Endosonographic diagnosis of advanced neoplasia in intraductal papillary mucinous neoplasms
Andrew Eiterman, Ali Lahooti, Somashekar G Krishna
Abstract
Key words: Pancreatic cyst; Confocal endomicroscopy; Microforceps biopsy; Cyst fluid molecular analysis; Endoscopic ultrasound; Intraductal papillary mucinous neoplasms
INTRODUCTION
Advances in abdominal imaging techniques have led to an increase in detection and diagnosis of pancreatic cystic lesions (PCLs)[1,2]. Although the majority of pancreatic cysts are asymptomatic and do not progress to adenocarcinoma, the ability to differentiate precancerous versus benign lesions and further estimate the risk for malignant transformation of precancerous lesions remains a challenge in the field[1,3].Among all solitary PCLs, branch duct (BD) intraductal papillary mucinous neoplasms(IPMN) are the most common type of precancerous lesions, and are often referred for surveillance due to their low chance of progressing to cancer[1,4]. However, even with the use of international consensus guidelines (ICG) for the evaluation of mucinous PCLs, advanced neoplasia (high grade dysplasia or adenocarcinoma) is frequently missed[5]. Moreover, surgery for PCLs must be highly selective since the risk of longterm significant morbidity is 30% with a 2.1% mortality rate[1,6]. Thus, a sensitive and accurate method measuring risk for advanced neoplasia in BD-IPMNs is critical to determine an optimal timeline for intervention for lesions that can potentially progress to adenocarcinoma.
Reflecting on morbidity rates for cyst resection, the recommendation of resection is becoming more heavily scrutinized. Determining the presence of low-grade or highgrade dysplasia informs the need and urgency for intervention, but evaluating dysplasia with current assessment methods remains a challenge[1]. A study by Sahoraet al[7]found that an estimated 75% of resected IPMNs were evaluated as low grade dysplasia that could have instead been monitored[7]. Additional studies have reported that for patients identified as having high-grade dysplasia, early identification significantly improved survival rates and eliminated or reduced risk for recurrence[1,8,9]. Thus, advances in identifying the risk and rate of transformation to advanced neoplasia could inform the ideal point for surgical intervention[10].
Risk assessment techniques in the field are lacking methods that can be used with frequency, possess excellent sensitivity and specificity, and are minimally invasive.First line assessment includes imaging techniques, followed by more invasive imaging or biopsy methodsviaendoscopic ultrasound (EUS)[1]. Computed tomography and magnetic resonance imaging technology provide abdominal imaging to characterize the cyst type and preemptively assess for the presence of high-risk or concerning features[1,11]. Cyst morphology on EUS imaging has similar accuracy in diagnosing a benign from malignant cyst to that of magnetic resonance imaging but is more invasive[1]. Additional methods that use EUS-guided technology can include fine needle aspiration (FNA), through-the-needle microforceps biopsy (EUS-MFB), and needle-based confocal laser endomicroscopy (EUS-nCLE). Advances in these techniques aim to address the critical need to assess risk for malignant transformation in IPMNs. These techniques and their feasibility for clinical adaptation will be summarized in this review.
HOW DO THE GUIDELINES PERFORM?
Clinical guidelines assist in the identification of advanced neoplasia in PCLs. The major society guidelines include 2012 and 2017 revised ICG, 2015 American Gastroenterological Association (AGA) guidelines, and the 2018 American College of Gastroenterology guidelines[11,12]. Changes in guidelines have improved identification of advanced neoplasia in IPMNs. Sighinolfiet al[13]retrospectively validated the 2006 ICG, 2012 ICG, and AGA criteria, where the 2012 ICG and AGA showed significantly improved specificity (58.2% and 62.4%, respectively) and diagnostic accuracy (67%and 68%, respectively) for advanced neoplasia compared to the 2006 ICG (specificity 32.7%, diagnostic accuracy 46%)[13]. Additionally, Kanget al[14]have demonstrated superior diagnostic performance of the 2017 ICG [area under the curve (AUC) 0.78]compared to 2012 ICG criteria (AUC 0.74) for the detection of malignancy in BD-or mixed-type IPMNs[14]. Yet, these consensus opinions remain suboptimal, and there is need for improved cyst surveillance methods and timing of surgical intervention.
