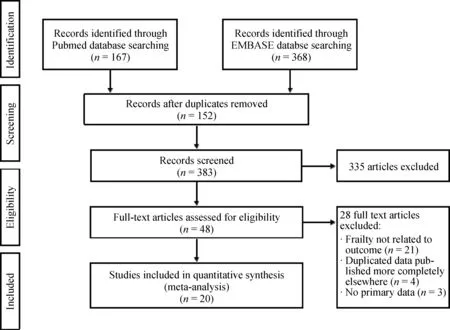
Figure 1. Search methodology and selection process.

Table 1. Baseline characteristics and summary of included studies in meta-analysis.
3.2 Definitions of frailty
Frailty was identified by authors using objective assessments. Various frailty-defining tools were used. Details of each tool are provided in supplement Table 2S. There was no subjective frailty, which was based on judgments of research team alone included in our meta-analysis.
3.3 Meta-analysis results
Twenty-one studies from August 2011 to February 2019 were included in this meta-analysis involving 143,301 subjects with AMI, mean age was 75.33-year-old and 60.0%were male. Ten thousand and two subjects (6.98% of total subjects) were determined as frail. Nine studies involving 5995 subjects with AMI (30.43% of subjects were frail)revealed an increased early (< 1 year) all-cause mortality among AMI patients[17-25]with one of nine studies[19]not achieving statistical significance. The pooled analysis demonstrated a significantly increased risk of early all-cause mortality in AMI patient who frail compared to those nonfrail group, with a pooled HR of 2.07 (95% CI: 1.67-2.56,P< 0.001,I2= 41.2%). A forest plot of this meta-analysis is shown in Figure 2.
In subgroup analysis among type of AMI, four studies were included in STEMI[17,20,23,25]involving 1,802 subjects(322 patients with frailty status and 1480 patients with non-frailty status). All four studies revealed statistical significantly increased early all-cause mortality among frail STEMI patients. The pooled analysis demonstrated a significant increased early all-cause mortality in frail STEMI patient compared to those without frail status (HR = 3.36,95% CI: 1.43-7.88,P= 0.005,I2= 55.7%). In NSTEMI cohorts, three studies were included in the analysis[18,22,23]involving 3507 subjects (1996 patients were frail and 1551 were non-frail). The pooled analysis again demonstrated a significant increased risk of early all-cause mortality in NSTEMI patients with frailty status compared to those without frailty status (HR = 1.99, 95% CI: 1.59-2.50,P<0.001,I2= 48.9%). A forest plot of this meta-analysis is shown in Figure 3.
For late all-cause mortality (≥ 1 year), there were 11 studies available involving 1400 frail and 6693 non-frail patients.[18,26-35]One of eleven studies did not demonstrate a statically significant association between frailty and late all-cause mortality.[31]However, the pooled analysis demonstrated a statistically significant increased risk of late allcause in frail group compared to non-frail group with HR =2.30 (95% CI: 1.70-3.11,P< 0.001,I2= 65.8%). Forest plot of this meta-analysis is shown in Figure 4.
To access the association of significant bleeding as the outcome, seven studies that reported bleeding as an outcome were analyzed, involving 134,640 patients (6.24% of patients were frail).[17,21,23,26,32,36,37]The pooled analysis also demonstrates a statistically significant increased risk of severe bleeding in frail patients compared to non-frail patients with the pooled HR 1.34 (95% CI: 1.12-1.59,P= 0.001,I2=4.7%). Forest plot of this meta-analysis is shown in Figure 5.
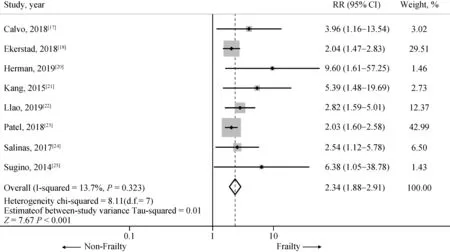
Figure 2. Risk of short term (< 1 year) mortality in included studies. Summary meta-estimate calculations based on random-effects model analysis.
