Seasonal changes in sperm parameters, testicular histology and circulating levels of reproductive hormones in the male African straw-colored fruit bat (Eidolon helvum)
Clifford N Abiaezute, Chike F Oguejiofor, Innocent C Nwaogu, Ikechukwu R Obidike, Udensi M Igwebuike
1Department of Veterinary Anatomy, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Nigeria
2Department of Veterinary Obstetrics and Reproductive Diseases, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Nigeria
3Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Nigeria
ABSTRACT
KEYWORDS: Eidolon helvum; Spermatozoa; Reproductive characteristics; Seasonal changes
1.Introduction
Chiroptera, the second largest mammalian order (after rodents),with almost global distribution consists of temperate and tropical bats that exhibit numerous reproductive specializations[1,2].In male mammals, seasonal breeders are known to show drastic changes in their hormonal profiles and testicular morphology during their seasonal cycle, which changes cyclically between active and inactive stages.These cyclical changes between active and inactive periods are modulated by the hypothalamic-pituitary-gonadal axis in such a way that reproduction is activated during breeding periods and halted during resting periods[2-4].Reproductive cycles in temperate male bat species are linked with hibernation and arousal,with synchronous or asynchronous breeding pattern occurring during a restricted breeding season.On the other hand, tropical male bat species do not undergo hibernation but exhibit highly variable breeding patterns that may be synchronous or asynchronous, single or multiple seasonally-restricted or seasonally-extended, or nonseasonal continuous breeding[2,5].
The African straw-colored fruit bat, Eidolon (E.) helvum, is a tropical Megachiroptera (megabat) of the family Pteropodidae which are known as ‘Old World fruit bats' or ‘flying foxes;' and are confined to Old World tropics[2].It is a common and conspicuously migratory species with an extensive distribution across Sub-Saharan Africa[6,7].It is also a non-hibernating seasonal breeder that copulates mainly during the breeding season[8].However, a recent large-scale study in Africa observed regional asynchrony of reproductive timing across several study sites[7].In Western Nigeria,mating was reported to occur in June/July and gestation from October/November to births in March[9,10].Hence, implantation was timed with the onset of the dry season and births with the onset of the rainy season.
Most of the available information on testicular dynamics, endocrine control of the reproductive pattern, and breeding cycles of male bats were derived from studies on temperate and neotropical bat species[2,11].However, tropical male bats are known to exhibit great variation in annual reproductive patterns that may be influenced by the female gametic cycle, geographic location and seasonal variations in photoperiod, temperature, rainfall and feeding resources[2].Apart from the works of Danmaigoro et al[12,13]on the gross anatomy and histology of the reproductive organs, there is scanty information on the reproductive characteristics of tropical African bats including the male E.helvum.Moreover, there is a major threat to the population of E.helvum due to loss of habitat and heavy harvesting for bush meat and traditional medicine in West and Central Africa[14,15].Consequently, it has been listed as a near-threatened species of bat by the International Union for Conservation of Nature, necessitating a conservation strategy[14,15].Therefore, adequate understanding and characterization of the seasonal reproductive characteristics of the male E.helvum will assist in the preservation of the species.
The aim of this study was to investigate spermatozoal characteristics in the testes and epididymides, testicular histology and circulating levels of reproductive hormones in male African straw-colored fruit bat (E.helvum) during the rainy and dry seasons of the year.
2.Materials and methods
2.1.Animals
The adult male African straw-colored fruit bat (E.helvum) used for this study were captured from Obiagu community, Enugu State, South-East Nigeria, located on latitude 6°26′14.7408″ N and longitude 7°30′13.3092″ E within the tropical rain forest belt.A total of 48 male bats were captured in 2019: sixteen bats each in late January (peak dry season), late May (early rainy season) and late September (late rainy season), respectively.Bats were captured 2-3 times within a 10-14 days interval during each period of the season.The bats were captured by using mist nets at nightfall near their roosts in the community.Upon capture, they were transported to the laboratory with minimal stress in dark carton cages with plant twigs hanging on the roofs to mimic a roosting environment.Taxonomic identification was done at the Zoology Department, University of Nigeria Nsukka.Adult male bats (24 months of age and above)were identified morphologically by the presence of bright orange collar[6]and fusion of the epiphyseal plates of the fourth metacarpalphalangeal bones[16].Age was also assessed by confirming full body size and weight within the range expected for an adult male, together with the presence of developed testicles[12].
