Endoscopic ultrasound-guided injective ablative treatment of pancreatic cystic neoplasms
Chen Du, Ning-Li Chai, En-Qiang Linghu, Hui-Kai Li, Xiu-Xue Feng
Abstract
Key words: Endoscopic ultrasound-guided ablation; Pancreatic cystic neoplasm; Ethanol;Paclitaxel; Lauromacrogol; Gemcitabine
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) used to be ignored because most are asymptomatic[1]. With the development of cross-sectional imaging modalities and the increasing attention being paid to physical examinations, the prevalence of PCNs is estimated to be nearly 20%[2-6]. However, PCNs comprise a broad differential spectrum of tumors that are difficult to distinguish because of their varied biological behaviors[7,8]. In general, PCNs can be categorized into four types: Serous cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs), intraductal papillary neoplasms (IPMNs), and other types. IPMNs are subcategorized into branch duct IPMNs (BD-IPMNs), main duct IPMNs (MD-IPMNs), and mixed IPMNs according to the type of pancreatic duct that is connected to the cysts. Other types of PCNs mainly include solid pseudopapillary neoplasms (SPNs) and cystic pancreatic neuroendocrine tumors (NETs). SPNs and pancreatic NETs should be surgically resected to eliminate their malignant potential. SCNs are regarded as benign lesions, while mucinous cysts, such as MCNs and IPMNs, are related to malignancy or have malignant potential. Unfortunately, it is difficult to achieve an accurate diagnosis of the type of PCN, making clinical decisions difficult. Histological accuracy could be improved by the development of techniques, such as endoscopic ultrasound-guided fine needle biopsy (EUS-FNB), single-operator cholangioscopy (SOC), and EUSguided through-the-needle biopsy (EUS-TTNB)[9-12]. However, these examination methods are challenging to perform, and this affects their wide application.
Surgical resection is an excellent way to prevent PCNs from evolving to malignancy; however, the morbidity and mortality rates related to a major pancreatic resection of a cystic lesion are 10%-40% and 1%-3%, respectively[13-16]. Long-term surveillance may not only increase the financial burden on and psychological stress in patients but could also result in a missed malignancy. The survival rate of patients with malignant pancreatic lesions is very low[2,17]. Therefore, a minimally invasive treatment, EUS-guided ethanol ablation, was reported by Ganet al[14]in 2005 as an effective way to treat PCNs. The effectiveness and safety of EUS-guided injective ablative treatment has been verified for over 15 years. Several ablative agents,including ethanol, paclitaxel, lauromacrogol, and gemcitabine, have been effectively used to treat PCNs[2,15,18]. While several reviews about EUS-guided ablation have been published[19-23], no systematic review has evaluated this method in detail from patient preparation to follow-up. In the present review, we describe EUS-guided injective ablation for the treatment of PCNs with regard to the indications and contraindications, preoperative treatment, endoscopic procedure, postoperative care, efficacy and safety outcomes, and current controversies and future perspectives.
INDICATIONS AND CONTRAINDICATIONS
EUS-guided injective ablation could be considered for the following patients: (1)Those with a presumed or confirmed diagnosis of an MCN or those with an enlarging or symptomatic SCN; (2) Those with a mass diameter of at least 1 cm; (3) Those with six or fewer locules (i.e., unilocular or oligolocular cystic lesions); (4) Those with an expected life expectancy; and (5) Those who provided informed consent.
The following patients were also considered for enrolment: (1) Those with a presumed or confirmed diagnosis of BD-IPMN; (2) Those with a multilocular cyst with more than 6 locules; and (3) Those with multiple pancreatic cysts. However, the treatment response in these patients may not be as promising, and the procedure might be more challenging.
The relative contraindications for this procedure are as follows: (1) Patients with a high risk of malignant transformation, including jaundice, an enhancing mural nodule> 5 mm, a main pancreatic duct (MPD) diameter > 10 mm, an MPD stricture with pancreatic tail atrophy, and a significant solid component[3,24]; (2) Those with a history of acute pancreatitis; and (3) Those with a short life expectancy.
The absolute contraindications were as follows: (1) A presumed or confirmed diagnosis of MD-IPMN or mixed IPMN; (2) Pregnancy; (3) Irreversible coagulopathy;(4) A high-risk operation; (5) Evidence of active acute pancreatitis or pancreatic necrosis, and (6) An inability to eliminate pancreatic cancer or signs of malignancy.
