干旱胁迫下小麦根部蛋白表达变化的双向电泳分析
邓艳君 王聪 赵利利 连娟 宋芳媛 刘娜 赵宝存
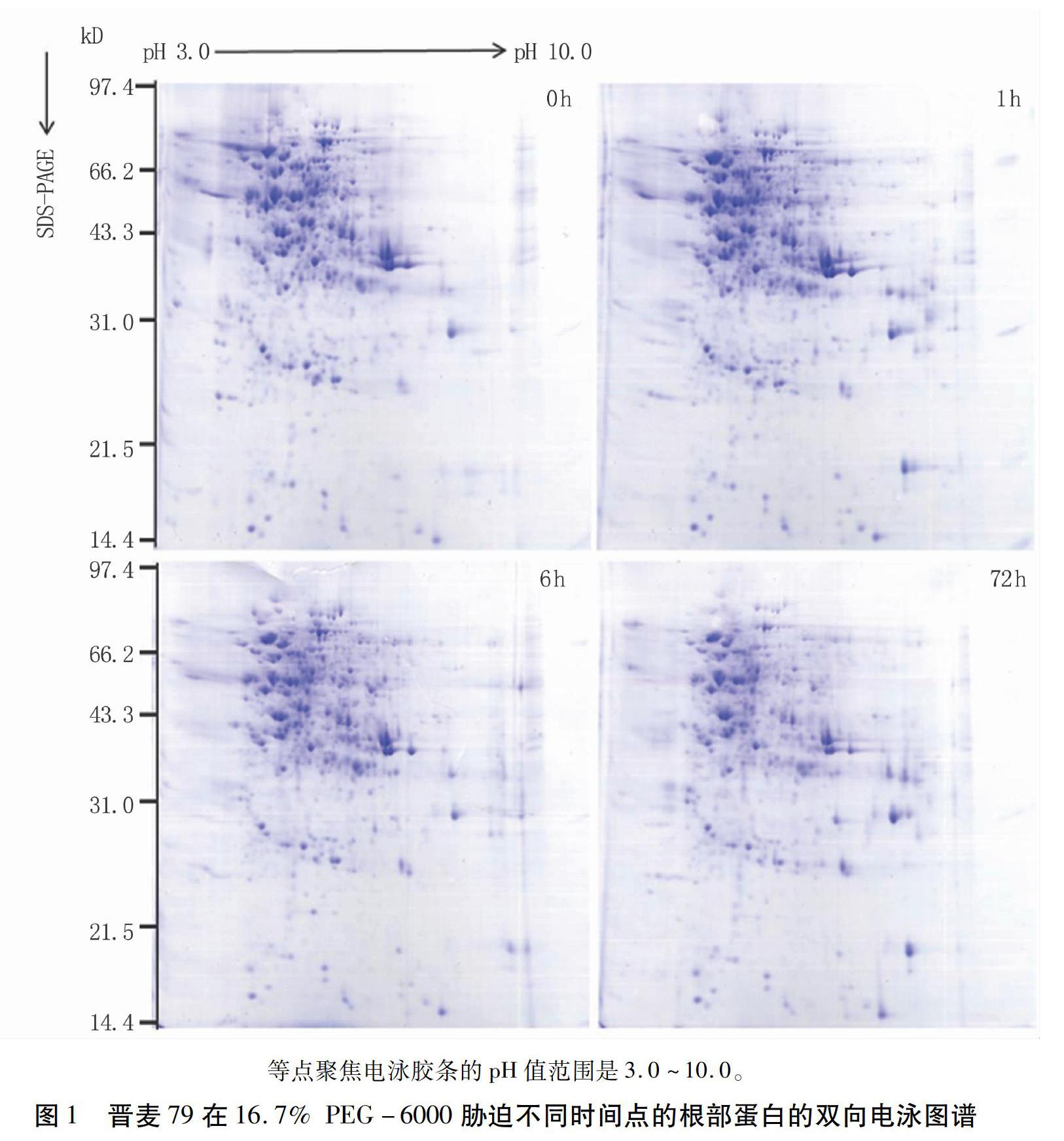
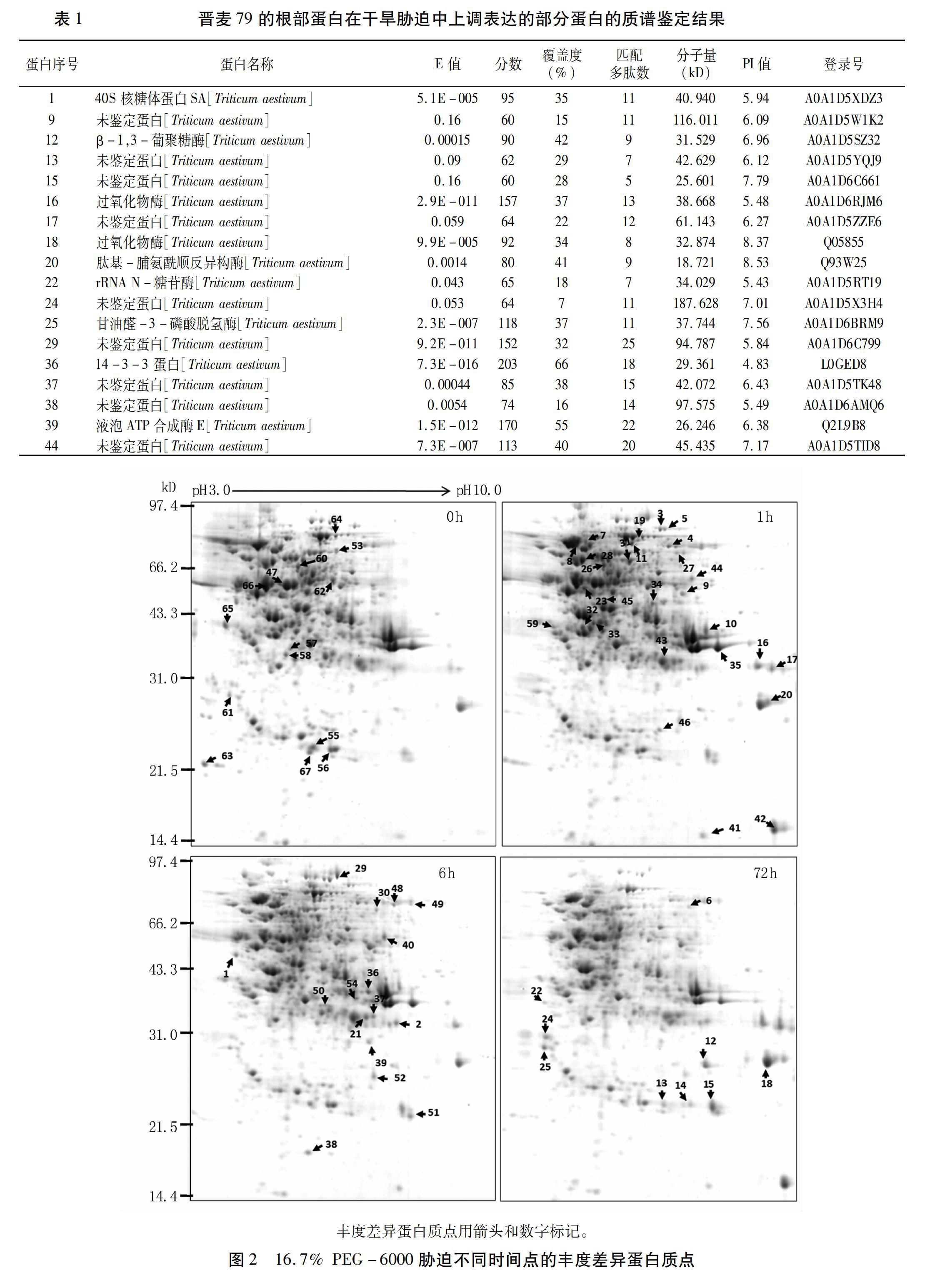
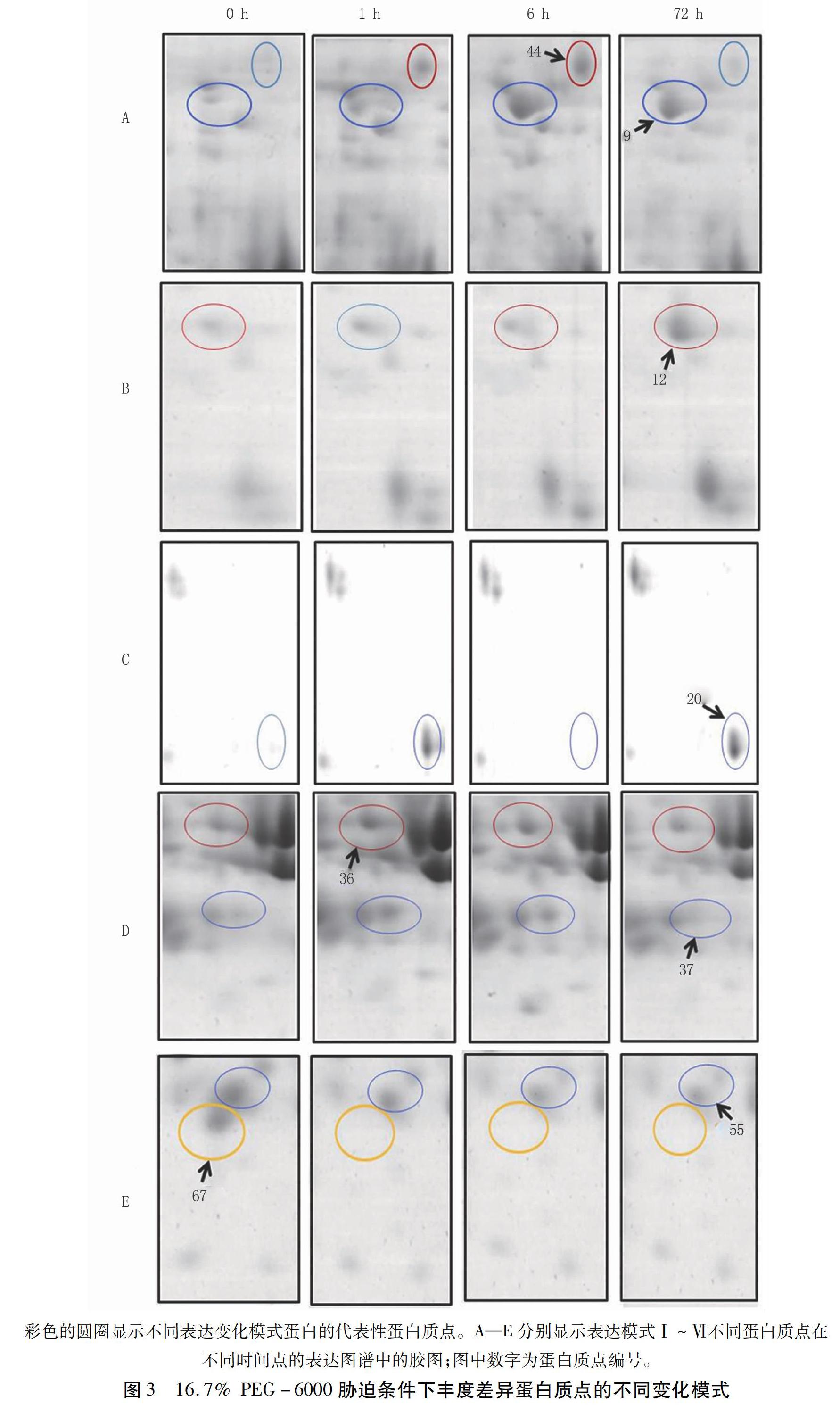
摘要:干旱是影响小麦生长和产量的主要环境因素之一,研究小麦耐旱机制对提高小麦产量保证粮食安全有重要的意义。本研究以耐旱小麦晋麦79为材料,利用双向电泳技术,对其两叶一心期幼苗在16.7% PEG-6000胁迫0、1、6、72 h的根部蛋白质表达谱进行分析,比较不同胁迫时间点的小麦蛋白质表达谱的差异。结果表明,相对于0 h的表达谱,67种蛋白质在不同的胁迫时间点改变了其表达丰度。对其中至少在某一个时间点上调表达2倍以上的20个蛋白质点进行基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF-MS)分析,质谱结果中得到了18个阳性蛋白质点的信息,包括9个功能已知的蛋白和9个未鉴定的蛋白。已知功能的蛋白涉及到能量代谢、胁迫耐受性、信号转导和蛋白质合成/代谢等生理生化过程,表明植物在干旱胁迫下调节多种蛋白质的表达,综合调控其耐旱性。同时,干旱胁迫下未鉴定的丰度差异蛋白质点(DAPs)为克隆新的干旱相关基因和进一步研究小麦的耐旱机理提供了有价值的信息。本试验结果为进一步研究小麦耐旱机理奠定了基础。
关键词:小麦;根部蛋白;表达;耐旱;双向电泳;MALDI-TOF-MS
中图分类号:S512.1+1 文献标识号:A 文章编号:1001-4942(2020)02-0007-08
