Genistein improves the vaginal epithelium thickness in a rat model of vaginal atrophy through modulation of hormone and heat shock protein 70 levels
Pribakti Budinurdjaja, I Wayan Arsana Wiyasa, Ika Kustiyah Oktaviyanti, Djanggan Sargowo
1Doctoral Program in Medicine, Faculty of Medicine, Universitas Brawijaya, Malang, East Java, Indonesia
2Department of Obstetric and Gynecology, Faculty of Medicine Universitas Lambung Mangkurat/Ulin General Hospital, Banjarmasin, South Kalimantan, Indonesia
3Department of Obstetric and Gynecology, Faculty of Medicine Universitas Brawijaya/dr.Saiful Anwar General Hospital, Malang, East Java, Indonesia
4Department of Cardiology and Vascular Medicine, Faculty of Medicine Universitas Brawijaya/dr.Saiful Anwar General Hospital, Malang, East Java, Indonesia
ABSTRACT
KEYWORDS: Vagina; Cellular stress; Hypoestrogen; Pelvic organ prolapse
1.Introduction
Pelvic organ prolapse is an anatomical change characterized by a drop in the pelvic organs and other vaginal compartments[1].The incidence of pelvic organ prolapse is 40% in all menopausal women.Fifty percent of women who have given birth will suffer from pelvic organ prolapse.The etiology of this disorder is multifactorial, and includes age, hypoestrogenism, genetics, race, various conditions that trigger an increase in intra-abdominal pressure, parity, and connective tissue disease[2,3].Pelvic organ prolapse treatments include pelvic wall exercises, vaginal pessarium, lifestyle modification, smoking cessation, constipation treatment, avoidance of impactful exercise, and operative correction[4,5].
Pregnancy and vaginal delivery cause the weakening of the vaginal structure[6].Furthermore, menopausal women are at the highest risk of pelvic physical changes due to loss of estrogen.Estrogen depletion causes vaginal dryness and atrophy[7].Vaginal atrophy is a symptom that is often overlooked in menopausal women.These structural and vaginal molecular changes have been identified in women with pelvic organ prolapse and are involved in the pathogenesis of pelvic organ prolapse[8,9].The standard treatment for vaginal atrophy is estrogen therapy, but many women do not use it due to due to complications associated with the therapy[10].
Various approaches in the form of stem cell therapy and phytoestrogens have been used to treat vaginal atrophy.Stem cell therapy includes the use of adipose-derived mesenchymal stem cells[11].Phytoestrogens are plant-derived active compounds with structural and functional similarity to estradiol[12].Phytoestrogen containing licorice vaginal creams can improve the vaginal maturation index, vaginal mucus cell changes, and vaginal acidity[13].Treatment with isoflavone isolates cannot improve vaginal dryness.Phytoestrogens cannot improve the vaginal epithelium, although these results are inconsistent[14].
Genistein is the main flavonoid in leguminous plant.It is a phytoestrogen that is both an agonist and antagonist.Genistein targets the estrogen receptorsαandβbut has a higher affinity for estrogen receptorβ. Various studies have proven the potential of genistein in prevention and treatment, but its use in the treatment of vaginal atrophy is rare[15,16].The efficacy of isoflavone products is also unclear[17].Therefore, this study aimed to investigate whether administration of genistein can improve hormonal changes [estradiol and follicle stimulating hormone (FSH)], heat shock protein 70(Hsp70) expression, and thickness of vaginal epithelial cells in rat models of vaginal atrophy.
2.Materials and methods
2.1.Animal
This study used female rats (Rattus norvegicus) with a history of giving birth three times and hypoestrogenism due to ovariectomy.The age of rats was 11 months, with average weight was 300-350 g.All animals were maintained in individual cage, received feed and drink ad libitum.Standard laboratory setting was adjusted at 12/12 h light/dark cycle (light on at 7:00 a.m.) at an average temperature of(25±1) ℃.