2.2.Sample collection
Blood samples were collected via thoracotomy and cardiac puncture of the animals[17]using terminal anaesthesia with ketamine hydrochloride (Ketmin®, Laborate, Panipat, India) at 13 mg/kg body weight.The bat was properly restrained on dorsal recumbency and blood collected with a sterile 2 mL syringe was transferred into plain vacutainer tube and allowed to clot.Serum was harvested and stored at -20 ℃ prior to hormonal analysis.A midline incision was then made from the xyphoid cartilage to the pelvic symphysis to gain access to the reproductive organs of the bat.The testes and epididymides were dissected and weighed.The left testis and epididymis of each animal were utilized for sperm evaluation, while the right ones were processed for histology.
2.3.Histology and hormonal assay
After surgical excision, small portions of the testes and cauda epididymides were cut and immersed in Bouin's fixative solution for 24 h, followed by preservation in 70% ethanol.The fixed tissues were dehydrated in a series of graded ethanol baths, cleared in xylene, embedded in paraffin wax, and subsequently cut into sections of 5 µm thicknesses.Tissue sections were stained with hematoxylineosin and histology was evaluated at ×100 and ×400 magnifications using a light microscope (Motic B3; Motic, Carlsbad, CA, USA).
The serum concentrations of the reproductive hormones:testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured by using a microplate reader(StatFax 4200; Awareness Tech, Palm City, FL, USA) and enzyme immunoassay (EIA) test kits (Monobind; Lake Forest, CA, USA)for testosterone (catalog #: 3725-300A), FSH (catalog #: 425-300A) and LH (catalog #: 625-300A), in accordance with the manufacturer's instructions.The testosterone EIA was based on the principle of competitive binding between testosterone in the bat serum (10 µL) and testosterone-horseradish peroxidase conjugate(50 µL) for a constant amount of anti-testosterone biotinylated rabbit immunoglobulin G (IgG) (50 µL) in Streptavidin-coated wells.The FSH and LH EIAs utilized 50 µL of bat serum and a biotinylated mouse anti-FSH(-LH) IgG for immobilization in Streptavidin-coated wells, and another mouse anti-FSH(-LH) IgG in the antibody-horseradish peroxidase conjugate, with the antibodies directed against distinct and different epitopes of FSH (LH).The EIA test systems had lower limits of detection of 0.0576 ng/mL,0.134 mIU/mL and 0.054 mIU/mL for testosterone, FSH and LH,respectively.The intra-assay coefficients of variation were 5.2%,9.5% and 8.1% for the respective testosterone, FSH and LH assays.
2.4.Sperm evaluation
Spermatozoal characteristics were evaluated in accordance with the methods described previously[18,19].Sperm was harvested from a sectioned part of the cauda epididymides.Sperm motility was evaluated following sperm diffusion in phosphate buffered saline (pH 7.4, 37 ℃).Sperm motility (%) was determined at ×400 magnification by using a phase-contrast microscope (Motic B3;Motic, Carlsbad, CA, USA) equipped with a stage slide-warmer(TCS-100; Amscope, Ivrine, CA, USA) set at 37 ℃.Sperm vitality(%) was evaluated following Eosin-Nigrosin staining, and sperm were categorized as live (unstained) or dead (marked pink-stained) under light microscopy at ×1 000 magnification.Sperm morphology was evaluated at ×1 000 magnification by using eosin-nigrosin staining and phase-contrast microscopy.Sperm abnormalities were recorded as percentage individual abnormality, and also as percentage total abnormalities.All values in percentages were derived by examining 200 sperm cells in duplicates.Cauda epididymal tissue sample was weighed, homogenized in phosphate buffered saline and used to determine epididymal sperm concentration by counting with a hemocytometer (Weber, England).Sperm concentration was expressed as number of spermatozoa per gram cauda epididymis.The same method was used to prepare a homogenate from a sample of the testis parenchymal tissue.Testicular spermatozoa were counted by using a hemocytometer and the value was expressed as number of spermatozoa per gram testis.
