Early exposure to food contaminants reshapes maturation of the human brain-gut-microbiota axis
Elodie Sarron, Maxime Pérot, Nicolas Barbezier, Carine Delayre-Orthez, Jérôme Gay-Quéheillard,Pauline M Anton
Abstract
Key words: Perinatal repeated low-level exposure; Gut homeostasis; Brain gut microbiota axis; Food contaminants; Non-communicable chronic diseases; Epigenetics
INTRODUCTION
Intrauterine and post-partum periods belong to the very sensitive time periods at the origin of the adaptation of the body to environmental stimuli also described as adaptative behavior thanks to developmental plasticity. This is phenomenon by which a single phenotype can give rise to a range of different physiologic or morphologic states in response to different environmental conditions during development. However, this adaptation ability is somehow restraint to a certain point.Indeed, since Barkeret al[1]described the Developmental Origin of Health and Diseases (DOHaD), several original publications have pointed out that a poor fetal and postnatal settlement of gut microbiota will necessarily remodulate neuroimmuno-endocrine maturation of the gut. This will then be responsible for the modification of the establishment of the brain neuronal connections at the origin of a higher risk of developing later in life non communicable chronic diseases affecting the gut and the brain functions (for review see[2]).
Environmental factors are known to impact placental development at the origin of fetal growth retardation and postnatal major modification of the neuro-immunoendocrine functions maturation and cross communication leading to chronic diseases.However, while the impact of food contaminants on the appearance of these diseases is not to be debated, investigation related to their impact on the brain gut microbiota axis development and maturation during perinatal life are needed to correlate all the events for the nutrition of pregnant women and infants.
This review is aimed at describing the actual understanding of the indirect and direct repeated low-level exposure of respectively the fetus and the infant to major categories of food contaminants and the consequences on development and maturation mainly of the brain gut microbiota axis through their impact on neuroimmuno-endocrine modulation.
SETTLEMENT AND MATURATION OF THE BRAIN-GUTMICROBIOTA AXIS
Intestinal homeostasis settlement is defined as the establishment of an equilibrium between gut microbiota, immune system and epithelial cell integrity[3]. This event occurs during the 1000 first days of life, from fetal stage to 2 years old[4]. Because gut microbiota plays a central role in the maturation of gut mucosa and immune system,many studies focused their interest on where and when will start maternal microbiota transmission. Early 20thcentury, the idea that gut microbiota acquisition in infant starts at delivery, following contamination with vaginal and fecal bacteria from maternal flora, was accepted. However, new methods based on independent-cultured molecular technics suggest that bacterial transfer from the mother to their infant beginsin utero[5]although the exact mechanisms of this event are not well understood.In 2005 and 2008, Jiménezet al[6,7]detected the presence of bacteria in amniotic fluid,umbilical cord and meconium or first stool of a newborn, by polymerase chain reaction. More recently studies were performed on the identification of microbiome in human placenta following termed and pretermed gestations.Firmicutes, Tenericutes,Proteobacteria, Bacteroidetes, Fusobacteria phylaand nonpathogenic commensal microbiota were found and associated with antenatal infection[8,9]. Nevertheless, even if bacteria patterns could be transmitted from mother to children in the womb,differences of newborn microbiota composition were observed depending on the mode of delivery, preterm birth and the use of antibiotics[10-13]. Currently, it has been acknowledged thatStaphylococci, Streptococci, Enterococci, LactobacillusandBifidobacteriaare the first bacteria which will colonize our gastrointestinal tract from vaginal and fecal flora and some of them come from lactation[14]. Before the third year,the interindividual variability is higher than in adults, but food diet diversification generates gut microbiota evolution by increasing their richness/diversity and will tend to reach a stability in time also called eubiosis state[15]. Gut microbiota contributes in health benefits such as metabolism of food, competition against pathogen, growth of intestinal cells and stimulation of the immune system among others[16,17]. During this period, child microbiota remains very sensitive to various influences that can impact its composition and cause long term effects on our physiology[18]. Nonetheless,a modification of gut microbiota inducing dysbiosis is usually associated with many diseases as inflammatory bowel disease, autism, type 2 diabetes, obesity, autoimmune diseases and allergy[19,20]. For example, clinical trials provide proofs concerning the relationship between allergy development and microbiota dysbiosis[21]in function of delivery mode in infants[22]. The behavior of children in the first 3 years of life clearly promotes significant exposure to microbes: Breastfeeding and direct contact with maternal skin, permanent introduction of objects, hands or feet into mouth, especially during crawling and early walking stages when hands are in contact onto floor surfaces. However, the actual excessive hygiene of our environment decreases the exposition to microbes in early life impacting immune system maturation. This has been mentioned in the rise of immune disorders (autoimmune diseases, allergies,etc.)for more than 40 years as “hygiene hypothesis”[23,24].
By contrast, as the intestinal microbiota of preterm infants at birth is less diverse than in full-term infants, it presents an immature intestine with underdeveloped peristalsis, barrier function and immunity. The intestine is therefore, a potential source of inflammation and infections by pathogenic bacteria such asEnterobacter,
Enterococcus, andStaphylococcus[25,26].
Mucosal tissue homeostasis also results from the perinatal establishment of mucosal-induced immune tolerance, phenomenon regulated by a set of signals provided by innate immune cells that shape the adaptative immune responses.Through its barrier function, cell contact-mediated signals and production of cytokines, this regulatory immune network is controlled by the mucosal epithelium.Perinatal maturation of the mucosal immune system may also be modulated by direct or undirect effect of environmental factors whichever route of exposure considered,During pregnancy, maternal and fetal immune system communicate in a bidirectional manner. The development and maturation of the immune system starts early in fetal life and continues throughout the infancy and early childhood. These are windows of high susceptibility and vulnerability to environmental insults such as malnutrition, stress or environmental contaminants. The development of fetal immune system is characterized by the presence of several distinctive features that promote fetal active tolerance against bothin uteromaternal antigens and exogenous antigens, such as infectious agents. It is well established that endogenous and exogenous factors shape the development and maturation of the intestinal immune system. In rodents and human, the formation of secondary lymphoid structures, such as Peyer’s patches or mesenteric lymph nodes (MLNs), occurs before birth. However,their size and development of germinal centers depend on the postnatal microbial colonization[27,28]. Myeloid-derived cells, such as macrophages, dendritic cells (DCs) or brain microglial cells, begin to populate the tissues as early as gestational weeks 4 to 7 and continue to increase in number throughout the second trimester[29]. Then from week 8 to 18, the thymus will be populated by lymphoid cells through the process of lymphocyte maturation and positive and negative selection (central tolerance). The population will continue to expand within the thymus as gestation proceeds[29]. The first mature T cells are seen in peripheral tissues between weeks 10 and 12 of gestation and a significant number are circulating at the end of the second trimester[30]. In mice,innate cells such as lymphoid tissue induced cells, natural killer cells or T helper 2 like cells, migrate from fetal liver to the gut mucosa thanks to endogenous signals within the first 4 wk after birth[31]. Bacterial colonization will stimulate the recruitment of forkhead box protein 3 (FOXP3) and regulatory T cells (Treg) to the gut mucosa and secretion of the anti-inflammatory cytokine interleukin-10 (IL-10)[31,32]. By this mechanism, Treg cells keep pro-inflammatory Th cells under control to preserve the epithelial barrier. It has been observed a great abundance of Treg cells in human fetal MLNs[33]and their homing seems to be particularly important in infancy[34].Bifidobacterium infantis, largely present in the gut lumen, strongly induces FOXP3+Treg cells in human infants[35]. Reversely, with the development of the innate immune response most of microorganisms colonizing the small intestine and the colon will be anaerobes. The small intestinal mucosa of human newborns has a mature crypt-villus architecture, with continuous stem cell proliferation, and epithelial cell migration and differentiation. Changes in the composition of antimicrobial peptides and in antibacterial activity during early postnatal period have also been observed in the intestinal lumen of human neonates[36]. Intestinal immune homeostasis is also established thanks to a close cooperation between the intestinal epithelial cells (IECs)and the components of sub-epithelial gut associated lymphoid tissue. In the intestinal mucosa, molecules frequently associated with pathogens may be recognized by surface pattern recognition receptors and among them, Toll-like receptors (TLRs).Upon activation of TLR signaling, IECs will release transforming growth factor beta(TGFβ) by which tolerogenic phenotypes of DCs are elicited[37]. DCs then secrete IL-10 at the origin of an immune response dominated by Treg cells. In parallel, Th1 immunity may be induced and anti-inflammatory cytokines will be secreted[38,39].Generation of FOXP3+ Treg and concomitant establishment of immune tolerance result from two steps. On the one hand, a founder pool of FOXP3+ Tregs is produced by the activation of antigen specific naive T cells in gut-draining MLNs. On the other hand, homing of activated Treg to intestinal lamina propria thanks to intestinal macrophages. Homing FOXP3+ Treg cells propagate, and immune tolerance is irreversibly installed. To provide passive immunity to the fetus or the neonate,maternal antibodies are transferred to the fetus by the placenta (starting as early as 6 wk of gestation with a rapid increase by week 24-26) and then to the infant through breast feeding. Peripheral activation of the immune system with production of cytokines can affect the central nervous system (CNS) directlyviacytokines crossing the brain blood barrier or indirectly by activating the hypothalamic pituitary adrenal axis[40].