A total of twenty-five rats were randomly divided into five study groups, namely the control group (without ovariectomy), the ovariectomy only group, the ovariectomy group 1 treated with genistein at a dose of 0.045 mg/kg body weight/day, the ovariectomy group 2 that received genistein at a dose of 0.090 mg/kg body weight/day, and the ovariectomy group 3 given genistein at a dose of 0.180 mg/kg body weight/day.All genistein was administered to the rats via subcutaneous injection.These dose was adopted from previous study[18].
2.2.Genistein
Genistein from Glycine max (soya bean) was purchased from Sigma Aldrich, Singapore, with catalog number 06776.Genistein was dissolved in 1% aqueous carboxymethyl cellulose as vehicle[19].Genistein was administered via subcutaneous injection.Genistein treatment began 28 days after ovariectomy and for a duration of 28 days.
2.3.Ovariectomy
Bilateral ovariectomy was performed seven days postpartum.Bilateral ovariectomy was performed under ketamine anesthesia at a dose of 35 mg/kg of body weight and xylazine at 5 mg/kg of body weight, delivered intraperitoneally.Before surgery, we cleaned the hair at the incision site by disinfecting the area.After that, a 1 cm incision was made over the ovary.The ovary was then removed and ligated to the oviduct.Finally, the incision site was closed.In the control group, the procedure was carried out, without removal of the ovaries[20].
2.4.Analysis of estradiol levels
Analysis of estradiol levels was performed by using the enzymelinked immunosorbent assay technique.The analysis procedure was carried out according to the manufacturer's instructions (Bioassay Technology Laboratory, Shanghai, China; Catalog No E0174Ra).
2.5.Analysis of FSH levels
Analysis of FSH levels was carried out by using an enzyme-linked immunosorbent assay.The analysis procedure was carried out according to the manufacturer's instructions (Bioassay Technology Laboratory, Shanghai, China; Catalog No E0182Ra).
2.6.Analysis of Hsp70 expression
Immunohistochemistry was used to analyze the expression level of Hsp70.The Hsp70 antibodies used were purchased from GenetTex(GeneTex Inc, Taiwan, China; catalog number GTX25442).
2.7.Tissue isolation and analysis of vaginal epithelial thickness
After genistein treatment was completed, euthanasia was carried out for the removal of organs and blood.The vagina was removed and then rinsed in physiological saline to remove blood and a dissection of the surrounding tissue was performed.The vagina was then weighed and fixed for 48 h in a 10% neutral formalin buffer and stored in 70% ethanol at 4 ℃.After dehydration and embedding in paraffin wax, tissue slices were carried out at a thickness of 5 µm and then stained with hematoxylin eosin for histological analysis[21].Measurement of the thickness was performed by using a light microscope (Olympus BX51, Japan) at magnification ×400 and expressed in micrometer.Measurements were made with dot slide software (Olympus Soft Imaging Solutions GmbH, Germany)starting from the basement membrane of the superficial epithelium in 10 fields of view.
2.8.Statistical analysis
Data were analyzed by using analysis of variance and least significant difference-post hoc tests.Data were presented as mean±standard deviations (mean±SD).P<0.05 was considered to be statistically significant.
2.9.Ethics statement
This research was conducted through a research ethics study and was approved by the Research Ethics Committee, Faculty of Medicine, Universitas Lambung Mangkurat, Banjarmasin, South Kalimantan, Indonesia (ethics approval No.590/KEPK-FK UNLAM/EC/2018).
3.Results
3.1.Levels of estradiol
Estradiol levels were significantly lower in the ovariectomy only group than in the control group (P<0.05).The various doses of genistein increased estradiol levels as compared with the ovariectomy only group (P<0.05), achieving a value comparable to the control group for lowest dose (0.045 mg/kg) of genistein(P>0.05).Genistein at dose of 0.090 or 0.180 mg/kg body weight/day significantly increased estradiol level compared with the control group or the ovariectomy only group (P<0.05).There was no significant difference of estradiol level between these two highest doses (P>0.05) (Figure 1).