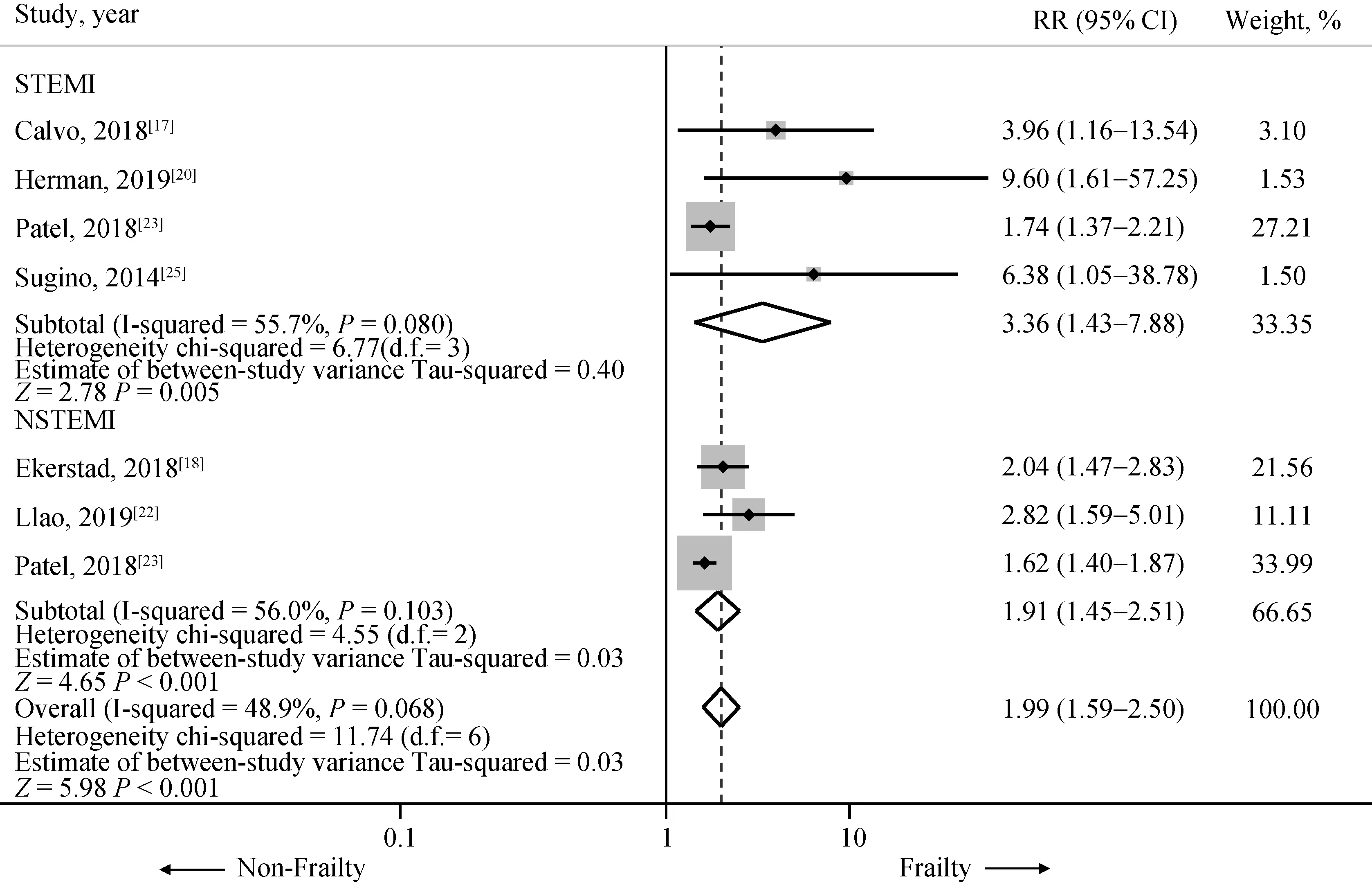
Figure 3. Risk of short term (< 1 year) mortality in included studies in STEMI and NSTEMI subgroup. Summary meta-estimate calculations based on random-effects model analysis. NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction.

Figure 4. Risk of long term (≥ 1 year) mortality in included studies. Summary meta-estimate calculations based on random-effects model analysis.
3.4 Sensitivity analysis
To assess the stability of the results of the meta-analysis,we conducted a sensitivity analysis by excluding one study at a time. None of the result was significantly altered, as the results after removing one study at a time were similar to that of the main meta-analysis indicating that our results were robust (Supplement Figure 1-3).

Figure 5. Risk of significant bleeding in included studies. Summary of meta-estimate calculations based on random-effects model analysis.
3.5 Publication bias
To investigate potential publication bias, we examined the contour-enhanced funnel plot of the included studies in assessing change in the log odd ratio of early all-cause mortality, late all-cause mortality or bleeding. The vertical axis represents studies size (standard error) while the horizontal axis represents effect size (log odds ratio). The distribution of studies on both sides of the mean was symmetrical.