2.5.Statistical analysis
Data were analyzed with the one-way analysis of variance(ANOVA) tool using GraphPad Prism version 6.01 (GraphPad Software, Inc.), and the results presented for each season as mean±standard deviation (mean±SD).Where ANOVA showed significant difference, differences between means were confirmed using the Tukey's honestly significant difference post hoc multiple comparison.Individual epididymal sperm abnormalities were analyzed by using Kruskal-Wallis test with Dunn's multiple comparisons test, and P values were adjusted for multiple comparison.Values were expressed as median (interquartile range)i.e.median (Q1, Q3).Results were considered significant when P<0.05.
2.6.Ethics statement
Animal welfare was observed in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals[20]and the protocol was approved (FVM-UNN-IACUC-2019-0710) by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine, University of Nigeria Nsukka.
3.Results
3.1.Hormonal analysis
There were statistically significant differences (P<0.05) in the mean serum testosterone concentrations of E.helvum during the three seasons studied (Table 1).Serum testosterone concentration was lowest in peak dry season compared with the other two seasons.It was highest in early rainy season but lower in late rainy season.Serum FSH concentration was not significantly different (P>0.05)during the three seasons.The serum concentration of LH was not different during peak dry season and late rainy season, but was significantly higher (P<0.05) during early rainy season.
3.2.Sperm analysis
There were changes in specific sperm parameters of E.helvum during the different seasons (Table 2).Epididymal sperm total motility differed significantly (P all<0.05).It was lowest during peak dry season but increased markedly in early rainy season.It then declined in late rainy season.Epididymal sperm vitality was also low(P<0.05) in peak dry season.It increased during early rainy season but declined in late rainy season (P<0.05).Phase-contrast microscopy revealed that the morphology of E.helvum spermatozoa was similar to the typical mammalian sperm cell, comprising of a head and a tail (Figure 1A-D).The head was flattened with a rounded-spatula shape, and was surrounded at the anterior region (over the acrosome)by an amorphous plasma membrane that was attached at the distal part of the head (Figure 1D).Epididymal sperm total abnormalities was highest (P<0.05) in peak dry season.It was lowest in early rainy season but showed an increase in late rainy season (P<0.05).When sperm individual abnormalities were evaluated (Table 2, Figure 1A-D), there were significant differences during the three seasons.There were significantly (P all <0.05) higher percentages of sperm with detached head, fractured neck, bent midpiece, coiled midpiece,bent tail and coiled tail in peak dry season compared to the other two seasons.There were lower percentages (P<0.05) of sperm with detached head and coiled tail in early rainy season compared to late rainy season, but the percentage of fractured neck, bent midpiece,coiled midpiece and bent tail abnormalities did not differ (P>0.05)between the two seasons.There was no difference in the percentage of sperm with proximal cytoplasmic droplets between the early rainy season and late rainy season, but this abnormality was not observed in peak dry season (P<0.05).The percentage of sperm with distal cytoplasmic droplets was highest (P<0.05) during early rainy season but did not differ significantly (P>0.05) between peak dry season and late rainy season.Epididymal sperm concentration was significantly different during the three periods (P all <0.05).It was lowest in peak dry season but highest in early rainy season.Testicular sperm concentrations was also significantly different during the three periods (P all <0.05), with the lowest value in peak dry season and the highest in early rainy season.

Table 1.Seasonal changes in serum levels of reproductive hormones in the male African straw-colored fruit bat (E.helvum).