ENDOSCOPIC ULTRASOUND-GUIDED METHODOLOGY FOR RISK STRATIFICATION OF INTRADUCTAL PAPILLARY MUCINOUS NEOPLASMS
Molecular analyses of cyst fluid
Cystic fluid carcinoembryonic antigen (CEA) acquired from EUS-FNA reflects a pooled 66% specificity and 65% sensitivity in identifying mucinous PCLs, yet it is not helpful in differentiating low-vshigh-grade dysplasia[1,15,16]. Overall, EUS-FNA has moderate diagnostic strength in identifying benign and malignant lesions, but lacks ability to monitor risk and progression of advanced neoplasia in the absence of obvious EUS-imaging morphological features of malignancy[17]. However, building on the use of this technique, as well as addressing its limitations, recent research has advanced to integrate the use of molecular and genetic testing from acquired cyst fluid as a potential solution for risk assessment[18].
Using analysis of DNA or miRNA mutationsviaalgorithms developed with wholegenome sequencing techniques, distinct genetic profiles have been developed to identify risk for malignant transformation from cystic fluid (Table 1)[1,19,20]. Mutations inKRASandGNAShave been specifically targeted, with 100% sensitivity and 96%specificity for the detection of IPMN. In addition, these mutations resulted in an 89%sensitivity and 100% specificity for IPMN and mucinous cystic neoplasms combined[1,18].
CombinationKRAS/GNASwith additional mutations inTP53,PIK3CA, andPTENshowed 88% sensitivity, 97% specificity for IPMN with advanced neoplasia[18].Additional mutations inTP53,PIK3CA, andPTENtesting showed 79% sensitivity,96% specificity for all mucinous pancreatic cysts with advanced neoplasia, showing potential for the role of molecular analyses in risk stratification of cysts. Other genes that have proven useful in risk stratification of IPMNs includeSMAD4,RNF43, andCDKN2A[18,21].
The benefits of such a technique address some of the previous sampling limitations of EUS-FNA CEA analysis, as molecular analysis uses a very small amount of cyst fluid (0.25 mL fluid) and can be helpful when aspirated volume is an issue[22].However, challenges with this technique include - reproducibility (replicating single center results in multicenter prospective studies), and feasibility for general hospitals[23]. Further, studies to date exploring the molecular markers have often used post-resected biologic specimens, thus creating the possibility for bias in the pathology of samples. Additional studies are needed to identify an adequate panel of genes for assessment of pancreatic cysts[18]. Results were also cross-validated using standard techniques such as cytology/CEA, whose sampling errors often results in discordant pairs of specimens for correlation, given challenges in diagnostic yield[17].Major advancements in this method have yet to solve the problem of monitoring risk for progression to advanced neoplasia, but its feasibility for repeated usage in clinical surveillance is optimistic[17].
EUS-guided microforceps biopsy
A relatively novel procedure, EUS-MFB utilizes through-the needle microforceps to acquire a tissue biopsy from PCLs for histologic analysis (Table 2)[24]. There is paucity of large, randomized trials evaluating EUS-MFB in PCLs; however, multiple systematic reviews and meta-analyses have sought to further evaluate the utility of this device. Most of these studies (Table 2) address only diagnostic yield without the estimation of diagnostic accuracy. Tacelliet al[25]reviewed 9 studies evaluating EUSMFB; technical success was achieved in 98.5% [95% confidence interval (CI): 97.3%-99.6%,I2(measure of heterogeneity) = 88.6%], specimen adequacy was achieved in 86.7% (95%CI: 80.1%-93.4%,I2= 84.3%), the pooled diagnostic yield was 69.5%(95%CI: 59.2%-79.7%,I2= 84.7%;P< 0.001), and pooled rate of adverse events was 8.6%[25]. Another recent meta-analyses by Facciorussoet al[26], involving 11 studies,revealed that specimen adequacy was 85.3% (95%CI: 78.2%-92.5%,I2= 41.5%), pooled diagnostic accuracy (8 of 11 studies) was 78.8% (95%CI: 73.4%-84.2%,I2= 28.4%), andrisk of adverse events was 8.98%[26]. In a study by Larghiet al[27], where EUS-MFB specimens (n= 40) were retrieved and evaluated by pathologists (n= 6), the interobserver agreement among pathologists was almost perfect for specimen adequacy (Gwet’s AC1 0.82, 95%CI: 0.79-0.98), and substantial for the diagnosis of PCL (AC1 0.62, 95%CI: 0.57-0.67)[27].