3.2.Levels of FSH
There was a significant increase in FSH levels in the ovariectomy only group as compared with the control group (P<0.05).Various doses of genistein reduced FSH levels as compared with the ovariectomy only group (P<0.05), even reaching a value comparable to the control group (P>0.05).There were no significant differences in FSH level between groups of genistein treatment (Figure 2).
3.3.Expression of Hsp70
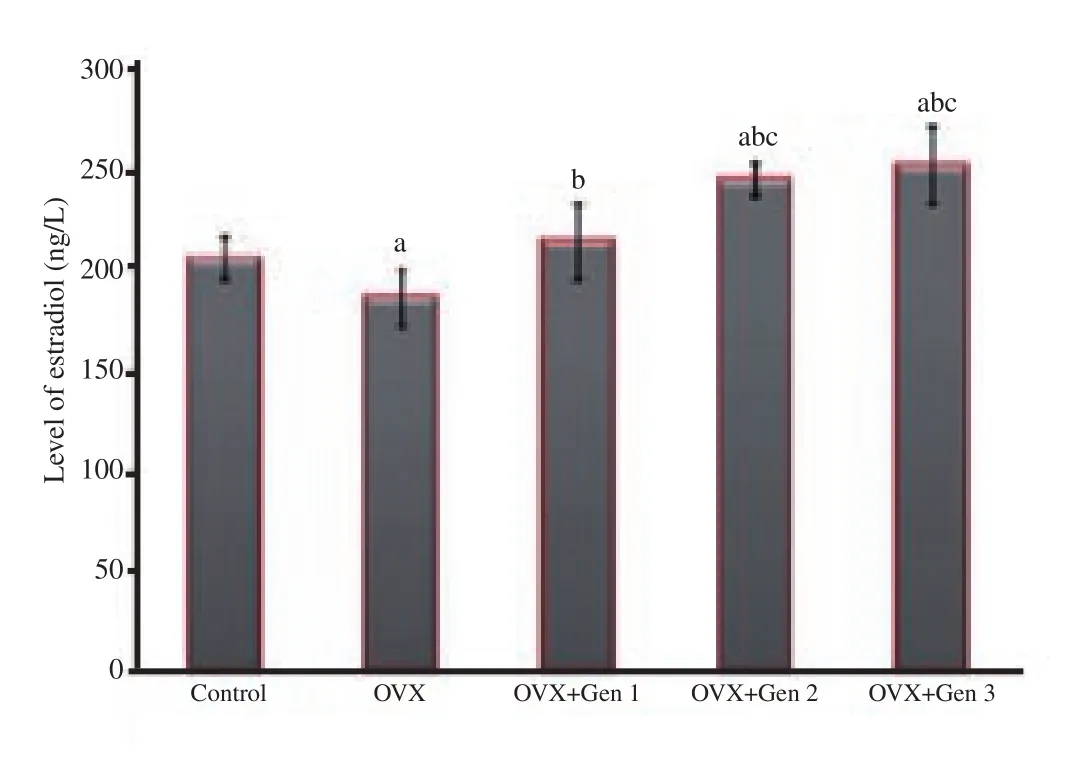
Figure 1.Levels of estradiol in different treatment groups.a: There are significant differences compared with the control group (P<0.05); b:there are significant differences compared with the ovariectomized only group (P<0.05); c: there are significant differences compared with the ovariectomized group that received genistein at the 0.045 mg/kg body weight/day dosage level (OVX+Gen 1) (P<0.05).
The expression of Hsp70 was significantly higher in the ovariectomy only group than in the control group (P<0.05).All three doses of genistein administration significantly reduced Hsp70 expression as compared with the ovariectomy only group (P<0.05),achieving expression levels comparable to the control group for the lowest dose (0.045 mg/kg) (P>0.05).The expression of Hsp70 was significantly lower in genistein group at dose of 0.090 or 0.180 mg/kg body weight/day compared with the control group or the ovariectomy only group (P<0.05).There was no significant difference of Hsp70 level between these two highest doses (P>0.05)(Figure 3).