Pancreatic cysts with six or fewer locules and measuring 2 to 6 cm in diameter are predicted to respond best to ablation[24]. The presence of too many locules affects the lavage procedure, causing some areas of the cystic wall to remain free from ablative solution, potentially leading to an unexpected result. Moreover, it is time-consuming to treat multilocular cysts because needle puncture is supposed to create communication between locules through the septum so that the ablative agent enters into each locule. There seems to be no consensus regarding the limit that cyst diameter makes a cyst unsuitable for EUS-guided ablation. It is challenging and dangerous to use this method in cysts with a diameter less than 1 cm; therefore, the maximum diameters of the cysts should be at least 1 cm[25]. The upper limit of the cystic diameter for successful ablation also remains controversial. Most previous studies enrolled patients in whom the maximum diameter of the cyst was smaller than 5 cm[16,25-27]; however, cysts as large as 11.9 cm have also been reported to be safely treated using this method[28]. The risk of malignancy is significantly increased in patients with large cysts[4], and surgical resection seems to be more suitable than EUSguided injective ablation for large PCNs. However, further studies are warranted to define the upper limit for cyst size for this procedure. Some authors have suggested that the presence of an IPMN is not an optimal indication for EUS-guided injective ablation[2,29]. Because of the communication between the cyst and the pancreatic duct of an IPMN, the ablative agent might escape the cyst, resulting in a poor treatment response and a higher risk of pancreatitis. In addition, IPMNs larger than 3 cm have a greater malignant potential and are not suitable for EUS-guided ablation. Some authors have suggested EUS-guided injective ablation as a promising method to treat non-neoplastic cysts, mainly pseudocysts (PCs)[30].
PREOPERATIVE TREATMENT
Patients suspected of having PCNs should be sent for blood tests [e.g., amylase, lipase,and tumor markers (mainly CEA and CA199)] prior to ablation. Enhanced pancreatic computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography, and EUS should also be conducted to obtain an accurate diagnosis and evaluate the size, location, wall thickness, and number of septations of the tumors; the morphology of the pancreatic duct; the presence of papillae or an associated mass; and the blood supply. EUS-guided fine-needle aspiration (EUS-FNA)is also recommended for obtaining cyst fluid for biochemical and cytological examinations to aid in the diagnosis of the cyst using 19-gauge or 22-gauge needles[4,24]. Enhanced EUS and FNB and SOC performed under EUS guidance can provide useful information for diagnosing pancreatic cysts[9,10]. EUS-TTNB allowed a high rate of adequate specimens to be obtained for histology with an overall histological accuracy rate of 86.7%[11]. However, its complication rate was slightly higher than that of standard EUS-FNA. It can be applied in selected patients by experienced operators.
There is some controversy regarding the use of prophylactic antibiotics. Some studies have recommended that prophylactic antibiotics be used to prevent postprocedural infection[5,24,30,31], while other studies performed without prophylactic antibiotic administration did not have increased complication rates[2,15,32,33].
EUS-GUIDED INJECTIVE ABLATIVE PROCEDURE
EUS-guided injective ablation is conducted with patients lying in the left-lateral position under intravenous anaesthesia. First, the cystic lesion is reidentified and recharacterized. Second, transgastric or transduodenal puncture of the cyst is performed using a 19-gauge or 22-gauge needle. A 19-gauge needle has one advantage in that it allows the aspiration of more viscous material than can be obtained using a 22-gauge needle and can be used as a tunnel for EUS-FNB,potentially resulting in a more accurate pathological diagnosis. However, the larger diameter of the needle might also increase the possibility of procedure-related complications, such as bleeding. The use of a 22-gauge needle produced better results in small cysts (those less than 2.5 cm), while the 19-gauge needle was more suitable for cysts > 2.5 cm[18,34]. The cyst fluid should be aspirated as much as possible, and a small amount of fluid around the tip of the needle is left within the cyst to prevent the possibility of pancreatic wall injury and ablative agent extravasation. Then, the ablative agent is injected into the cyst.
Several ablative agents, such as ethanol, paclitaxel, lauromacrogol, and gemcitabine, are available, and several ablative methods can be performed using these agents (Table 1). Ethanol was the first solution used as an ablative agent to treat PCNs. The concentration of ethanol reported in previous studies ranged from 80% to 100%, with 80% and 99% being the most commonly used concentrations[25,27,32,35]. In 2008, paclitaxel was injected after ethanol lavage as a novel treatment for PCNs[5].Later, an ethanol-free ablation protocol that used a cocktail of paclitaxel and gemcitabine as an ablative solution was demonstrated to be safe and feasible[34].Ethanol lavage was evaluated to determine whether it could prevent malignancy in pancreatic cysts[25]. Additionally, lauromacrogol, as a sclerosant, was first reported for the ablation of PCNs with the aid of EUS in 2017 by Linghuet al[2], who demonstrated it to be effective and safe.
The ablation procedures differ slightly among these agents. When using ethanol,the cyst cavity is lavaged for 3 to 5 min, with the cavity alternately filled and emptied[14]. The injection volume remains unknown. Some studies have recommended that the ethanol volume should be equal to that originally aspirated[28,32], while in other studies, the injected ethanol volume was equivalent to 50% and 90% of the fluid extracted from the cyst[25,33]. Because ethanol should be retained for 20 to 40 min while the position of the patient was rotated to ensure that the cyst wall was completely ablated, this procedure was slightly complicated and time consuming[24]. Ethanol must then be fully aspirated. In some studies, paclitaxel was then infused at a concentration of 2 mg/mL[26,27], 3 mg/mL[5,15,31], or 6 mg/mL[15,28,31]after ethanol aspiration. The volume of the paclitaxel solution administered was the same as that of the cyst fluid that was aspirated[5,15,29,31], and the dose of paclitaxel has been reported to range from 1.5-30 mg[5,15,28,29]. Finally, paclitaxel is left in the cyst cavity.