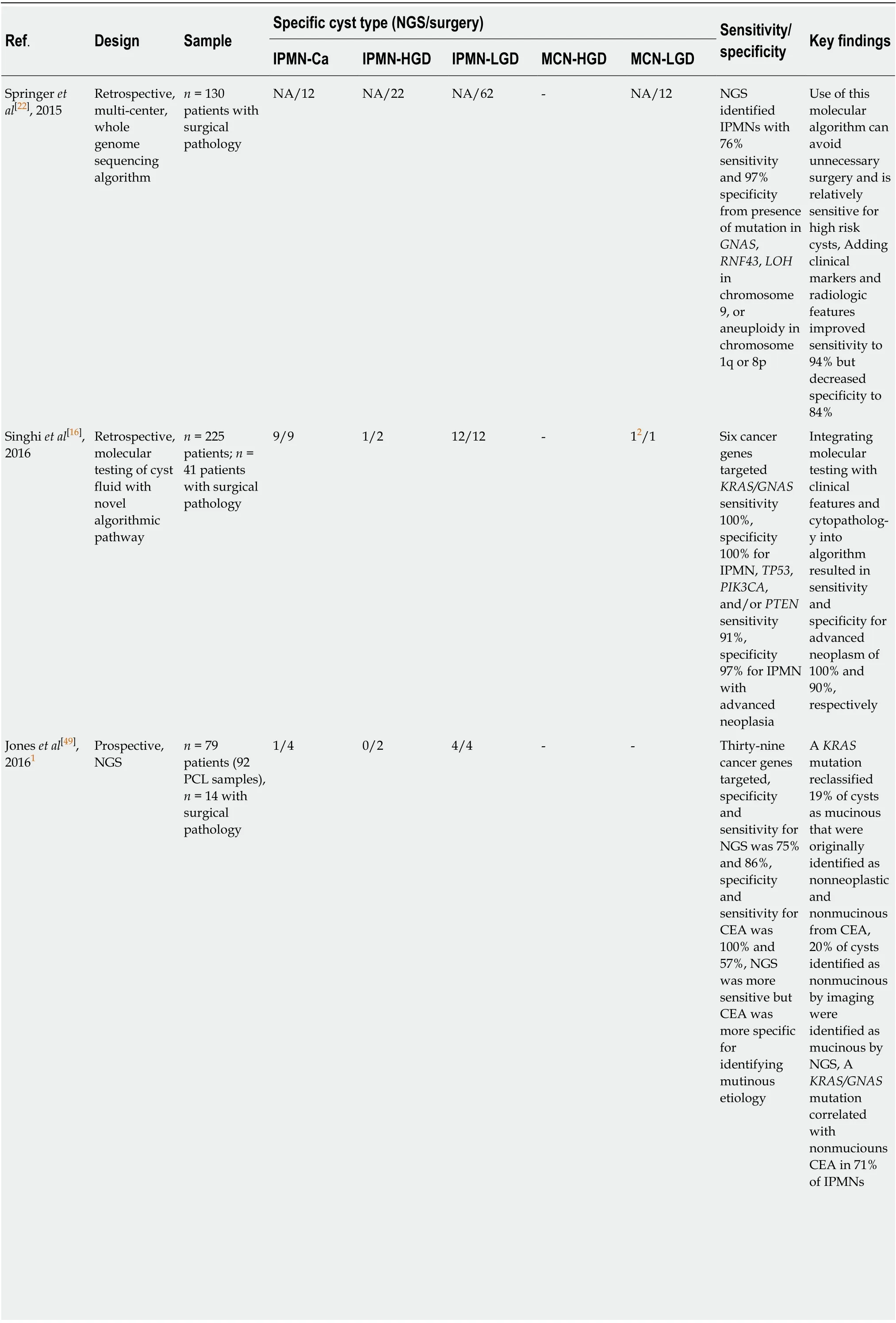
Table 1 Comparison of molecular analyses to resected specimen[23]
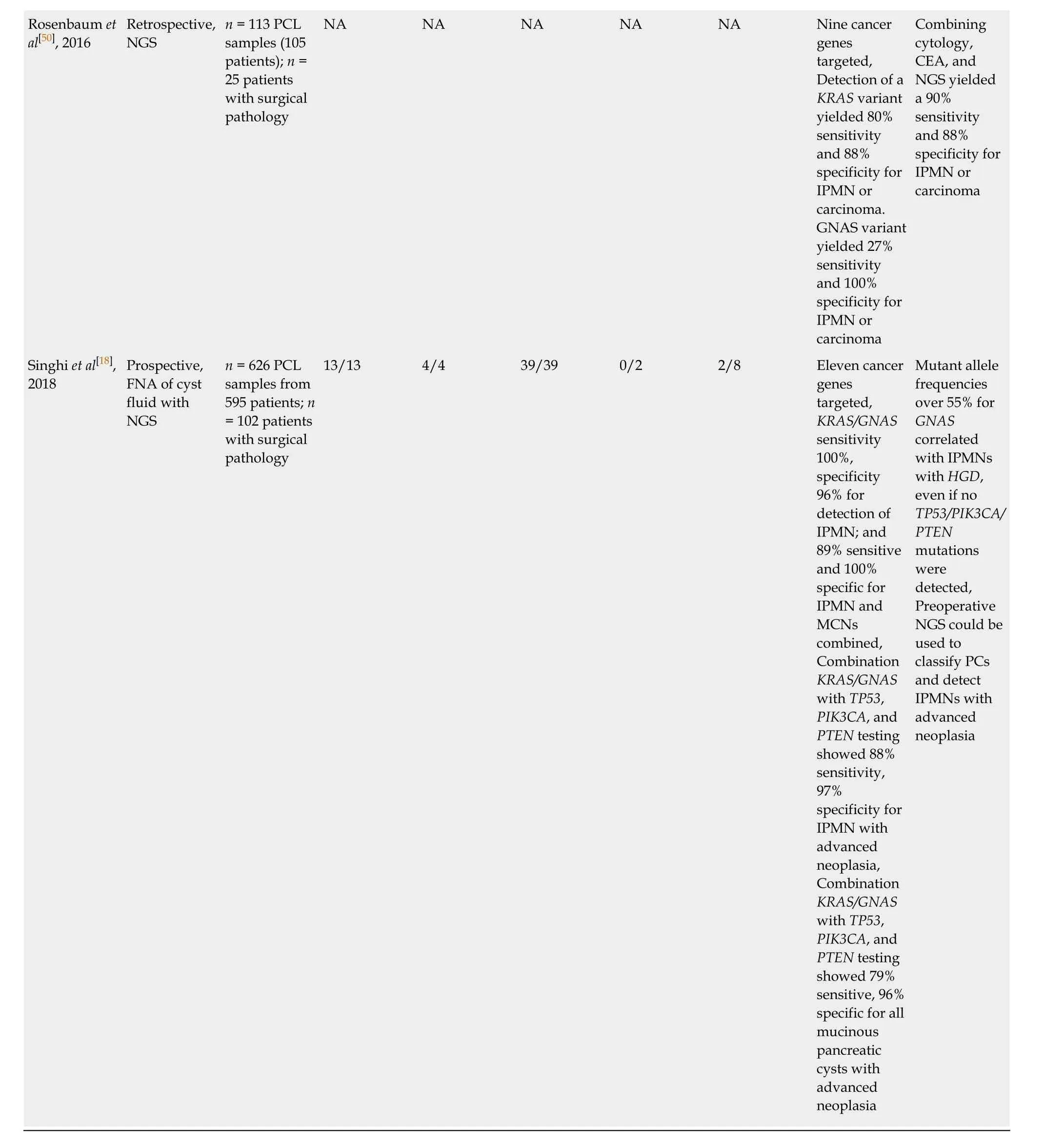
1No reported mutant allele frequencies which may impact risk stratification for 2 high-grade dysplasia intraductal papillary mucinous neoplasm.2Identified by alterations in TP53, PIK3CA and/or PTEN; not KRAS or GNAS. IPMN: Intraductal papillary mucinous neoplasm; IPMN-Ca: IPMN with adenocarcinoma; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; NGS: Next-generation sequencing; CEA: Carcinoembryonic antigen; NA: Not available.