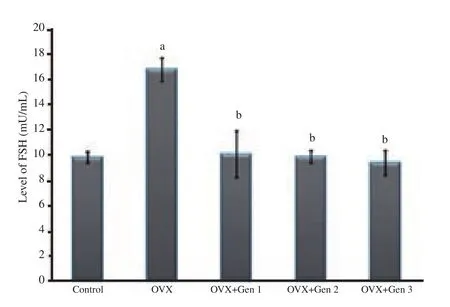
Figure 2.FSH levels in different treatment groups.a: There are significant differences compared with the control group (P<0.05); b: there are significant differences compared with the ovariectomized only group(P<0.05).Note: OVX: ovariectomy; OVX+Gen 1: Rats are ovariectomized and receive a genistein dose of 0.045 mg/kg body weight/day; OVX+Gen 2:Rats are ovariectomized and receive a genistein dose of 0.090 mg/kg body weight/day; OVX+Gen 3: Rats are ovariectomized and receive a genistein dose of 0.180 mg/kg body weight/day.
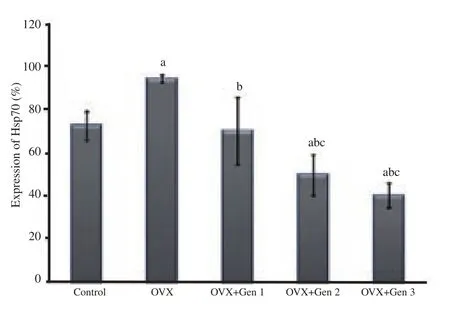
Figure 3.Expression of Hsp70 in different treatment groups.a: There are significant differences compared with the control group (P<0.05);b: there are significant differences compared with the ovariectomized only group (P<0.05); c: there are significant differences compared with the ovariectomized group received to genistein at the 0.045 mg/kg body weight/day (OVX+Gen 1) (P<0.05).
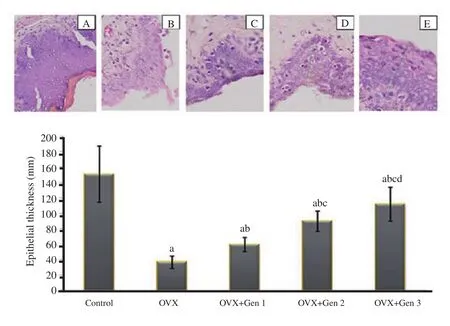
Figure 4.Histology of epithelial vaginal tissue in the control and treatment groups (hematoxylin eosin; magnification ×400)(upper panel).A: the control group; B: the ovariectomized only group; C: the ovariectomized group receiving genistein at the 0.045 mg/kg body weight/day dosage level (OVX+Gen 1); D: the ovariectomized group receiving genistein at 0.090 mg/kg body weight/day dosage level (OVX+Gen 2); E: the ovariectomized group receiving genistein at 0.180 mg/kg body weight/day dosage level (OVX+Gen 3).Vaginal epithelial thickness in different treatment groups (lower panel).a: There are significant differences compared with the control group (P<0.05); b: there are significant differences compared with the ovariectomized only group (P<0.05); c: there are significant differences compared with the ovariectomized group received to genistein at the 0.045 mg/kg body weight/day dosage level (P<0.05) (OVX+Gen 1); d: there are significant differences compared with the ovariectomized group received to genistein at the 0.090 mg/kg body weight/day dosage level(P<0.05) (OVX+Gen 2).