At last, the homeostasis of maternal and fetal endocrine milieu is also important to allow normal development and prevent adverse effects arising from early insults.Alteration of gestational endocrine environment by diseases will then perturb hormonal homeostasis and may be at the origin of fetal programming of altered endocrine profile in fetus. An important pathway by which gut microbes and their metabolites communicate with CNS involves the cells making up the endocrine system of the gut[41]. Enteroendocrine cells are intersped between gut epithelial cells throughout the length of the gut and are able to release different types of molecules that can enter the systemic circulation and have an incidence on the CNS regulatory pathways. 5-Hydroxytryptamin or serotonin (5-HT), the main regulating hormone of gastrointestinal motility and secretion, is produced by the enterochromaffin cells and stored in these cells and enteric neurons but also in CNS. Most of the molecules contributing to the neuroendocrine signaling within the brain-gut-microbiota axis originate from the gut microbiota metabolism of food as it is the case for tryptophan, a precursor to the neurotransmitter 5-HT[42].
While neuronal connections start to settle during the fetal period, their complete development won’t be achieved before adulthood. This is the reason why brain/periphery neuronal organization will remain very sensitive during a wide lifespan window. Thanks to epidemiological observation, the developing brain is known to be very sensitive to both external and internal environmental cues as early as prenatal life. During this period, maternal metabolic and immune activities will be dependent of the external environment. Their reactivity will interfere with the neurodevelopment in the womb and any alteration of maternal endocrine or immune response may have strong irreversible consequences on fetal development through epigenetic modifications. These studies linked neurodevelopmental disorders such as schizophrenia or autism with microbial infection during fetal life[43-45]. While placenta is considered to protect the fetus placing him in an almost sterile environment, the maternal environment will, by many ways, condition fetal brain development.Indeed, maternal microbiota metabolites may cross the placental barrier and affect the fetal brain construction programming which is dependent on placental neurohormones such as serotonin, essential for the forebrain development[46]or metabolic hormones such as adipokines or steroids controlling cell proliferation,differentiation, neurodevelopment[47,48]. Furthermore, maternal gut microbiome regulates serotonin production by enterochromaffin cells[49]. Its depletion results in uttered brain development[50]. Maternal dysbiosis will also influence blood brain barrier formation[51].
Brain development will continue postnatally, and metabolic programming of energy expenditure will be conditioned by a proper settlement of the Arcuate nucleus/hypothalamic connections that take place the first couple of days after birth.Synaptogenesis begins shortly after birth and reaches a maximal level around 2 years of age before synaptic refinement during mid-adolescence to get adult neuronal organization[52].
This period of neuronal development parallels the gut microbiota settlement and maturation a process known to be essential for the establishment of a proper immune function[56-60], the neuroendocrine system[61]and metabolic regulation[15,62]. Gut microbes communicate with the CNS through at least three parallel and interacting channels involving endocrine, nervous and immune signaling mechanisms. As thus,the settlement of this bidirectional communication is very crucial for preserving health according to a large body of literature. Alteration of this cross talk will be at the origin of the pathogenesis and pathophysiology of classic brain/gut disorders such as gastrointestinal disorders such as irritable bowel syndrome or inflammatory bowel diseases but also a growing list of psychiatric disorders or autism[63,64], Parkinson’s disease[65]or chronic pain[66]. One can suspect a mis settlement of this bidirectional communication to be at the origin of all of these chronic non-communicable diseases the alteration of gut microbiota settlement early in life.
During a sensitive window of development, environmental chemical exposure to chemicals, particularly through nutritional route, will act when tissues are forming, to affect the phenotype, shift the immune-metabolism-microbiota interactions, with consequences on the neuro-immuno-endocrine pathways at both the gut and the brain levels and this will then impact organ functions and chronic diseases development susceptibility later in life[67]. Furthermore, many mechanisms presented here are under genetic control including specific gene expression at each developmental and maturation stage. These mechanisms are allowed by particular transcription factors that interact with DNA to activate or inhibit gene expression.Epigenetic mechanisms are linked to accessibility of DNA to these transcription factors and are not transmitted with the classical mendelian genetics. Epigenetic is linked to biochemical modifications that affect DNA compaction and accessibility,essentially methylation of DNA on the specific nitrogen base cytosine and post translational modifications of Histone proteins. miRNA are another epigenetic mechanism where these small RNAs from 21 to 24 nucleotides are able to hybridise mainly in the 3’-UTR region of mRNA and block translation or cause mRNA degradation[68]. Alterations of epigenetic modifications are presented as key events of developmental programming[69,70]. Epigenetic mechanisms are inherently malleable and may accumulate over time which will, during the developmental programming of individuals, have profound effects on gene expression and predispose to the development of disease phenotypes profile[71].
The “Trialogue” among bacterial inhabitants, intestinal barriers and gut -associated neuro-immuno-endocrine components should normally ensure genesis and preservation of a stable symbiotic relationship consolidating the gut homeostasis.However, if the organism gets into contact with environmental toxicants, carried inside the lumen through food intake, these conditions could be at the origin of instability increasing the risk to develop chronic disorders, especially in critical windows of life such as perinatal stage.
ROLE OF FOOD CONTAMINANTS ON FETAL PROGRAMMING
Based on all this literature information, one can question whether prenatal or postnatal food contaminants exposure could somehow reprogram the fetus or infant overall development and more especially may interfere with the microbiota settlement, reconditioning the maturation of the gut mucosa not only at the immune but also at the neuroendocrine level. One can speculate that all of these modifications will naturally impact the developing connection between the gut and the brain. This has clearly been evidenced that exposition to certain chemical substances during postnatal life and early childhood will predispose to significant and irreversible damage in the developing brain. Pregnancy is a peculiar state of high vulnerability for the woman and the fetus because of the necessary adaptation to a putatively noxious environment which may modify the symbiotic interaction between mother and baby and could, in turn, influence the fetal programming process. This could result in some permanent, often subtle alterations in different organs among which gut or brain.Many factors during and after pregnancy may influence the future health status of the human being: Genetic status, inflammation during pregnancy, but also diet. Indeed,food not only provides nutrients but is also the most important source of environmental contaminants of diverse origins including heavy metals[72]or persistent organic pollutants (POPs) coming from industrial activities and found in food and particularly in fatty fish[73]. Some other, such as pesticides, may be detected in harvested crops[74]or may appear during storage which is the case for fungi or bacteria toxins[75]or during food processing and particularly heating with the appearance of Maillard reaction products (MRPs)[76]. Fetuses are exposed to contaminants from their mother’s diet. Since all of these substances are unintentionally found in food products,they can impair health under certain circumstances. Human fetus is not a small adult.This vulnerable subpopulation is probably at a greater risk from food contaminant because of a higher absorption rate, a poor detoxification elimination capacity, rapid cell proliferation, and immature repairing mechanisms. In this part, we will review the consequences of food contaminants exposure of the mother during pregnancy on the maturation of fetus and then on infant and child.