Lauromacrogol is a sclerosant that has been widely used in the treatment of esophageal and gastric variceal bleeding. It was initially reported to treat PCNs by Linghuet al[2]in 2017. Lauromacrogol (Lauromacrogol Injection, 10 mg/mL; Tianyu Pharmaceutical Co. Ltd., Shanxi, China) lavage was also performed for 3 to 5 min to increase its concentration in the cyst. However, it was not necessary to retain lauromacrogol in the tumor for 20-40 min. Approximately 2-10 mL of pure lauromacrogol was left in the cyst cavity.
Paclitaxel (3 mg/mL) and gemcitabine (19 mg/mL) were mixed to make a paclitaxel-gemcitabine cocktail. The cocktail was infused using a 30-cc syringe custom fitted to a high-pressure gun to allow timely infusion of the cocktail. The upper limit for the volume of the chemotherapeutic cocktail was 8 mL, as per Food and Drug Administration stipulations[18,34]. Two prospective, randomized, double-blinded studies were reported to evaluate whether ethanol is necessary for EUS-guided pancreatic cyst ablation. Patients underwent lavage with either 80% ethanol or normal saline, followed by the infusion of an admixture of paclitaxel and gemcitabine. These studies demonstrated that ethanol is not required for effective EUS-guided pancreatic cyst ablation but is likely to cause more complications[18,34].
Finally, the needle was retrieved, and the needle puncture on the gastric or duodenal wall was carefully examined.
POSTOPERATIVE CARE AND FOLLOW-UP
After ablation, patients should be carefully monitored to record any problems or symptoms. Complications, such as abdominal pain, abdominal distention, fever,vomiting, hypotension, hematemesis, hematochezia, and bleeding, should be recorded. Serum amylase and lipase levels and complete blood counts should be assessed the morning after the procedure. However, in some studies, the patients were discharged from the hospital only 2 h post procedure without any blood tests[18,25,26,32].
Whether proton pump inhibitors (PPIs) should be used in these patients is controversial. Most studies have not mentioned the use of PPIs[18,26,28,32,35], however,Linghuet al[2]intravenously administered PPI for 3 d, followed by oral PPI intake for 3 to 7 d. Whether the use of PPIs decreases the possibility of pancreatitis related to EUSguided ablation remains unknown. In addition, there is no consensus regarding the use of antibiotics. Some studies did not use any antibiotics[5,15,25,31,32], while others included the administration of intravenous or oral antibiotics[2,26,35]. Octreotide was intravenously administered for at least one day until the serum amylase level returned to normal in a study of lauromacrogol[2]. Patients suffering from severe pain or suspected pancreatitis are recommended to undergo abdominal ultrasound or CT.
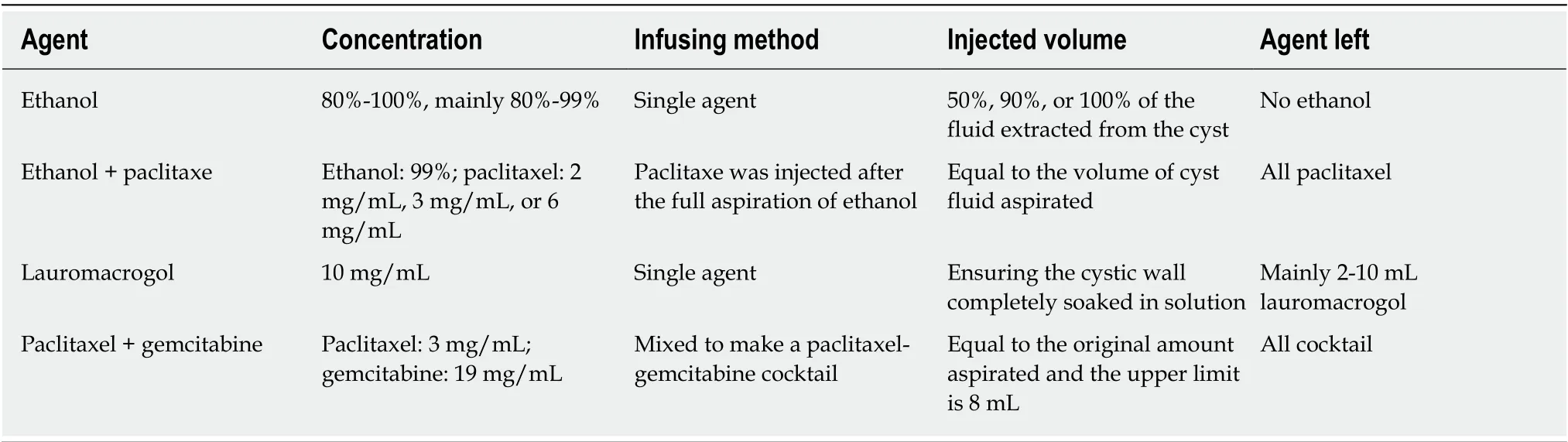
Table 1 Different injective ablative treatment methods
This procedure had a shortened hospital time of 2 h postprocedure[18,25,26,32,34,35], and several studies reported that patients could be discharged 2 d after the procedure if no complications were noted[5,15,28,31].