4 Discussion
In our systematic review and meta-analysis, we explored the association between frailty and outcome of elderly with AMI, including early mortality, late mortality, and bleeding in 20 studies comprising 143,301 patients with frail elderly.We have made notably important observation. First, frail AMI patients have over twice as high risk of both early and late mortality than non-frail patients. The effect of frailty is even more prominent in STEMI subgroups. Second, a variety of assessment tool was used to determined frailty resulting in a wide range of prevalence of frailty in studied cohorts. However, all study observed a similar association between frailty and interested outcomes.
Frailty has become an emerging consideration in cardiovascular medicine due to the aging population. Not only AMI, but frailty also related to increased mortality in congestive heart failure, cardiac surgery as well as transcatheter aortic valve implantation.[10,38-40]On the other hand, the Women’s Health Initiative Study showed that patients with coronary artery disease were more likely to develop de novo frailty.[41]Thus, the American Heart Association and European Society of Cardiology have emphasized that assessment of frailty is crucial in managing elderly patients with AMI.[42,43]Intervening frailty status of patients is might be as vital as preventing and managing AMI.
Advanced age is considered a risk factor of impaired outcomes in patients with AMI and also included in wellaccepted risk scores such as the GRACE risk score and TIMI risk score.[44,45]However, the elderly population is heterogeneous and under-represented in the derivation and validation cohorts. Interestingly, Hermans,et al.[20]revealed that patients with at least one signs of frailty, according to VMS score had a nearly ten times higher risk of early mortality regardless of age and clinical characteristic. Hence,frailty assessment needs to be integrated with the prognostic score for better risk stratification, especially in the geriatric population.
The decision for invasive or conservative treatment of frail elder patients with AMI, especially NSTEMI, is frequently challenging to make. There is no recommendation or guideline specifically to AMI with frailty. Several studies observed increased bleeding risk only in frail patients who underwent catheterization, but not those treated with medical management.[36,37,46]Besides, major bleeding may lead to other consequences such as transfusion reaction, cessation of anti-thrombotic therapy, prolonged hospitalization, and even death.[47,48]Randomized control trails need to be performed in frail elderly patients with AMI to determine if invasive management could be favorable in this unique population.
Despite a significant impact of frailty to AMI and other cardiovascular diseases, there is a lack of consensus of validating frailty assessment tools. In our included studies, the prevalence of frailty varied from 5.3% to 53.7%. Numerous definition and assessment score were used. All of the included frailty tools are consisted of at least one of five core domains (slowness, weakness, low physical activity, exhaustion and shrinking). Comprehensive geriatric assessment of every elderly individual before managing AMI is not realistic for daily practice; therefore, adapting most available measures according to resources and circumstance may be reasonable. Nevertheless, frailty tool that easy to use,reproducible and accurate is warranted not only for guiding AMI management and excelling outcome but also leading to further research on this critical field.
Frailty is not modifiable in an acute setting. So far, exercise is the most promising intervention in a frail elderly population.[49,50]Unfortunately, Flint,et al.[29]revealed that patients in the frail group were less likely to be referred to cardiac rehabilitation (CR) and even with a referral, they less participated after AMI. Individualized designed of CR program that is appropriate to older adults is imperative.Other interventions to mitigate the risk of adverse outcome,especially bleeding include radial access and avoid excess dosing of anticoagulants.[51-54]
4.1 Limitation
We recognize that there are limitations to our metaanalysis. First, studies with different methodology and demographic data were included and thus might be potential sources of heterogeneity, but the sensitivity analysis revealed no significant alteration in the result. Second, we could not perform the subgroup analysis comparing both early mortality, late mortality, and bleeding outcome between patients who underwent invasive strategies and medical management. Finally, extracted data from included studies were not always adjusted for other variables.
4.2 Conclusion
The value of frailty as a prognostic factor of AMI in the geriatric population is well demonstrated. Frailty should be included in prognostic tools of AMI. A standardized frailty assessment tool that simple, accurate and reproducible is needed not only for clinical practice but also for future researches.
 Journal of Geriatric Cardiology2020年5期
Journal of Geriatric Cardiology2020年5期
- Journal of Geriatric Cardiology的其它文章
- Long-term follow-up of antithrombotic management patterns in patients with acute coronary syndrome in China
- Relationship between high sensitivity C-reactive protein and angiographic severity of coronary artery disease
- Association between serum uric acid level and endothelial dysfunction in elderly individuals with untreated mild hypertension
- Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients
- Ischemia/hypoxia inhibits cardiomyocyte autophagy and promotes apoptosis via the Egr-1/Bim/Beclin-1 pathway
- What is the cause of the neck hematoma? A rare complication of percutaneous coronary intervention of acute coronary syndrome: a case report