Fetal exposure to antibiotics
As mentioned above, maternal microbiota is transmitted to the fetus during pregnancy through mechanisms partly understood and, during natural parturition,microflora is ingested into the neonatal gut, establishing the initial microbial population provided that delivery was realized without caesarean, without disinfectants, intrapartum antibiotics, antiseptic creams,etc[77]. As thus, perturbations of vaginal maternal microflora (diet, body composition, infection, antibiotic treatment,stress, probiotics,etc.) will definitely have significant consequences on the offspring.During pregnancy, vaginal bacterial infections may alter early neurodevelopment and seems to be related with autism spectrum disorder[78]and following the delivery it may alter the microbial assembly of the neonatal gut. This will decrease diversity and stability of the microbiota populations that will influence fermentation, digestion and absorption of metabolites, increase of antibiotic resistant bacteria such asKlebsiella,Citrobacter,Enterobacter,E. coli[79-81]. Altogether, these effects will alter the development of the neonate and the infant and will be increasing its disease risk.
In rodents, exposure of dams to antibiotics during lactation is responsible forLactobacilliabundance depletion, an increased fat mass and altered secretion of metabolic hormones in their offspring[82,83].
Fetal exposure to pesticides
Impacts of pesticides on the offspring after maternal exposure has been widely documented in the literature. However, few studies evidenced their deleterious effects at repeated low-level exposure. Many of these have long been described as endocrine disruptors. Organophosphates and carbamates such as chlorpyriphos(CPF), malathion, diazinon, carbaryl share cholinesterase inhibitory activities.Moreover, these organophosphate do have a strong incidence on microbiota balance[84]. It has been evidenced that exposing dams to diazinon results in behavioural disturbances in their offspring[85]. It is acknowledged that environmental chemicals, especially endocrine disrupting factors, will induce functional modification of gene expression and when these changes occur in fetal life this may be at the origin of increased risk of dysfunction and disease later in life although any phenotype change will be observed at birth[67].
Carbofuran, an anticholinesterase carbamate, is commonly used as an insecticide,nematicide, and acaricide in agricultural practice throughout the world. Carbofuran and/or its major metabolites can cross the placental barrier and produce serious effects on the maternal-placental-fetal unit. Carbofuran's toxicity can be potentiated by simultaneous exposure with other cholinesterase inhibitors[86].
Chlorpyrifos [O,O-diethyl-O-(3,5,6-trichloro-2-pyridinyl)phosphorothioate: CPF] is an organophosphate insecticide used worldwide to treat fruit and vegetable crops.The most widely studied organophosphate, CPF has been clearly identified as a neuron killer, neural cell migration and brain connection disruptor and its residues are often detected in food and drinking water[87]. Although the digestive tract is the first organ to come into contact with food contaminants, little is known about CPF’s impact on the epithelial barrier. Studies from Joly Condetteet al[88], have shown that chronic CPF-exposure during critical pre- and postnatal periods of organ development and maturation alters epithelial barrier function, which in turn is associated with elevated permeability and bacterial translocation. Furthermore, the barrier dysfunction is associated with changes in tight junction’s protein expression.In the same rat model, rat pups exposed to 5 mg/kg/d CPF were both significantly smaller (body length) and lighter than controls. Exposure to CPF was associated with changes in the histological structures (shorter and thinner intestinal villi), an intestinal microbial dysbiosis and increased bacterial translocation in the spleen and liver. These significant microbial changes in the gut were associated with impaired epithelium protection (mucin-2) and microbial pattern recognition receptors (TLRs 2 and 4) genes expression[84]. In summary, pesticide residues in food may impact the digestive tract function and its ability to adapt to environmental changes. In rats, this effect appears to be even greater at the time of weaning (i.e., when food diversification occurs).Increasing evidence indicates that CPF is involved in metabolic disorders. Data from Reygneret al[89], indicate that developmental exposure to CPF interferes with metabolism with dose related effects evident at adulthood. Today, as developmental intellectual disorders affect one out of six children in industrialized countries, there is a growing interest in identifying impacts of pesticides on brain development and maturation and in the etiology of intellectual impairments. A study from the same French team assessed whether maternal ingestion of low CPF dose in rats could impair the cerebral function of their progeny[90]. According to the results, the progeny of CPF-treated dams showed slower negative geotaxis as neonates, lower novelty exploration as juveniles and faster startle reflex as adolescents and adults. This data suggests that developmental CPF relevant to human exposure may impair noveltyrelated activity and sensori-motor functions, thus adaptability to the environment.This data supports the hypothesis that CPF may contribute to behavioural disorders including acquisition retardation and consequences as an adult. Another organophosphate compound has also been studied for its potential health impacts.Acephate is a pesticide that targets insects which also belongs to the organochlorines.As for CPF, a Wistar rat animal model suggests that acephate exposure during pregnancy and lactation causes alterations in maternal glucose metabolism and programs the offspring to be susceptible to type 2 diabetes at adulthood[91].
Organochlorine pesticides (OCPs) are environmental contaminants that persist in the environment and bioaccumulate through the food chain in humans and animals.The results from Yamazakiet al[92]support the hypothesis that prenatal exposure to OCPs, especially cis-heptachlor epoxide, may have an adverse effect on the neurodevelopment of infants at specific ages, even at low levels. Chlordecone is a persistent OCP that was used in the French West Indies until the early 1990s for banana weevil borer control. In a prospective longitudinal study conducted in Guadeloupe (Timoun mother-child cohort study), exposure to chlordecone was measured at birth from an umbilical cord blood sample and from a breast milk sample collected at 3 mo postpartum. The results suggest that prenatal exposure to chlordecone is associated with specific impairments in fine motor function in boys,and add to the growing evidence that exposure to OCPs early in life impairs child development[93]. Similarly, in the same birth cohort Timoun in Guadeloupe, another objective of the study was to examine the association between prenatal exposure to chlordecone and fetal growth measured by birth weight[94]. They found a significant U-shaped association between birth weight and chlordecone exposure, within the upper quartiles of gestational weight gain or excessive gestational weight gain.Chlordecone exposure may affect fetal growth, particularly when excessive gestational weight gain is present. Other pesticides such as dichlorodiphenyltrichloroethane and its highly toxic metabolite dichloro-diphenyl-dichloroethylene(DDE) are persistent OCPs which are lipophilic environmental pollutants that accumulate in the food chain. These chemicals have recently been under scrutiny for their possible health hazards such as cancer and reproductive outcomes including low birth weight. The study from Khanjaniet al[95]investigated whether mothers with a higher contamination of pesticides were different from mothers with low contamination in relation to their offspring's birth outcomes such as birth weight,small for gestation age, prematurity, head circumference, sex ratio, and previous miscarriage or still birth. In this case, those pesticides were not found to be associated with adverse birth outcomes in contaminated mothers in the range of contamination of our population (< 7.5 mg/kg lipid in maternal milk), although there is weak evidence that sex ratio may be affected. Maternal exposure to Great Lakes sportcaught fish contaminated by DDE suggest that fetal DDE exposure (as indicated by maternal serum DDE concentration) may decrease birth weight[96].