However, the appropriate follow-up period remains controversial. Most studies have recommended that follow-up pancreatic CT or MRI should be performed 3 mo after the last planned cyst lavage and then at 6-mo intervals and then annually thereafter[2,5,15,16,27,29]. More frequent follow-up CT scans were recommended for patients with a persistent cyst at the first follow-up, in whom scans should be performed at 3-mo intervals instead of 6-mo intervals[15]. EUS was also regarded as a follow-up examination in some studies[16,27,33]. An international expert panel stated that patients should undergo cross-sectional imaging at 6-mo intervals for the first year and then annually thereafter[24]. Another study suggested that abdominal imaging should be repeated 3-4 mo and 12 mo after the second EUS[26]. In several studies,patients were discharged 2 d after ablation if no complications were noted[15,31].
EVALUATION METHOD
Treatment responses can be divided into three levels: Complete resolution (CR),partial resolution (PR), and persistent cyst. Several methods can be used to evaluate the treatment response; these include a decrease in the cystic surface area[32,35],volume[2,5,15,18,26,29,31,34], or diameter [Response Evaluation Criteria in Solid Tumors(RECIST)][14,16]. However, the RECIST aim to evaluate the treatment response of solid neoplasms, and therefore seem unsuitable for cystic lesions[6]. It appears that it may be more convincing to determine the effectiveness of ablation based on changes in cystic volume. The volume method was assessed by comparing the volume recorded before the procedure [original volume (OV)] with that obtained at the follow-up [final volume (FV)]. CR was defined as a FV < 5% of the OV; PR was defined as a FV ranging from 5% to 25% of the OV; and a persistent cyst was defined as an FV > 25%of the OV. However, studies exploring whether a decrease in the volume is related to low malignant potential are lacking.
As the OV evaluation method is less detailed, we suggest a new method. In this method, the treatment response can be divided into five levels, with CR defined as a FV ≤ 10% of the OV; PR as 10% of the OV < FV ≤ 25% of the OV; fair resolution as 25%of the OV < FV ≤ 75% of the OV; persistent cyst as 75% of the OV < FV ≤ 100% of the OV; and progressive cyst as an FV ≥ 100% of the OV. However, this method needs to be verified.
Re-ablation could be considered for patients with PR, persistent cysts, or progressive cysts if the cysts are larger than 1 cm. However, it remains unknown whether surgical resection should be recommended if the effectiveness of EUS-guided re-ablation is unsatisfactory. For patients who did not achieve CR, the follow-up can involve more frequent appointments and last longer.
TREATMENT EFFICIENCY
In all, 16 original articles have been reported on EUS-guided injective ablation; of these, six used ethanol, seven used ethanol lavage followed by paclitaxel, one used lauromacrogol, and two used the paclitaxel and gemcitabine cocktail (Table 2).
In the first study to explore EUS-guided cyst ablation with ethanol, Ganet al[14]enrolled 25 patients with cysts of a mean diameter of 19.4 mm to be treated by 5%-80% ethanol. CR was achieved in eight (34.8%) of the 23 patients who completed a follow-up period of 12 mo. Later studies reported a CR rate ranging from 8.7% to 84.6%, with study sizes ranging from 13 to 42 patients[25,32,33,35]. Although the CR rate for ethanol ablation varies widely, 3 studies reported a CR rate of approximately 35%.
The effectiveness of ethanol alone seems limited[36]. To improve treatment responses, paclitaxel, a widely used chemotherapeutic agent, has been used as an agent following ethanol lavage to treat PCNs. After a follow-up period of 9-72 mo, the CR rates for this treatment have ranged from 50.0% to 78.6%[5,15,26-29,31], with the average CR rate for EUS-guided ethanol lavage with paclitaxel injection being approximately 60.0%, which is slightly higher than that of ethanol ablation.
Lauromacrogol is a sclerosant with a mild anesthetic effect that was initially reported as a treatment for PCNs by Linghuet al[2]. Twenty-nine patients underwent EUS-guided ablation with lauromacrogol; of these 7 underwent a second ablation,leading to a total of 36 treatments. Among the 36 treatments, 29 completed a followup at 3 mo after the first or second ablation, and 11 (37.9%) achieved CR (Figures 1 and 2). The CR rate for lauromacrogol was similar to that of ethanol and slightly lower than that of ethanol lavage with paclitaxel injection. However, Linghuet al[2]evaluated ablative treatment based solely on 3-mo imaging examinations after ablation despite a mean follow-up period of 9 mo. Moreover, the response to each ablative treatment rather than that of each patient was documented, leading to an underestimation of ablative effectiveness. Further studies with longer follow-up periods are needed to evaluate the effectiveness of this promising agent.