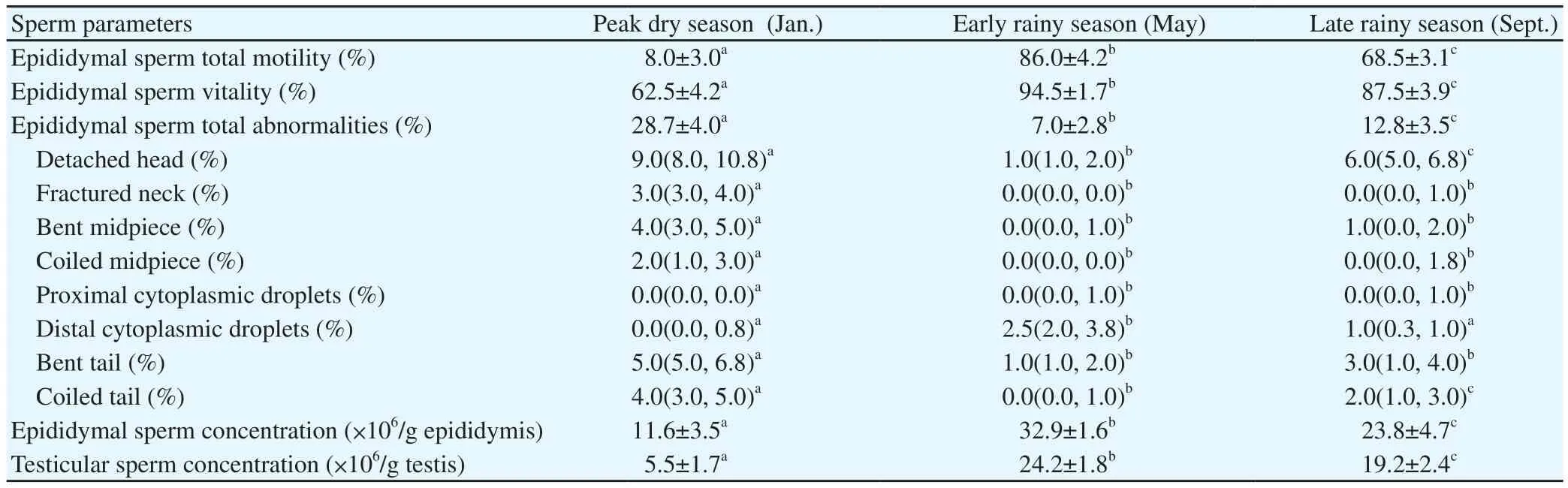
Table 2.Seasonal changes in sperm parameters in the male African straw-colored fruit bat (E.helvum).
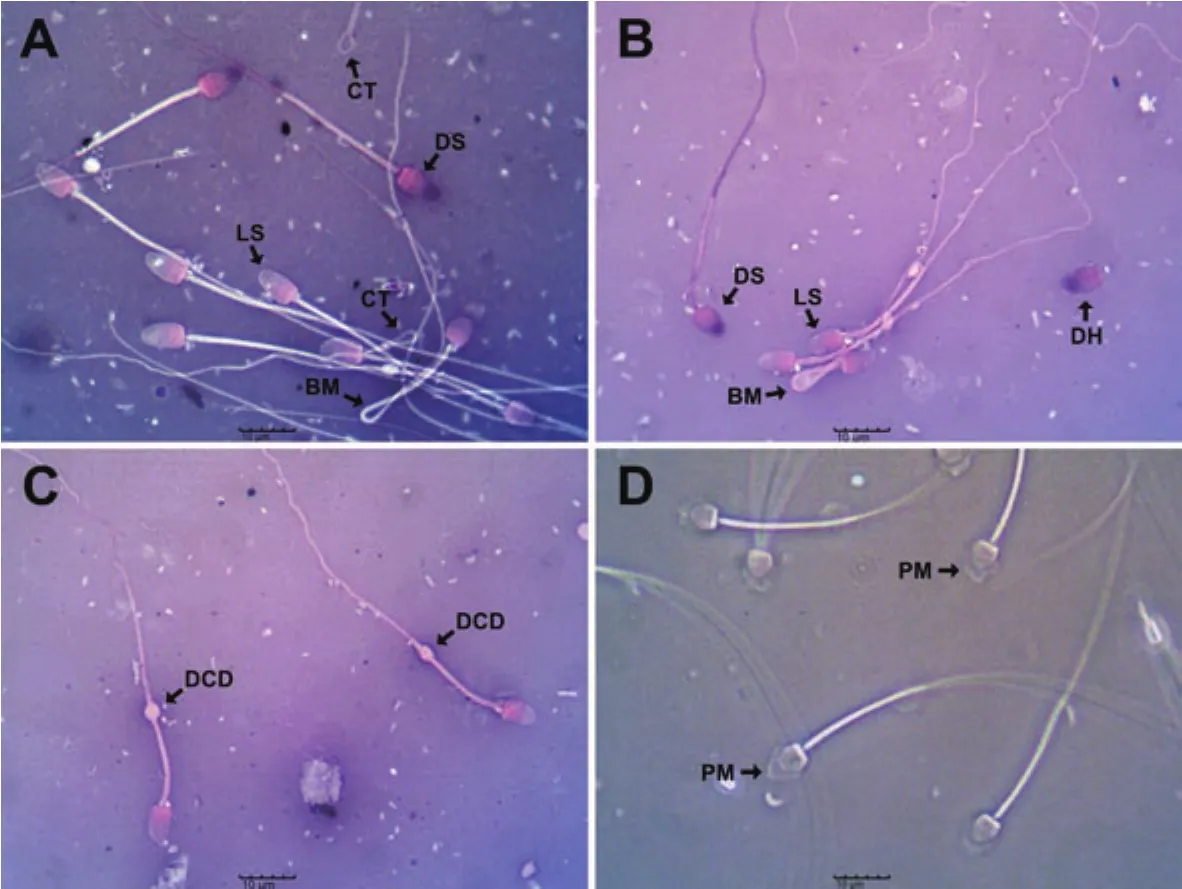
Figure 1.Micrographs of the caudal epididymal spermatozoa of African straw-colored fruit bat (E.helvum) (scale bar: 10 µm).A-C: Evaluation of sperm vitality and morphology is performed by using eosin-nigrosin staining.Note the dead sperm (DS) and live sperm (LS), and sperm with detached head (DH),bent midpiece (BM), coiled tail (CT) and distal cytoplasmic droplets (DCD).D: Evaluation of sperm morphology is done by using phase-contrast microscopy.Note the presence of an amorphous plasma membrane (PM) at the anterior potion (over the acrosome) that is attached at the distal part of the head.