Amitraz is an active plant protection product, which has an antiparasitic effect, and which belongs to the chemical family of formamidines. Kimet al[97]investigated the potential adverse effects of amitraz on the initiation and maintenance of pregnancy in Sprague-Dawley rats as well as its effects on embryo-fetal development after maternal exposure during the entire pregnancy period. Amitraz was administered to pregnant rats by gavage from days 1 to 19 of gestation at dose levels of 0, 3, 10, and 30 mg/kg/d. All dams underwent a caesarean section on day 20 of gestation and their fetuses were examined for any external, visceral, and skeletal abnormalities. At 30 mg/kg, maternal toxicity manifested as an increase in the incidence of abnormal clinical signs and a lower body weight gain and food intake. Developmental toxicity included an increase in the fetal death rate, a decrease in the litter size, and a reduction in the fetal body weight. In addition, there was an increase in the incidence of fetal external, visceral, and skeletal abnormalities. At a dose below 10 mg/kg,maternal toxicity observed included a decrease in the body weight gain and a decrease in food intake associated to a minimal developmental toxicity. This includes a decrease in the fetal body weight, an increase in the visceral and skeletal aberrations, and a delay in fetal ossification. These results show that amitraz administered during the entire pregnancy period in rats is embryotoxic and teratogenic at the maternally toxic dose (i.e., 30 mg/kg/d) and is minimally embryotoxic at a minimally maternally toxic dose (i.e., 10 mg/kg/d).
Diuron is widely used as a weed killer to kill unwanted grasses and other annual and evergreen broadleaf weeds, especially in viticulture. It is also used in gardens and for weeding roadsides or railways. A perinatal exposure to diuron [3-(3,4-dichlorophenyl)-1-1-dimethylurea] might exert adverse effects on rat lymphoid organs. Pregnant Sprague-Dawley rats were exposed to diuron at 500, 750 or 1250 ppm in the diet from gestational days 12 to 21 (GD 12-21) and during lactation. Flow cytometric analysis revealed a significant reduction in B lymphocytes (CD45RA+) in male pups but T lymphocytes (CD4+, CD8+ and CD4+/CD8+) were not markedly affected. Thus, data suggest that Diuron-induced maternal toxicity in dams exposed to high dose and perinatal exposure to this herbicide produced spleen toxicity as evidenced by a reduction in B lymphocyte number in male Sprague-Dawley pups[98].
Fetal exposure to mycotoxins
Because of the growing evidence of the deleterious effects of pesticides on health,more and more people are choosing to consume organic products. However, there is growing attention in western countries related to the increasing consumption of organic products since the actual means of crop storage conditions may not prevent from mycotoxins food contamination, especially when it relates to organic products.This concern is even more important when it comes to pregnant women and infants.Indeed, cereals intended for human consumption may be contaminated by fungi species of the generaAspergillus,Penicillium, FusariumandAlternaria, which are able to produce highly noxious secondary metabolites: Mycotoxins[75]. The most relevant worldwide mycotoxins in cereals are aflatoxins (AF), ochratoxin A (OTA), patulin(PAT), fumonisins, zearalenone (ZE) and trichothecenes including T-2 toxin and deoxynivalenol (DON)[99]. Based on current available evidence, it is probable that a placental transfer of mycotoxins occurs as early as gestational stage and accumulates in the fetal circulation towards the end of pregnancy. Mycotoxins from human maternal contaminated food consumption can cross placental barrier, be metabolize by the developing fetus and can affect fetal systems[100]. This vulnerable population is probably at a greater risk from carcinogenic environmental toxins because of high absorption rate, poor elimination capacity, rapid cell proliferation, and immature repairing mechanisms[101]. Few studies are carried out on the effect of mycotoxins on the fetus during gestation. Moreover, an occupational exposure to mycotoxins in grain induces labor at an early stage of pregnancy[102].
Only one human study investigated AF exposure and prematurity, but animal studies suggest AF exposure may increase risk for prematurity and pregnancy loss.The fetus could be affected by maternal AF exposure through direct toxicity as well as indirect toxicity,viamaternal systemic inflammation, impaired placental growth, or elevation of placental cytokines. The cytotoxic and systemic effects of AF could plausibly mediate maternal anemia, intrauterine growth restriction, fetal loss, and preterm birth[103]. A study realised on a specific Human population submitted to AF exposition showed perturbation on epigenetic modifications. Precisely, it has been shown a correlation between AF rate in mother during gestation and a differential methylation pattern of many genes in child after birth, susceptible to affect gene expression[104]. Indeed, another study on cells from the digestive tract showed that exposition to another mycotoxin fumonisin B1 altered methylation of gene promotors,showing that this mechanism is possibly general[105].
Wangikaret al[106], 2014 studied the effect of OTA and aflatoxin B1 (AFB1)mycotoxins administered orally to pregnant rats, revealing that these mycotoxins caused visceral anomalies such as gastroschisis. Aroraet al[107], reported that a single dose of AFB1given in a pregnant female by the stomach tube caused protrusion of intestines after exposure on gestation day 8. Treatment of pregnant rats with AFB1resulted in the formation of benign and malignant tumors in the liver, stomach,intestine, endocrine organs, and the central and peripheral nervous system in the offspring.
ZE, an estrogenic mycotoxin produced byFusarium graminearumorF. roseum, is one of the most common contaminants of cereal grains world-wide. The objective of the study from Collins and colleagues was to determine the effects of ZE onin uterodevelopment of rats[108]. Pregnant female Charles River Sprague-Dawley rats were gavaged once daily with ZE (in corn oil) at doses of 0, 1, 2, 4, or 8 mg/kg body weight on GD 6-19. In summary, ZE was maternally toxic and fetotoxic but not teratogenic.
Fetal exposure to other organic pollutants
Bisphenol A (BPA) is an organic compound in the aromatic family, used primarily in the manufacture of plastics and resins. It is pointed out as a risk factor in development of food allergy and food intolerance, two adverse food reactions increasing worldwide. Menardet al[109]evaluated the consequences of perinatal (end of gravity to weaning) exposure to low doses of BPA on immune-specific response to the food antigen ovalbumin (OVA) at adulthood. When BPA-treated OVA-tolerized rats were orally challenged with OVA, colonic inflammation occurred, with neutrophil infiltration, increased IFNγ, and decreased TGFβ. They show that perinatal exposure to BPA altered oral tolerance and immunization to dietary antigens (OVA). In summary, the naive immune system of neonate is vulnerable to low doses of BPA that trigger food intolerance later in life.
POPs are highly resistant chemicals, including dioxins, furans, polychlorinated biphenyls and OCPs, created by industrial activities and found in food with the highest levels found in fatty fish[73]. These highly toxic compounds have been found to cross the placenta and to be excreted in breast milk[110]. Early life exposure to POPs is associated with developmental immunotoxicity and neurodevelopmental disorders like autism spectrum disorders (for review see Dietertet al[111]). This complex interaction between the immune system, environmental pollutants and neurodevelopment has been described by different authors although the exact mechanisms are still not well understood[112]. However, prenatal and postnatal exposure to POPs are clearly associated with brain damage[113,114]. At last, most of POPs and particularly tetrachlorodibenzofluranes, polychlorinated bisphenyls (PCBs) or chlorothalonil, another OCP, has been described to have strong incidence on gut microbiota composition[115]but this has still to be confirmed in infants.