To evaluate whether ethanol is required for effective PCN ablation and related to complication rates, Moyeret al[18]performed a prospective, double-blind trial of 39 patients with MCNs and compared the effects of 80% ethanol (control group) to those of normal saline (ethanol-free group). All enrolled patients in the two groups were then infused with an admixture of paclitaxel and gemcitabine. The authors concluded that ethanol was not required for effective EUS-guided pancreatic cyst ablation because the CR rates in the two groups were similar, and the removal of ethanol decreased the complication rate. Their results also demonstrated that the paclitaxelgemcitabine cocktail provided no advantages over the current standard consisting of alcohol lavage followed by paclitaxel alone.
Several comparative studies have further evaluated factors that may predict improved effectiveness. DeWittet al[35]designed a multicenter, randomized, doubleblind study to determine whether EUS-guided ethanol lavage performed better than saline lavage. They concluded that EUS-guided ethanol lavage decreased the size of the PCNs to a greater extent than was achieved by saline lavage and did not increase the complication rate. DiMaioet al[32]evaluated the effectiveness of multiple ethanol lavage sessions and found that the size and surface area of the treated PCNs decreased more following two ethanol lavage treatments than with only one ethanol lavage treatment.
Multiple ethanol lavage sessions might lead to a high rate of image-defined cyst resolution. Some studies compared the CR group and the non-CR group[2,15,16,18,25,27,29].Cyst diameter was reported to be a predictive factor for CR in three studies, indicating that a small cyst may be ablated effectively[15,16,29]; however, Moyeret al[18]and Linghuet al[2]reported that the initial diameter did not affect the treatment response. The study of Ohet al[15]revealed that cystic volume predicted an ablative response,inconsistent with other reports[2,25]. Most studies have reported that the diagnosis of PCNs has no effect on the ablative results[2,15,18,29]; however, Parket al[16]professed doubt in these findings. They found that patients with IPMNs were less likely to achieve CR than those with other tumor types. In the univariate and multivariate analyses, Choiet al[29]found that a unilocular form predicted CR; however, other studies described inconsistent results[2,16,18,25]. No significant difference was revealed in age, sex, or cyst location between the CR and non-CR groups[2,16,25,29]. Kimet al[27]prospectively studied sonographic and cyst fluid cytological changes after EUSguided ablation and found that there was no significant difference in the frequency of sonographic or cytological features between CR and non-CR patients.