EUS-MFB likely solves the problems of low cellular cystic fluid acquisition by instead sampling tissue from the epithelium lining the cyst wall, as well as tissue beyond the epithelium[25,28]. It has been demonstrated that obtaining two macroscopically visible tissue samples reached optimal histologic adequacy and specifically, identification of stroma beyond the epithelium greatly assists in diagnosisof mucinous cystic neoplasms[29,30]. Additionally, a study by Kovacevicet al[31]posited EUS-MFB may be used to subtype IPMNs according to mucin expression, leading to more accurate risk stratification, but the scope of the study was limited and additional research and follow up is needed[31].
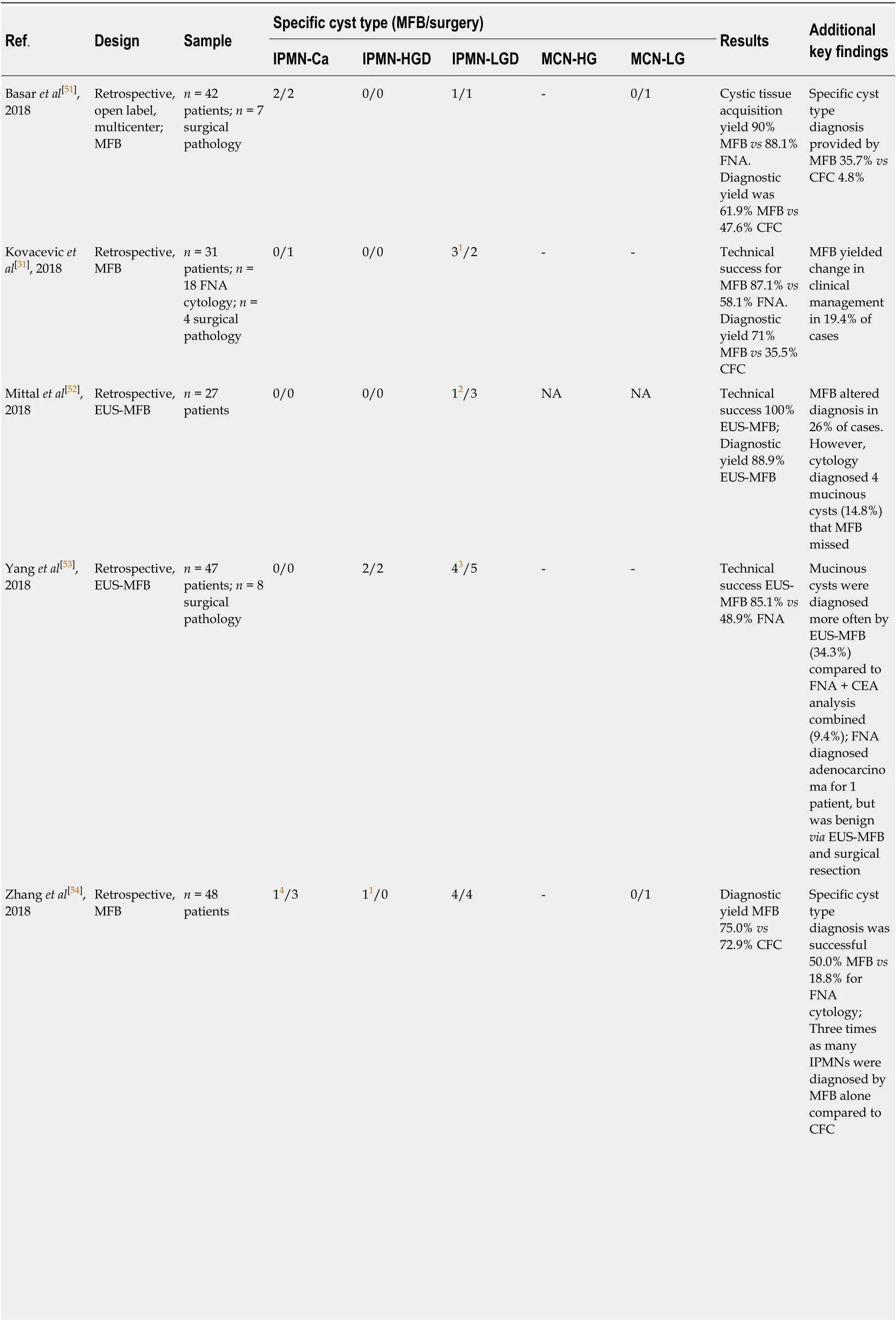