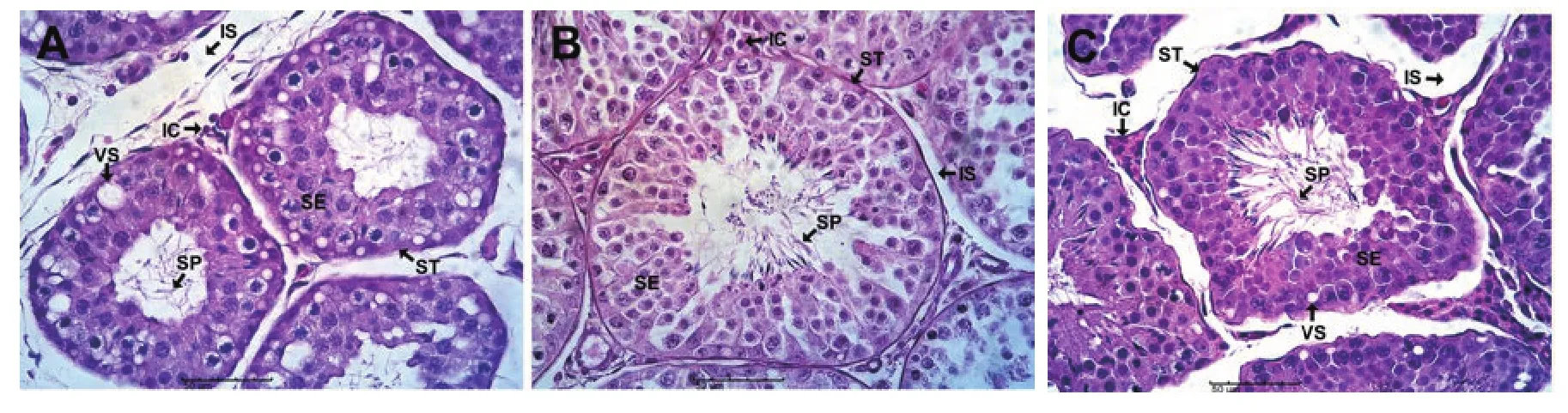
Figure 2.Histology micrographs showing seasonal effects on the testes of the African straw-colored fruit bat (E.helvum) (H & E stain; scale bar: 50 µm).(A)Peak dry season: Smallest diameter of seminiferous tubules (ST), decreased interstitial cells (IC), increased intertubular spaces (IS), numerous vacuole-like structures (VS) in the seminiferous epithelia (SE), and few spermatozoa (SP) in the tubular lumen are seen.(B) Early rainy season: Largest diameter of ST,multilayered SE, prominent IC, little IS, and abundant luminal SP are present.(C) Late rainy season: Reduced diameter of ST, prominent IC, increased IS,appearance of VS in the SE and abundant luminal SP are observed.