Mutant DNA appeared to be eliminated after EUS-guided ethanol lavage with paclitaxel[26]. In another study, four patients with MCNs underwent surgical resection after ablation and histologic findings showed cyst epithelial ablation ranging from 0%(saline solution alone) to 50% to 100% (1 or 2 ethanol lavages), and no evidence ofdysplasia or malignancy after resection was observed[35]. Ohet al[15]found that the histopathologic extents of epithelial lining denudation were 25%, 40%, 100%, and 0%in four patients who underwent surgical resection after ablation. The study by Choiet al[29]enrolled 164 patients, 12 of whom received surgical resection. The histopathological extents of epithelial lining denudation were 25% (n= 2), 40% (n= 2),100% (n= 7), and 0% (n= 1)[29].
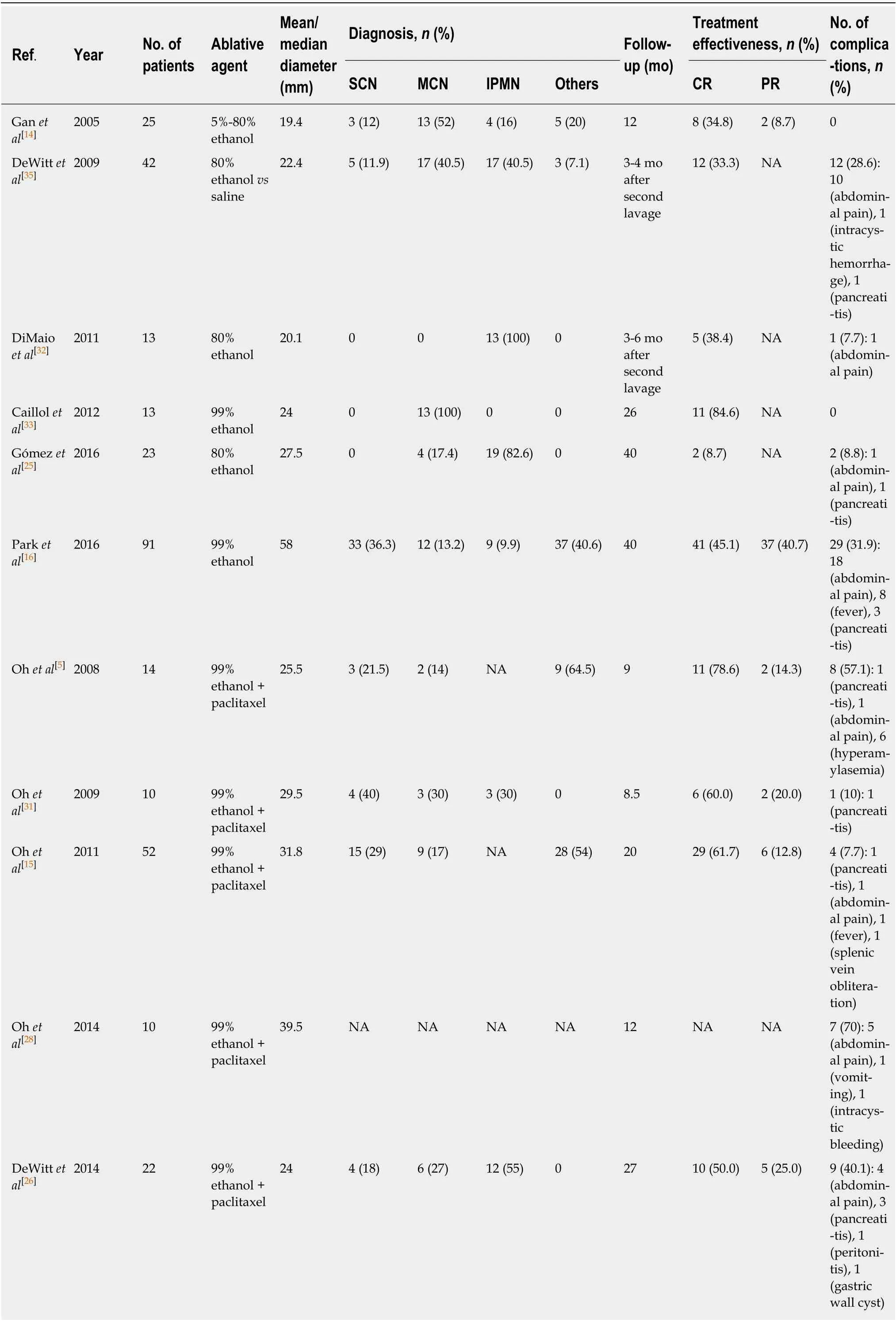
Table 2 Studies of EUS-guided ablation using different agents
SCN: Serous cystic neoplasm; MCN: Mucinous cystic neoplasm; IPMN: Intraductal papillary neoplasm; CR: Complete response; PR: Partial complete; NA:Not available.
In a study on the largest patient sample to date (n= 164), 114 (72.2%) patients achieved CR, and 112 (98.3%) remained in remission at the 6-year follow-up[29]. The author concluded that EUS-guided ablation was effective and durable and had a high CR rate and low recurrence rate during the long-term follow-up.
SAFETY PROFILE
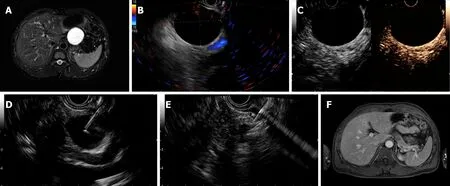
Figure 1 Complete resolution was achieved in a patient with a serous cystic neoplasm. A: Magnetic resonance imaging (MRI) before endoscopic ultrasoundguided (EUS-guided) ablation showing a 52 mm × 52 mm × 41 mm cyst located in the pancreatic body; B: EUS evaluation of the cyst showing a 46.0 mm × 39.0 mm cyst in the body; C: Enhanced EUS view showing no obvious enhancement of the cystic wall; D: EUS-guided fine needle aspiration to aspirate cyst fluid; E: Injection of the ablative agent through the needle; F: Follow-up MRI at 4 mo after ablation showing complete resolution.
Although EUS-guided injective ablation is regarded as a minimally invasive and safe procedure[37], some procedure-related complications have been reported (Table 2).Abdominal pain is the most common complication, followed by pancreatitis.Intracystic hemorrhage has also been reported in some studies[27-29,35]. Some rare complications, such as fever, splenic vein obliteration, portal vein thrombosis,hyperamylasemia, vomiting, peritonitis, gastric wall cyst, PC, abscess, pericystic spillage, and pancreatic duct stricture, have also been reported.