Table 2 Comparison of endoscopic ultrasound-guided microforceps biopsy to resected specimen[23]
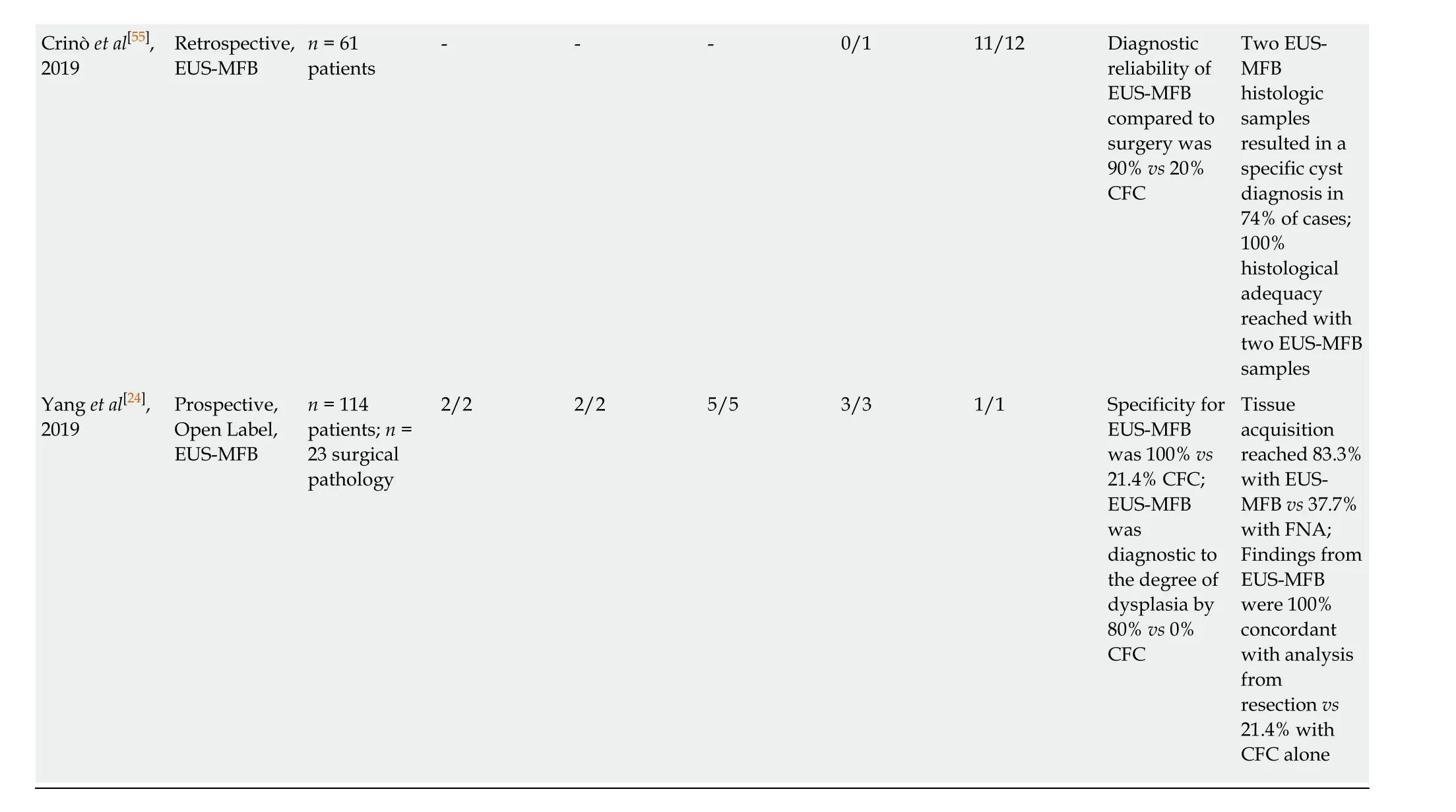
1False negative by micro-forceps biopsy, surgery identified as adenocarcinoma.2Cysts classified as mucinous, but not differentiated into intraductal papillary mucinous neoplasm or mucinous neoplasm.3Intraductal papillary mucinous neoplasms with no dysplasia subtype were grouped as intraductal papillary mucinous neoplasm low-grade dysplasia.4Undiagnostic on micro-forceps biopsy. IPMNs: Intraductal papillary mucinous neoplasms; IPMN-Ca: IPMN with adenocarcinoma; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; MFB: Micro-forceps biopsy; CFC: Cystic fluid cytology; FNA: Fine needle aspiration; MCN: Mucinous neoplasm;NA: Not available.