3.3.Histological findings
Histological evaluation revealed significant changes in the testis of E.helvum during the different seasons (Figure 2A-C).Seminiferous and spermatogenic activities as demonstrated by seminiferous tubular diameter and epithelia were lowest in peak dry season,highest in early rainy season but decreased in late rainy season.Intertubular spaces were most abundant in peak dry season, scanty in early rainy season but increased in late rainy season.Interstitial cells were also scanty in peak dry season but prominent in early rainy season and late rainy season.Vacuole-like structures in the seminiferous epithelia were abundant peak dry season but scanty in early rainy season and late rainy season.Spermatozoa were relatively fewer in the tubular lumen in peak dry season but more abundant in early rainy season and late rainy season.
4.Discussion
This study has provided novel evidence of changes in the sperm characteristics, testicular histology and circulating levels of reproductive hormones in the African straw-colored fruit bat (E.helvum) during the different seasons of the year in the study area.There were high serum concentrations of testosterone in early rainy season and late rainy season and LH in early rainy season, but the concentrations of both hormones were lowest during peak dry season.These hormonal changes corresponded with the testicular seminiferous and spermatogenic activities and testicular and epididymal sperm concentrations, which were also maximal during early rainy season and late rainy season but minimal during peak dry season.These observations reflected the already established roles of the hormones in spermatogenesis.The gonadotropin, LH is known to stimulate the production of testosterone from the testicular interstitial (Leydig) cells.The testosterone so produced is essential for spermatogenesis and the development/functioning of accessory sex glands, and development of secondary sexual behaviors including courtship, territoriality, aggressiveness, rank and mating successes[21-24].Therefore, the increased LH and testosterone concentrations which corresponded with increased testicular and epididymal sperm concentrations are in synchrony with early rainy season as the mating period in this species.Likewise, previous reports observed mating in E.helvum in the early rainy season[8].Seasonally-breeding tropical bats have also been reported to exhibit increased circulating testosterone levels during breeding season in synchrony with peak spermatogenesis and maximal function of accessory sex glands[25].Maximal level of LH secretion also coincided with the period of spermatogenic activity in some hibernating bats[26].Our findings are in contrast with the reports in certain temperate hibernating bats where the onset of breeding does not synchronize with period of peak plasma testosterone and spermatogenesis[2,5,27].In those temperate bats, maximal testosterone and spermatogenesis are synchronized and occur during the summer, while high libido and breeding takes place in winter when testosterone levels are minimal[2,27].
The LH concentrations during late rainy season and peak dry season were not significantly different, but the testosterone concentration was higher in late rainy season than peak dry season.The higher testosterone level may be responsible for the higher level of spermatogenesis as evident in the higher testicular and epididymal sperm concentrations observed during the late rainy season.It is also possible that the high testosterone level may stimulate continued reproductive behaviors in male E.helvum during the late rainy season.It has been reported that the fertilized egg of female E.helvum after mating in April-June, becomes arrested at the blastocyst stage until October-November when implantation occurs[2,8,9].Thus,the relatively high testosterone level and spermatogenesis suggest that mating processes may continue during the late rainy season, for instance in females that return to oestrus due to non-fertilization or loss of pregnancy.The low levels of LH and testosterone in peak dry season may be associated with testis regression, and lowered sperm concentrations in the testes and epididymides of the bats.This suggests that little or no reproductive activities may occur during the period.Serum FSH levels were not significantly different during the three seasons.In males, FSH stimulates Sertoli cells to secrete androgen-binding proteins, and is critical in the initiation and regulation of spermatogenesis[24,28].In hibernating bats, FSH secretion may be required for the initiation of testis reactivation, and may have a role in the stimulation of androgen production especially during winter dormancy[2,29].