In one study, the total complication rate of EUS-guided ethanol ablation was 21.2%,while that of EUS-guided ethanol lavage with paclitaxel was 15%[37]. EUS-guided lauromacrogol ablation was successfully performed in all 36 treatments, while mild pancreatitis occurred in two treatments and moderate fever in one treatment[2]. The complication rate was 8.3%. Moyeret al[18,34]performed two studies using EUS-guided ablation with an admixture of paclitaxel and gemcitabine. Abdominal pain and pancreatitis were noted, with the total complication rate ranging from 10% to 12.8%.The complications related to EUS-guided ablation were similar to those related to EUS-FNA. Most of these complications were minor and could recover with conservative management. However, Ohet al[38]reported one case in which portal vein thrombosis occurred after EUS-guided ethanol lavage with paclitaxel injection. In their study, this 68-year-old woman with a 5.2 cm × 4.5 cm cyst at the head of the pancreas died of portal vein thrombosis. Chunet al[39]reported a case of duodenal stricture induced by necrotizing pancreatitis following EUS-guided ethanol ablation.The 61-year-old patient was suspected of having BD-IPMN and treated with 99%ethanol lavage. He was conservatively managed for acute interstitial pancreatitis after ablation. Unexpectedly, aggravated abdominal pain and vomiting occurred, and abdominal CT demonstrated walled-off necrosis around the pancreatic head and duodenal stricture. A total of five consecutive sessions of endoscopic balloon dilatation were performed to relieve his obstructive symptoms. Clinicians should therefore operate carefully enough to avoid these rare but severe complications.
The incidences of abdominal pain in EUS-guided ethanol with and without paclitaxel were 4% and 14.5%, respectively[7,37]. Abdominal pain might result from the EUS operation, EUS-FNA procedure, or the use of ablative agents. Linghuet al[2]reported that because of its anesthetic effect, lauromacrogol performed better than ethanol and paclitaxel in relieving pain during and after ablation. No patients suffering from abdominal pain were noted in this study.
Pancreatitis was also common, with incidences of 5% and 2.4% in EUS-guided ethanol with and without paclitaxel, respectively[7,37]. MD-IPMN was regarded as an absolute contraindication for EUS-guided injective ablation mainly because of the strong possibility of procedure-related pancreatitis. BD-IPMNs were theoretically more likely to result in procedure-related pancreatitis than SCNs and MCNs;however, a study by DiMaioet al[32]demonstrated that EUS-guided ethanol ablation was safe in patients with BD-IPMNs, with only one (7.7%) patient experiencing postprocedure minor abdominal pain. When patients complained of severe abdominal pain with high levels of lipase and/or amylase, they were asked to undergo abdominal CT.
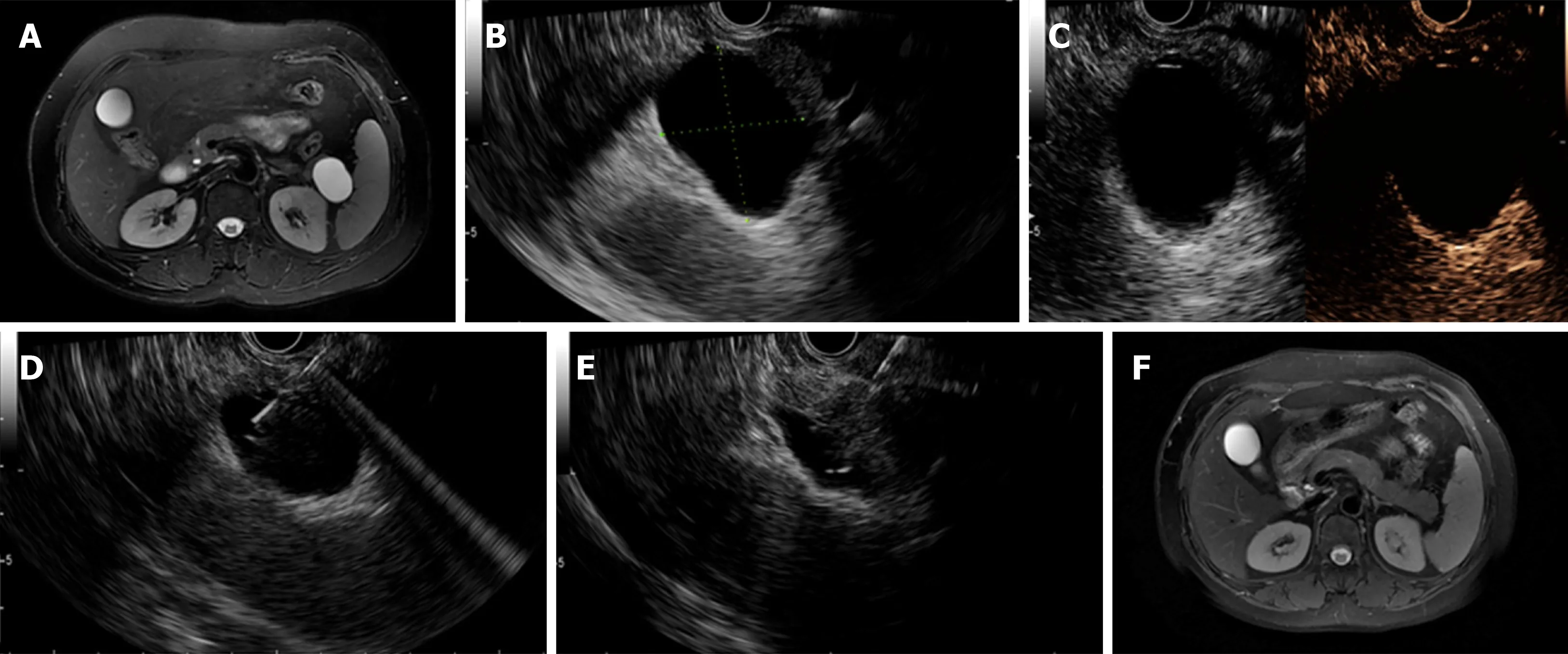
Figure 2 Complete resolution was achieved in a patient with a mucinous cystic neoplasm. A: Magnetic resonance imaging (MRI) before endoscopic ultrasoundguided (EUS-guided) ablation showing a 38.0 mm × 26.0 mm cyst located in the pancreatic body; B: EUS evaluation of the cyst showing a 37.0 mm × 32.0 mm cyst located in the pancreatic tail; C: Enhanced EUS view showing moderate enhancement of the cystic wall; D: EUS-guided fine needle aspiration to aspirate cyst fluid; E:Injection of the ablative agent through the needle; F. Follow-up MRI at 3 mo after ablation showing complete resolution.