Although EUS-MFB carries improvements in tissue acquisition and thus advances in diagnostic yield from samples, there are increased reports of adverse events(pooled estimate of 8.6%, with limited serious adverse events), but this might be related to operator skill[25]. The benefit for obtaining tissue for histology may lead to further advantages for this technique, however it is important to note that the biopsies are obtained from focal areas in the cyst, due in part to the stiffness of the 19-g needle with the forceps[25]. It is also noted that there are concerns for gastric seeding using this technique, and thus its clinical safety and feasibility imply the need for additional testing[11].
Studies utilizing EUS-MFB have addressed the diagnostic yield, feasibility,technical success, and associated adverse events. However, there are no comparative large prospective studies with assessment of diagnostic accuracies where the reference standard is surgical histopathology and the lack of this diminishes the full impact of this novel diagnostic modality in the management of PCLs.
EUS guided needle confocal laser endomicroscopy
EUS guided needle confocal laser endomicroscopy is a novelin vivoimaging technique that grants real-time, high-resolution microscopic imaging of the pancreatic cyst epithelium. Major trials (INSPECT, DETECT, CONTACT, and INDEX) have established feasibility, diagnostic image standard, and characteristic CLE patterns of pancreatic cysts routinely evaluated in clinical practice[32-37]. In the INDEX study,investigators confirmed thein vivonCLE features onex vivopost-resection specimens of IPMNs[38,39], and also validated EUS-nCLE image patterns of mucinous PCLs including IPMNs among blinded external CLE-expert observers[40]. In a post-hoc analysis of the INDEX study, quantification of the papillary epithelial width and darkness (pixel intensity) was able to predict advanced neoplasia in IPMNs (Figure 1).The study included 26 IPMNs (high-grade dysplasia-Ca in 16) with histopathological(n= 24) or cytological (n= 2) reference standard. For diagnosing advanced neoplasia,CLE features of increased papillary epithelial “width” with cut-off value of ≥ 50 μm revealed a sensitivity, specificity, and AUC of 87.5%, 100%, and 0.95, respectively.Similar diagnostic values of sensitivity, specificity, and AUC of 87.5%, 100%, and 0.95,respectively, were observed for papillary darkness (pixel-intensity) cut-off value ≤ 90 pixels (lower values being darker). The pathology of IPMNs presents a papillary configuration that includes a spectrum of dysplastic mucinous epithelium ranging from low-to-high grade dysplasia[41,42]. While low-grade dysplasia demonstrates a single flat layer of columnar cells with retained polarity, high-grade dysplasia demonstrates loss of nuclear polarity, increased nuclear-cytoplasmic ratio,stratification of both the columnar cells and their nuclei, and complex architecture[41].In parallel to these pathological changes, IPMNs with low-grade dysplasia reveal a thin layer of epithelium (Figure 1), whereas advanced neoplasia reveals thicker and darker epithelium since CLE imaging of the nuclei reveals dark spots, which when stratified imparts a darker hue to the epithelium. Thus, the consequence of a combination of cellular and nuclear stratification is thicker and darker epithelium of the papillae (Figure 1). Qualitative image interpretation by an endoscopist is susceptible to interobserver disagreement and quantitative estimation of papillary thickness and darkness can be laborious. Hence, computer-aided quantification utilizing machine learning and artificial intelligence would need to be adapted to reduce interobserver disagreement and endoscopist work load.