Fetal exposure to heavy metals
For the general population, the most common source of exposure to toxic heavy metals are through the air and dietary intake. Due to their high vulnerability,pregnant women and infants are even more concerned than any other by exposure to them since their effect on health are major. Mercurials are global environmental pollutants deriving from natural processes and anthropogenic activities. Most human exposure to mercury occurs through the intake of fish, shellfish, and sea mammals contaminated with methylmercury (MeHg)[116]. The neurotoxic hazard posed by MeHg to humans and the unique susceptibility of the developing brain have been well documented following the mass poisonings occurring in Japan and Iraq. Why the foetus displays different neuropathological effects and a higher sensitivity to MeHg relative to the adult is still unknown. Depending on the degree ofin uteroexposure,MeHg may result in effects ranging from foetal death to subtle neurodevelopmental delays. On the basis of epidemiological studies performed in populations having moderate chronic MeHg exposure, no definitive consensus has been reached to date on the safety level of maternal exposure during pregnancy[117]. In the study from Cambieret al[118], pregnant rat mothers were contaminated with environmentally relevant doses of 36 and 76 ng MeHg/g of food using diets containing naturally mercury-containing fish. Newborns were protected against Hg exposure by the placental barrier since in newborns from mothers fed the diet containing 76 ng MeHg/g of food, the concentrations of Hg in brain, kidney, liver and skeletal muscles represented 12, 3, 21 and 18% of those of their mother's tissues, respectively. These results suggest the existence, at least in rats, of a threshold level in terms of MeHg exposure above which the placental barrier collapses. In behavioural tests performed at 5 and 6 wk of age, MeHg-exposed rats showed a significant deficit in motor coordination in the rotarod test and a learning disability in the passive avoidance response test, compared with controls. Histopathologically, focal cerebellar dysplasia,including the heterotopic location of Purkinje cells and granule cells, was observed.These abnormalities may be induced by the effect of highly accumulated MeHg in the brain during the gestation period[119]. As shown above, foetuses are particularly sensitive to MeHg exposure and adverse effects on infant development have been associated with levels of exposure that result in few, if any, signs of maternal clinical illness or toxicity. High levels of prenatal exposure in humans result in neurobehavioral effects such as cerebral palsy and severe mental retardation. Prenatal exposure to MeHg in communities with chronic low-level exposure is related to decreased birth weight and early sensorimotor dysfunction such as delayed onset of walking[120]. Moreover, late pregnancy exposure to mercury intensified the toxic effects of lead contamination on infant neurodevelopment at 6 mo of age[121]. Cadmium is known to have endocrine disrupting activities[122].In uterocadmium exposure in human has been associated to modification in methylation of genes associated to lipid metabolism and bone mineralisation[123]. As a result, one can program increased systemic fat accumulation and increased incidence of bone fractures and osteoporosis later in life[123]. Arsenic is a potent toxicant and carcinogen and can be found in a large variety of food including fish and rice[124]. A clinical study, based on blood cord samples, revealed that exposure of pregnant women to arsenic was responsible for the increased expression of 12 miRNA predicted to be involved in regulation of signaling pathways commonly deregulated in cancer and diabetes mellitus[125]. This study thus confirmed the deregulation of miRNA expression following arsenic fetal exposure and partly explains how arsenic contributes to the development of these diseases.
Children from Faroe Island born from apparently healthy mothers who ate mercury contaminated fish during pregnancy showed altered neuronal connections evidenced by decreased scores on attentiveness and memory[126]. Lead exposure may cause increased risk of pregnancy hypertension and miscarriage[127]. Arsenic, lead and MeHg have been associated with reduced length of gestation or preterm birth[127-130]. A French group has reported results from cross studies of women before pregnancy and during the third trimester. They evidenced that exposure before pregnancy was higher than in the last trimester. The reason of this difference seems to relies partly on the increase of body weight that was not compensated by parallel increase in food intake[131]. This was one of the first study in France revealing the level of dietary exposure to food contaminants for the foetus and how it is affected by changes of food intake during pregnancy and seasonal variation. Dairy consumption is associated with lower mercury and lead levels in both pregnant women and children. Lead absorption is enhanced under calcium deficiency whereas lead can interfere with the cycle of calcium homeostasis and function[132,133]. This explains why high dairy intake,meaning high calcium intake, might reduce lead absorption and its related neurotoxic effects[134]. So far, very few studies evaluated joint exposure to heavy metals. To our knowledge, only one recent work illustrated that joint exposure to lead and mercury in late pregnancy negatively affected the neurodevelopment of infants aged 6 mo[121].
Fetal exposure to MRPs
Since the first human discovered the fire, our food habits evolved a lot and this evolution increased markedly this past century in parallel with deep modification of our way of life especially in northern modernized countries. Food cooking became necessary to improve food nutritional and organoleptic qualities, hygiene but also facilitates its storage on a longer period. However, this transformation process comes with the appearance of new compounds among which MRPs. This wide set of molecules appears through cooking and results from the reaction between the carbonyl group of a reduced sugar and a free amine group of amino acid, typically the ε-amino group of lysine residues in proteins. This initial reaction gives then the rise to a large bunch of molecules through multiple rearrangements at the origin of the taste of cooked food, the brownish to grilled color of food. However, advanced glycation end-products (AGEs), acrylamide or heterocyclic amine belonging to glycotoxins may also have negative effects on health[135]. Dietary AGEs get absorbed through the small intestine and their blood concentration increases lifelong and further exacerbates their endogenous production[136]. Exposure to high AGEs during fetal development could lead to inflammatory states at young age such as juvenile diabetes[137]. In rodents as well as human, consumption of AGEs rich diet (AGE-RD) negatively affects glucose homeostasis and favors the appearance of free radicals. Csongováet al[138], recently evidenced that AGE-RD consumption during pregnancy might accelerate the maturation of reflexes in their offspring. Furthermore, they showed that this diet would predispose the male progeny to weight gain and affect their glucose metabolism[138]. Unfortunately, the study did not indicate any impact on the digestive tract. Literature describes that endogenously produced AGEs are able to interact with the receptor for advanced glycation end products (RAGE) leading to prolonged activation of nuclear factor kappa B (NF-κB) which result in the transcription of proinflammatory cytokines, including TNFα[139,140]. Then, restricting AGE intake could be beneficial in reducing levels of some inflammatory markers, helping to limit the risks of developing later in life the progression of chronic conditions[141]. Two longitudinal studies have shown a relationship between maternal acrylamide exposure and lower birth weight or higher risk of having a small baby for gestational age[142,143].Furthermore, increasing maternal exposure to dietary acrylamide during pregnancy was associated with higher prevalence of children being overweight/obese at 3, 5 and 8 years of age[144]. Food acrylamide, largely found in fried potatoes, breakfast cereals,biscuits and coffee is extensively absorbed from the gastrointestinal tract and after reaching the systemic circulation us rapidly distributed into the tissues. Acrylamide is recognized as a neurotoxicant[145]and can exert reproductive and developmental toxicity effects[146]. This is rather critical since it has been estimated that 10%-50% of acrylamide passes the placental barrier[147,148]. Hormonal and endocrine effect of acrylamide is equivocal. Acrylamide modifies thyroid activity since it has been reported that urinary acrylamide metabolites were negatively associated with free thyroxine[149]in adolescents and young adults. Fetal thyroid function might as well have an impact on fetal growth[150]since thyroid hormones are essential for optimal growth. This could partly explain growth retardation observed in acrylamide exposed children. Diamanti-Kandarakiset al[151]showed that exposure of dietary AGEs affects metabolic and hormonal functions of female rats. Six months exposure to highly heated rodents chow rich in AGE increased: (1) Deposition of AGEs in tissues; (2)Expression of RAGE; and (3) Elevated plasma testosterone levels in theca cells of ovaries. Furthermore, female Wistar rats fed high-AGE diet had declined levels of estradiol and progesterone. These female sex hormones are crucial for implanting the fertilized egg in the uterus, maintaining pregnancy and also to regulate the estrous and menstrual cycles[151]. Such effects may then have severe repercussions onto the developing fetus. It has been evidenced that peripheral blood mononuclear cells of rats isolated from high-AGE diet-fed female rats in had increased expression of RAGE, the AGEs receptor associated with the proinflammatory pathways[152,153]. This might be a mechanism also stimulated in the developing child which could contribute to alteration of the programming function.