Ohet al[28]analyzed the plasma paclitaxel concentration after EUS-guided pancreatic cyst ablation and found that it was nearly undetectable and thus unlikely to cause systemic side effects. These findings revealed that a paclitaxel dose of 6 mg/mL was safe.
TIPS AND TRICKS
Several tips and tricks are recommended to facilitate the effectiveness and safety of ablation. First, a small amount of cyst fluid should be left around the tip of the needle before the ablation process is performed to prevent the needle from damaging the surrounding pancreatic wall[24]. Either 19-gauge or 22-gauge needles can be used in EUS-guided ethanol ablation. When the cyst fluid is too viscous to be aspirated,normal saline can be injected to dilute the fluid, and a 19-gauge needle is recommended in these cases. When the cyst is small or transduodenal puncture of the cyst occurs, a 22-gauge needle will perform well. The ablative agent should be used to lavage the cyst cavity for 3 to 5 min to increase the concentration of ethanol in the cyst. Finally, the agent concentration in the cyst should be roughly equal to its original concentration before being injected to the cyst.
CURRENT CONTROVERSIES AND PERSPECTIVES
Several technical challenges are associated with EUS-guided injective ablation. First,the optimal concentration used to achieve the best CR rate remains unknown.Different concentrations of ethanol have been used; their results have not been compared. Only one study has evaluated lauromacrogol ablation. Further studies are needed to determine the appropriate concentration of this drug to use. Second, no study has reported the lowest volume of agent that should be left in the cyst to achieve the best treatment response. Third, the optimal re-ablation time remains unknown. Parket al[16]reported that no more than 6 mo is needed for most patients undergoing EUS-guided ethanol ablation therapy to achieve CR. Another study by Ohet al[15]reported that CR was achieved 6-12 mo after ablation in 57.1% of patients. It remains unclear whether re-ablation should be considered if the patient does not achieve CR. Fourth, we used imaging changes to evaluate the treatment response.However, whether a decrease in size indicates low malignant potential remains controversial. Moreover, further double-blind, randomized controlled studies are needed to compare the effectiveness among ethanol, ethanol with paclitaxel,lauromacrogol, and the paclitaxel and gemcitabine cocktail.
Although several challenges are associated with EUS-guided injective ablation, it is a promising and minimally invasive method for treating PCNs that has excellent effectiveness. The surgical resection of pancreatic lesions can severely influence patients’ quality of life, especially when the lesions are located in the pancreatic head.Compared with surgical resection, EUS-guided injective ablation provides doctors and patients with a safer choice. We believe that with the optimization of this procedure, it will have a significant effect on PCNs. With the development of endoscopic equipment and research on new agents, this procedure may be indicated for other pancreatic lesions, such as SPNs, NETs, PCs, and even cancer.
CONCLUSION
EUS-guided injective ablation is a minimally invasive, effective, and safe treatment for PCNs in selected patients. Most procedure-related complications are minor and can recover with conservative management. Ethanol, paclitaxel, gemcitabine, and lauromacrogol have been used as ablative agents; however, it is difficult to state which is better. Further studies on EUS-guided ablation and additional randomized trials that compare different agents are warranted to optimize this treatment.
 World Journal of Gastroenterology2020年23期
World Journal of Gastroenterology2020年23期
- World Journal of Gastroenterology的其它文章
- Liver-directed therapies for liver metastases from neuroendocrine neoplasms: Can laser ablation play any role?
- Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection
- Endosonographic diagnosis of advanced neoplasia in intraductal papillary mucinous neoplasms
- Medications in type-2 diabetics and their association with liver fibrosis
- Pancreatic necrosis and severity are independent risk factors for pancreatic endocrine insufficiency after acute pancreatitis: A long-term follow-up study
- Impact of a national basic skills in colonoscopy course on trainee performance: An interrupted time series analysis