Similar to other EUS-guided evaluations, nCLE differentiation of dysplasia in IPMN needs to be reproducible and validated in prospective multi-center studies. If endomicroscopy assisted machine learning and artificial algorithms are generated,these would need to be validated in multicenter studies across multiple centers and endoscopists. Since the entire cyst is not imaged during CLE, theoretically the modality cannot be utilized to rule-out advanced neoplasia in IPMNs. Despite the limitations in the maneuverability of the EUS-FNA needle (19-g), approximately 30%-50% of the PCLs can be observed depending on the location of the neoplasm in the pancreas.
CONCLUSION
Pancreatic adenocarcinoma is a near fatal diagnosis with a < 10% 5-year overall survival rate, and there are no pragmatic investigations for screening with proven efficacy[43]. Analogous to other adenocarcinomas such as esophageal and colorectal cancer, the resection of a premalignant lesion such as IPMN should prevent development of pancreatic adenocarcinoma; however, the operative morbidity of pancreatic surgery is high, the natural history of BD-IPMNs (< 3 cm) has not been delineated, and it is challenging to diagnose BD-IPMNs with advanced neoplasia[11].Despite multiple revisions of the international consensus guidelines and various guidelines by other major societies[11,12,44,45], there continues to be range of percentage of patients (up to 2/3rdof resections at tertiary care centers) who have low-grade dysplasia on postoperative pathology[46-48].
Improved diagnostics are needed that are sensitive and accurate in identifying dysplasia, as well as informing an appropriate timeline for intervention when considering risk of malignant transformation. Each of the methods explored in this manuscript, through-the-needle tissue biopsy/MFB, molecular analysis, and nCLE,have attempted to address this need. Collectively these methods suffer from potential sample bias given the limited amount of surgical specimen pathology available to confirm diagnostic accuracy. There is inherent need for improved sample size to refine these methods, which will likely only be attained from large-scale multisite studies. These methods each have individual limitations, and it may be prudent to evaluate a combination of techniques to optimize clinical feasibility and diagnostic accuracy. In summary, great strides have been made in the evaluation of pancreatic cysts, but there is still a critical need for further research to classify the risk for malignant potential.
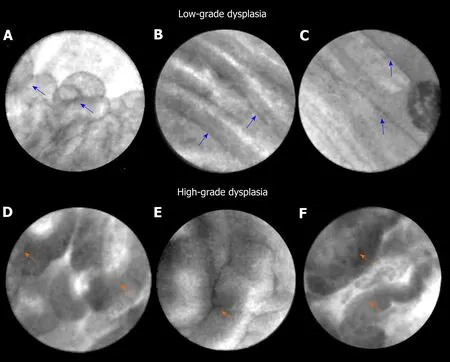
Figure 1 Endoscopic ultrasound-guided confocal endomicroscopy characteristics of intraductal papillary mucinous neoplasm. A-C: Intraductal papillary mucinous neoplasm with low-grade dysplasia. Papillae (blue arrows) reveal thin and translucent epithelium. The papillae are small with thin epithelium in panel A. The epithelium is flat in panels B and C; D-H: Intraductal papillary mucinous neoplasm with high-grade dysplasia. Papillae (orange arrows) show thicker and darker epithelium. In panel D, there is higher density of papillae.
 World Journal of Gastroenterology2020年23期
World Journal of Gastroenterology2020年23期
- World Journal of Gastroenterology的其它文章
- Liver-directed therapies for liver metastases from neuroendocrine neoplasms: Can laser ablation play any role?
- Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection
- Medications in type-2 diabetics and their association with liver fibrosis
- Pancreatic necrosis and severity are independent risk factors for pancreatic endocrine insufficiency after acute pancreatitis: A long-term follow-up study
- Impact of a national basic skills in colonoscopy course on trainee performance: An interrupted time series analysis
- Sodium glucose co-transporter 2 inhibition reduces succinate levels in diabetic mice