The characteristic feature of E.helvum spermatozoa head that is covered by an anteriorly-free and distally-attached amorphous plasma membrane has also been observed in the spermatozoa of other neo-tropical and sub-tropical non-hibernating bats such as Tadarida brasiliensis[30], Eptesicus furinalis[31], Artibeus jamaicensis and Sturnira lilium[32].The best sperm quality was observed during early rainy season which coincided with the active breeding period,while the least sperm quality occurred during the inactive, nonbreeding dry season.Sperm concentration was high mainly during early rainy season and also in late rainy season but declined in peak dry season.The epididymal sperm concentrations observed in E.helvum during the early rainy season and late rainy season in this study were higher than the values reported in some studies in other bats during the mating seasons[32-34].The disparities may be attributed to differences in bat species, reproductive pattern,testicular sizes, epididymal segments assessed and the methods used for assessment.The low sperm concentration in peak dry season is probably due to testis regression and decreased stimulation of spermatogenesis in the testis by the low level of testosterone observed during the period.The cauda epididymis is the site of sperm storage before ejaculation.Therefore, the lowered epididymal sperm concentration could be a reflection of decreased testicular spermatogenesis.There was histologic evidence that spermatogenesis did not cease completely during testis regression, but continued at a reduced rate during the dry season.Therefore, our findings do not support concurrent epididymal sperm storage with testis regression in E.helvum.This differs from other non-hibernating tropical and neotropical bats that exhibit testis regression and complete cessation of spermatogenesis during the dry season, concurrent with epididymal sperm storage[27,35-37].The banana bat, Pipistrellus nanus, was reported as the only tropical African bat known to store epididymal sperm[35].Other hibernating temperate bats experience complete involution of the seminiferous tubules and cessation of spermatogenesis,concurrent with prolonged sperm retention in the epididymis during the period of inactive spermatogenesis[2,5].
Epididymal sperm motility and vitality were high in early rainy season and late rainy season but low in peak dry season.Sperm morphological abnormalities were also low in early rainy season and late rainy season but elevated in peak dry season.Likewise, it was reported that semen quality increased from low during the inactive/resting period to high during the active/breeding period in other seasonally-breeding mammals[38,39].The higher percentage of sperm abnormalities noticed during the dry season may be related to testis regression during this period.An increase in sperm abnormalities,such as detached heads and fractured necks may be present in males that have not ejaculated for long periods as senescent changes[40].Increased proportion of sperm with distal cytoplasmic droplets in early rainy season may reflect frequent ejaculation during this mating period, as this defect is also known to increase in males during sexual overuse due to ejaculation of immature epididymal sperm[40].The sperm defects, which were mainly associated with the head, mid-piece and tail regions were similar to the defects reported in other bats[32,34]and other mammalian seasonally-breeding males[38,39].The highest percentage sperm total abnormalities observed in the study was approximately 29% during peak dry season.Other studies in bats reported 38% in Artibeus jamaicensis and 29% in Sturnira lilium[32], 59% in Hipposideros larvatus at the onset of spermatogenesis after hibernation and 19% at cessation of spermatogenesis before winter break[34].Moreover, the occurrence of sperm abnormalities may be influenced by differences in species, health status, nutrition, age, environment and exposure to toxicants[32].
A few limitations in the study may include unknown environmental effects on the bats during the different seasons.In addition, the evaluation of sperm morphologic changes was limited to the observations possible with light and phase-contrast microscopy.Nevertheless, there was increased testicular and epididymal sperm concentration and sperm quality, and increased levels of the circulating hormones, LH and testosterone during the mating period in early rainy season.However, these reproductive parameters were observed to decrease during the non-breeding, dry season in the bats.In conclusion, seasonal changes significantly altered sperm characteristics, testicular histology and circulating levels of reproductive hormones in E.helvum within the study area.
Conflict of interest statement
The authors declare that there is no conflict of interest.
 Asian Pacific Journal of Reproduction2020年4期
Asian Pacific Journal of Reproduction2020年4期
- Asian Pacific Journal of Reproduction的其它文章
- Prospects of diagnostic and prognostic biomarkers of pyometra in canine
- Hemodynamic changes in arterial flow velocities throughout the first six months of pregnancy in buffalo heifers by Doppler ultrasonography
- Genistein improves the vaginal epithelium thickness in a rat model of vaginal atrophy through modulation of hormone and heat shock protein 70 levels
- Process of becoming a mother in women receiving donated egg: Based on the grounded theory
- Food insecurity and other possible factors contributing to low birth weight: A case control study in Addis Ababa, Ethiopia
- Effects of nitric oxide on reproductive organs and related physiological processes