ROLE OF FOOD CONTAMINANTS ON INFANT AND CHILD DEVELOPMENT AND MATURATION
While fetal exposure to food contaminants may strongly condition developmental programming, one cannot exclude the postnatal exposure to these contaminants as an important factor contributing to modification of this programming. Disruption of the developing gut and of the establishing cross communication between the brain and the gut by food contaminants may lead to a reduced capacity to settle local and systemic neuro-immuno-endocrine functions and an increased risk to develop neurological and inflammatory disorders. Indeed, strong or novel evolutionary challenges may be at the origin of disruption of normal signaling pathways explaining the appearance soon or late of these disorders. However, while more and more studies evidence the consequences of maternal exposure during pregnancy on the fetus and the infant/child, very few studies focused on the direct exposure of the baby and the infant to food contaminants. This will be important to figure out all these effects since infants and children are very vulnerable to food contaminants since they consume high amounts of food in comparison with their weight, have a higher metabolic rate and thus get a higher exposure to them by contrast to adults. In this part of the review, we will summarize the actual state of the art, considering that most of the below cited studies will often be related to gut microbiota settlement but there are very few works related to neurotoxicity of food contaminants on juvenile individuals.
Postnatal exposure to antibiotics
Pharmaceutical products among which laxatives[154]or antibiotics[155]have a major influence on the gut microbiota activity and may have repercussions on the settlement of local and systemic interactions with the neuro-immuno-endocrine functions.Disrupting the overall eubiosis results in shift of abundance of resident gut bacteria and predisposes the child to certain inflammatory or functional disorders[156]. Use of antibiotics during the postnatal period may severely alter this fragile settling ecosystem especially when they are given very early and for long periods of time[157].This disrupted profile associated to a decreased microbial activity will be conditions favoring the settlement of enteric pathogens[158,159]. This has been associated with long term disruption with intestinal barrier function but will also impact the growth of dominant bacteria in the human gut[157], confirming that antibiotic exposure in early life car render the infant susceptible to numerous diseases later in life[160,161]. The perturbation of composition and biodiversity of the gut microbiota is often associated to an increase of Proteobacteria because of a higher content of antimicrobial resistance genes in this phylum[162]and the disruption of our microbiota caused by antibiotics favors the development of non-communicable chronic diseases (NCCDs)characteristic of dysbiosis. At last, the alteration of the microbiota composition observed in infants treated with antibiotics was close to the one of children from mothers treated with antibiotics before delivery, with consequences late in life[163].Moreover, antibiotics treatment has the main disadvantages of enriching our microbiota with antibiotics resistant genes which might become later on a problem towards the gut homeostasis. Furthermore, disrupting microbiota settlement will also disrupt its overall metabolizing activity which concerns its ability to transform food contaminants and limit their impact on our health (for review see Flandroyet al[164]).On top of this, although the use of antibiotics might be very important to prevent sepsis in preterm or newborn infants, they might, under some circumstances, interfere with neurotransmitters receptors and particularly those of gamma-amino butyric acid[165]or of serotonin[166], compromising correct central and peripheral neuronal circuitry which may under prolonged exposure, have serious consequences[167]. These toxicological effects have been evidenced for amoxicillin in juvenile rats[168].
Rotavirus infection is a very common cause of gastroenteritis since it targets mainly the gastrointestinal tract. In animals, several observations are concordant: Rotavirus infection decreases intestinal enzymes activities in both piglets[169]and young mice[170,171]. In rabbits, it impairs epithelial homeostasis by altering the intestinal brush border membrane[172]. Rotavirus infection in children results in the occurrence of a flat mucosa with total virus atrophy[173]. Exposition during early life to environmental microorganisms strongly affects protein expression at the brush border since it clearly stimulates TLR5 and TLR9 gene expressions of exposed new born mice[174]. This mismatch at birth will fragilize the maturation of the intestinal epithelium.
Postnatal exposure to pesticides
There is growing evidence from animal studies suggesting that early life exposure to toxic stress may magnify the effects of environmental chemical exposure on neurodevelopment[175]. Air pollution and pesticides may affect growth during infancy or childhood, and they are associated with neurodevelopmental and behavioral problems[176].
Organophosphates pesticides are neurotoxic at high doses and target the nervous system of children, and are associated with changes in neurotransmitters including serotonin, norepinephrine, acetylcholine, and dopamine. The associations of organophosphate pesticide and child IQ at 7 years old was noted by Steinet al[177]in 2016 and was already observed[178]as more important in children that also experience certain social adversities. Those who have the highest exposure to environmental toxicants are often the same populations and individuals who experience the greatest adversity. Children with high prenatal exposure to organophosphates who also experience specific early and persistent toxic stress may be at a greater risk for adverse cognitive development[177]. However, postnatal organophosphates studies are not numerous and most of the results are inconsistent[179].
Prolonged breastfeeding was associated with increased of OCPs in Norwegian children blood samples[180]. In studies that measured the levels of DDE and PCBs,organochlorine compounds postnatally, authors reported a slower growth and a higher risk of endocrine function alteration in children[181].
Glyphosate is one of the agro-chemical which should also deserve a special attention in infants and children. In fact, this herbicide is inhibiting the shikimate pathway, which, when present in bacteria, controls the synthesis of aromatic compounds such as tyrosine or tryptophan, key intermediaries in systemic effects of our microbiota (see the first section of this review)[164]. From now, there seems to be no direct effect of neonatal exposure to glyphosate on gut brain axis maturation.However, since it may alter uterus development, one can’t exclude any alteration of the gut homeostasis[182], since it has been evidenced that this herbicide could temporarily modify commensal bacterial community composition[183].
Postnatal exposure to mycotoxins
Infants and young children are more vulnerable to mycotoxins than adults, due to their constantly growing, poorly developed nervous, immune, reproductive, and digestive systems, high rate of metabolism, and restricted diet. Childhood exposure to mycotoxins, induced by the consumption of contaminated foods : Breast milk[184],infant formula or complementary foods with cereals[185], vegetables or nuts[186,187]. This has been associated with poor child growth and development[188], increased susceptibility to infections and many others effects like immune disorders[189], tumors development[190], precocious puberty[191,192],etc. Assessment of health risks related to mycotoxins ingestion during childhood has been evaluated in the last years. The presence of mycotoxins in the intestinal fluid of a child could cause more significant damage to the intestinal enterocytes because of the reduced dimension of the intestinal epithelium compared to that of an adult. An outbreak in the children population of Kashmir Valley was associated with the consumption of naturally contaminated bread with a varying quantities of trichothecene mycotoxins[193].Symptoms of gastrointestinal illness appeared 15 min to 1 h after consuming it. DON belonging to these trichothecenes, can act on the viability and the proliferation of immune cells. This event then inhibits proteins biosynthesis that prevent cytokine production[194]. The direct consequence of this event is a higher susceptibility to infection disease which is of peculiar importance on such vulnerable people that are babies and infants. Moreover, it was evidenced that DON was affecting the intestinal barrier function in chicken[195].