3.4.Vaginal thickness
The thickness of the vaginal epithelium in ovariectomized rats was significantly lower than in the control group (P<0.05).There was a significant increase in vaginal epithelial thickness in all three ovariectomy groups treated with genistein as compared with the ovariectomy only group (P<0.05), but was unable to reach a thickness in the control group (P<0.05).The vaginal thickness was significantly higher in the 0.090 mg/kg body weight/day group compared with the 0.045 mg/kg body weight/day group (P<0.05).The vaginal thickness was significantly higher in the 0.180 mg/kg body weight/day group compared with the 0.090 mg/kg body weight/day group (P<0.05) (Figure 4).
4.Discussion
In this study, ovariectomy suppressed the production of estradiol and significantly increased the FSH levels as compared with the control group.This finding suggests that ovarian loss causes suppression of estradiol release accompanied by an increase in FSH levels.This study is consistent with previous studies that ovariectomy triggers a significant decrease in serum estrogen levels compared to the control group[22,23].This increase in FSH levels was also confirmed in a previous study[24], which aimed to support the change of cholesterol into estrogen in ovariectomy rats[25].The lowest dose of genistein can increase the levels of estradiol to levels observed in the control group.In the case of FSH, all doses of genistein suppressed the increase in FSH, reaching levels similar to that in the control group.This finding indicates that genistein can normalize the hormones, estradiol, and FSH in vaginal atrophy.This study extends previous findings that genistein can control FSH levels depending on estrogen receptor activity[26].For hormone modulation,the normalization of estradiol levels is played by the lowest dose genistein.Meanwhile, for FSH modulation, all genistein doses can return to normal values.These findings indicate that genistein works in the biosynthesis of estradiol and FSH.Under these pathological conditions, the optimal dose is different for modulation of estradiol or FSH.
In this study, the thickness of the vaginal epithelium decreased significantly in the ovariectomy only group as compared with the control group.This finding shows the existence of thinning or atrophy of the vaginal wall in ovariectomy rats, which is consistent with previous studies[27,28].This depletion is thought to be caused by changes in the population of vaginal epithelial cells owing to decrease of cells proliferation, as proven in previous studies[29].We suspect that this atrophy mechanism occurs owing to an increase in stress due to estrogen deficiency, which is characterized by estradiol deletions and significantly increased expression of Hsp70 as compared with that in the control group.This result is consistent with previous findings that Hsp70 upregulation occurs under conditions of cellular stress[30].The mechanism of Hsp 70 changes due to ovariectomy and the effect of genistein in repairing the changes are thought to be played by heat shock factor[31].
In this study, administration of genistein increased vaginal thickness, but had not yet reached thickness in the control group.This finding indicates that genistein induces cell proliferation of vaginal epithelial cells.The mechanism of increased thickness of the epithelium is in part related to the mechanism of hormonal modulation and decreased cellular stress.Previous studies have shown that herbal contains genistein or vaginal genistein is able to improve vaginal atrophy via epithelial cells proliferation[16,32].
In conclusion, genistein is able to increase the thickness of the vaginal epithelium through hormone modulation and cellular stress suppression.Thus, genistein can be consumed as a herbal or alternative food for the improvement of vaginal atrophy due to menopause.
Conflict of interest statement
The authors declare there is no conflict of interest.
 Asian Pacific Journal of Reproduction2020年4期
Asian Pacific Journal of Reproduction2020年4期
- Asian Pacific Journal of Reproduction的其它文章
- Prospects of diagnostic and prognostic biomarkers of pyometra in canine
- Hemodynamic changes in arterial flow velocities throughout the first six months of pregnancy in buffalo heifers by Doppler ultrasonography
- Seasonal changes in sperm parameters, testicular histology and circulating levels of reproductive hormones in the male African straw-colored fruit bat (Eidolon helvum)
- Process of becoming a mother in women receiving donated egg: Based on the grounded theory
- Food insecurity and other possible factors contributing to low birth weight: A case control study in Addis Ababa, Ethiopia
- Effects of nitric oxide on reproductive organs and related physiological processes