AF may be generated byAspergillus Fungi. One of its metabolites, a 4-hydroxylated form, can be secreted in mammal milk after ingestion of the mycotoxin. As for DON,AF reduce anti-inflammatory cytokines IL-4 expression together with the increase of susceptibility to infection[196].
Another outbreak of diarrhea in children in Sweden was related to consumption of fruit contaminated with PAT[197]. Indeed, it was reported that consumption of high concentration of mycotoxins (DON and PAT) could cause gastroenteritis with vomiting in humans[198,199]. PAT is cytotoxic to enterocytes, increase permeability across intestinal Caco-2 monolayers and alter ion transport in intact intestinal mucosae[199,200].Fusariummycotoxins are quite frequently found in baby food in particular in the developing countries. Despite of efforts made to correct the heating process to limit or eliminate the mycotoxins from maize or soy-bean, it has been evidenced that this food transformation not only does not eliminate mycotoxins but rather help to generate many others thus presenting a higher risk for the consumer and particularly babies[184].
Other groups of metabolites produced by someAspergillusorPenicilliumare Ochratoxins. However, from the actual knowledge, it seems not to have any consequence neither on the gut nor on the grain but rather on kidneys.
At last, fumonisins, Fusarium mycotoxins, may also be found in baby and infant food. From the actual literature, it has not been so far determined that they are responsible for gut disease apart from a foodborne disease outbreak in India[193].However, they seem to alter the settlement of the nervous system since they are associated to neural tube defects in the Mexico-Texan border[201].
Postnatal exposure to other organic pollutants
Several POPs have been associated with increased children's behavior problems[202].Some studies have pointed out that exposition for 24 h to 5 pollutants [TCDD,deltamethrin, hexabromocyclododecane, Benzo(a)Pyrene and 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine] and one mixture of pollutants polycyclic aromatic hydrocarbures (PAHs) significantly shifted the microbial volatile pattern, suggesting that the activity of the microbiota might have been affected[203]. Moreover, their direct or indirect consequences on neuronal communication and network development has already been described[204]. Taking all these informations into account, one can’t exclude the possibility of misshaping the gut microbiota settlement in newborns and the sensible consequences on his/her health. POPs, PCBs, PAHs have been described as significantly altering the microbiota metabolism at the origin of the induction of a proinflammatory status of the gut[203]. 2,3,7,8-tetrachlorodibenzofurane, a POP, may shift the firmicutes/Bacteroidetesratio which is at the origin of alteration of bile acid metabolism[205]. Furthermore, PAHs can be transformed by the gut microbiota to estrogenic metabolites[206].
In the same line, phthalates, a category of food plasticizers, may strongly unbalance the settlement of gut brain microbiota axis. Indeed, exposure to di-ethylhexyl phthalate through lactation showed negative association with mental index[202].Phthalate metabolite concentrations were higher in the 2- and 5-years old children,compared to 11 years old ones. Postnatal high exposure to phthalic acid ester is related to attention deficit hyperactivity disorder symptoms in children[207], and particularly diethylphthalate are inducing gut dysbiosis and consistent weight loss.One of the mechanisms proposed was through the aryl hydrocarbon receptor activation[208]. Moreover, child exposition to Phthalates has been correlated with the decrease of DNA methylation of specific genes among which TNFα. As this demethylation is linked to a decrease of TNFα protein level, this could explain the gene expression deregulation observed during the inflammatory response as it was suggested for asthma[209]. These recent observations play in favor for a correlation that can be pointed out between contaminants and molecular pathways involved in a given disease.
Duration of breastfeeding was the main determinant and was significantly associated with increased POP concentrations in the children. Prolonged breastfeeding was associated with increased concentrations of POPs such as PCBs,and polybrominated diphenyl ethers in children blood samples[180]. In their study,Caspersenet al[180], revealed that concentrations of POPs in 3 years old children were 1.4-fold higher than in an independent sample of pregnant women.
Postnatal exposure to heavy metals
There are a dozen of studies concerning prenatal and postnatal exposure to heavy metals such as cadmium, arsenic, mercury or lead. Authors have reported in all of them a slower postnatal growth in children but due to a limited number of studies per specific heavy metals authors could not conclude on any other side effects[210].Exposure to mercury, cadmium and lead in early life can disrupt the development of the nervous system and such exposure can affect the child’s cognitive, motor and behavioral development[72]. The main route of exposure to cadmium are inhalation then ingestion due to consumption of cereals and vegetables grown on contaminated soils.
On 278 nine-year-old children, 21% had total toenail mercury concentrations (1.5 to 6 μg/g) higher than the United State Environmental Protection Agency recommended levels (1 μg/g Hg) for optimal health in children, associated with an aggressive behavior[211].
A decrease of intelligence quotient at 1.5 and 5 years was associated with childhood duration and amount of arsenic exposure[212]. From other studies, it was established in mice that gut microbiota could metabolize arsenic intro potent toxic metabolites[213]that will interfere with neuronal signaling, limit brain weight but also neurons and glia number[214].
Postnatal exposure to MRPs
Depending on length and temperature of food heating, the amount, type and diversity of MRPs may significantly change. No direct evidence of MRPs incidence on the brain gut microbiota axis development has been described so far although most of AGEs are absorbed through the gut[215]. However, there are a couple of evidences confirming the activation of an immune response in the presence of MRPs probably due to the fixation of these molecules on RAGE and its subsequent activation of the NF-κB pathway leading to the release of pro-inflammatory cytokines such as TNFα,IL-6 or IL-1β and the increase of tissue oxidative stress along with an inflammatory response[216]. We and others are currently working on the establishment of any correlation with perinatal events.
Babies exposed to acrylamide show growth retardation[217]. This has been partly explained by prenatal alteration of fetal programming of the thyroid. However,another window of susceptibility should be investigated, and child’s postnatal exposition should be considered in addition to prenatal exposition. Another possible mechanism linking acrylamide exposure and growth is through oxidative stress and inflammation. Indeed, acrylamide exposure can result in increased oxidative stress through increased expression of CYP2E1, resulting further in heightened perinatal inflammatory status[218,219]. Dietary AGEs are responsible for oxidative stress and inflammation in several tissues by interfering with vital hormones involving physiological reactions. Exposure to high AGEs during childhood is responsible for inflammatory status. This speculation is supported by the relationship between high maternal AGEs and infant plasma insulin levels[137]. New born and infants may be very concerned by AGEs especially those that need to receive hydrolyzed infant formulas that have very high levels of Nε-carboxy-methyllysine (CML) as compared to breast milk due to the heat treatment of milk proteins in presence of lactose[220]. In a recent review, we suggested that neoformed compounds from the Maillard Reaction and especially CML could be a new risk factor for allergy[221]since they may react with RAGE receptors expressed at the surface of numerous immune cells (mononuclear cells, DCs and T cells) present in areas where AGEs are often more abundant[222-224].
CONCLUSION
Consequences on risks of developing non-communicable chronic diseases
Nutrition is somehow conditioning the metabolic imprinting of each individual.Recent literature clearly established that qualitative early nutrition is a prerequisite to limit the risks of developing later in life chronic diseases (Figure 1). From the first studies describing the DOHaD, several original articles have pointed out that a poor fetal and postnatal settlement of gut microbiota will necessarily remodels neuroimmuno-endocrine maturation of the gut (Figure 2) that will, in turn, modify brain neuronal connections establishment leading to a significantly higher risk of developing later in life NCCD, either at the gut of the brain level see[2]for review.However, this poor establishment of an efficient gut barrier may have other deleterious effects that will result into other NCCD affecting the endocrine function:Diabetes[224], hypertension[1]but also general behavior. Indeed, it has long been evidenced that developmental exposure to testosterone produced by fetal testis programs male sexual behavior[225-227]while excessive exposure of female fetus to this hormone during critical windows of differentiation will masculinize the female brain[228,229]. Many other factors may also be responsible of fetal and postnatal major programming modifications which will increase the risk of developing NCCD.Mother undernutrition or malnutrition, poor placental development will be at the origin of growth retardation. Compensatory growth during early life is also a risk factor for the development of adult disease in the offspring. Moreover, maternal stress, conditioning corticotropin releasing hormone and cortisol release, has been evidenced to alter irreversibly perinatal programming as well as maternal disease state especially when associated with prescription drugs such as antibiotics. Mother lifestyle: Family size, living place, presence of pets in householdsetc. will also leave epigenetic marks at the origin of the remodeling of the programming of the offspring and the possibility of their transmission to the upcoming generation.
This past couple of years the qualification of some molecular epigenetics has clearly added strong insight into the effects of environmental stimuli during perinatal programming. It especially gave information on the importance of the timing of exposures to these stimuli on later human health. So far, investigation of the consequences of all these environmental factors is running on and gives interesting evidences of the mechanisms responsible for NCCD. This is the reason why we must explore the interaction of genetic and environmental components through lifetime with a special focus on early development. However, the main work to be undertaken will be to correlate all these events with each other to be able to improve recommendation to the population in terms of nutrition behaviors on the one side and lifestyle on the other side.
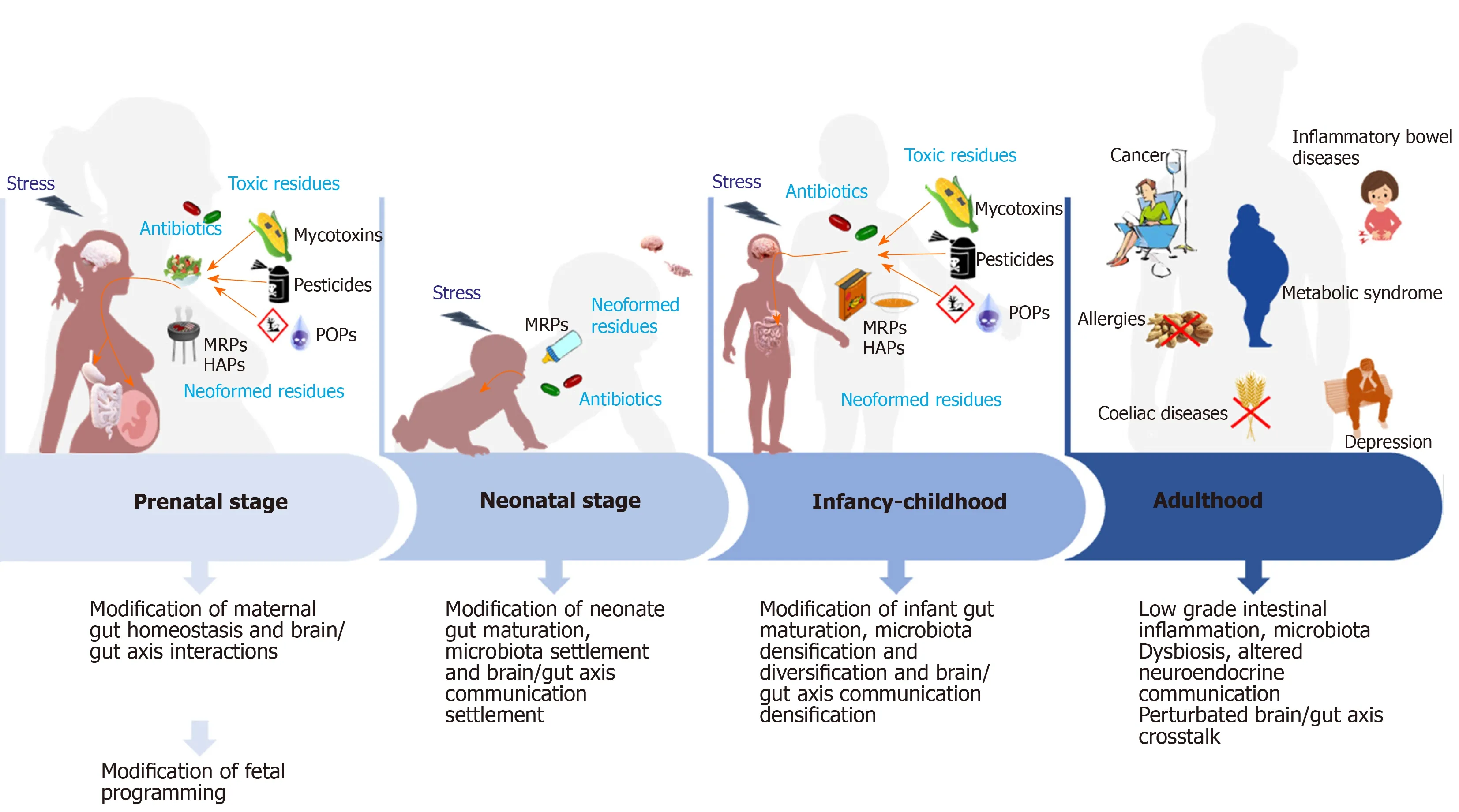
Figure 1 Hypothesis on the impact of food contaminants on prenatal and postnatal gut homeostasis mis-programming and its consequences on the etiology of non-communicable chronic diseases. During pregnancy and infancy, repeated exposure of fetus and then infant to food contaminants is presumed to be at the origin of altered gut homeostasis due to mis settlement of neuro-immuno-endocrine cross talks and microbiota diversification and densification. Furthermore,also due to these contaminants, the brain/gut axis exchanges might not settle properly. All these modifications during the perinatal period may, later in life, predispose to the appearance of non-communicable chronic diseases among which celiac disease, allergies, inflammatory bowel disease, metabolic syndrome, depression, etc.At the bottom of the figure, are presented the main consequences on gut homeostasis observed at each stage of life. MRPs: Maillard reaction products; POPs:Persistent organic pollutants.
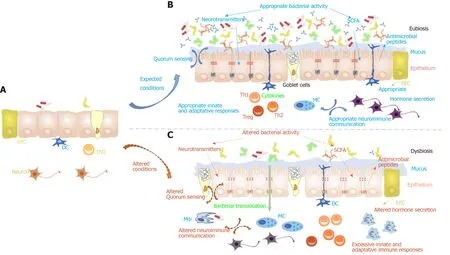
Figure 2 lmpact of food contaminants on gut homeostasis settlement and stability. A: At birth the intestinal epithelial environment is still immature with fairly no secretion, very few microorganisms and immature neuro-immuno-endocrine interactions; B: These interactions settle progressively during infancy and childhood to be fully functional at adulthood; C: However, when the conditions are not met and more especially if the child has been submitted during the fetal stage and infant stage to food contaminants, this might be at the origin of gut homeostasis misshaping observed at adulthood. This could then be responsible for gut permeability increase responsible for a mild “leaky gut”, predisposing to intestinal neuro-immuno-endocrine communications alterations. This will in turn be at the origin of systemic neuroimmuno-endocrine communication misshaping contributing to an alteration of crosstalk between the brain and the gut.
 World Journal of Gastroenterology2020年23期
World Journal of Gastroenterology2020年23期
- World Journal of Gastroenterology的其它文章
- Liver-directed therapies for liver metastases from neuroendocrine neoplasms: Can laser ablation play any role?
- Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection
- Endosonographic diagnosis of advanced neoplasia in intraductal papillary mucinous neoplasms
- Medications in type-2 diabetics and their association with liver fibrosis
- Pancreatic necrosis and severity are independent risk factors for pancreatic endocrine insufficiency after acute pancreatitis: A long-term follow-up study
- Impact of a national basic skills in colonoscopy course on trainee performance: An interrupted time series analysis