Abstract Drought stress is one of the major constraints to wheat growth and yield, so studying drought tolerant mechanisms has important significance to wheat yield and food security. In this study, the two-dimensional electrophoresis (2-DE) was used to analyze the protein expression profiles of Jinmai 79 seedling roots exposed to 16.7% PEG-6000 simulated drought stress. The results showed that the expression abundance of 67 proteins changed at different stress time compared with that at 0 h. Moreover, 20 upregulated protein spots, whose abundance levels increased more than 2-fold at a certain time, were identified by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS). The amino acid sequence information of 18 upregulated protein spots was obtained on the basis of the MS results including 9 reported proteins and 9 not identified proteins. The function reported proteins were involved in several physiological and biochemical pathways such as energy metabolism, stress tolerance, signal transduction, and protein synthesis and metabolism, which showed plant regulated expression of multiple proteins in response to drought stress. The unidentified DAPs under drought stress provided valuable information for cloning novel drought related genes and further studying the drought-tolerant mechanisms of wheat. This study also laid foundations for further research on wheat drought tolerant mechanisms.
Keywords Wheat; Protein in roots; Expression; Drought tolerance; Two-dimensional electrophoresis; MALDI-TOF-MS
小麥是我国主要的粮食作物,随着水资源危机的加剧,越来越多的小麦生产区受到干旱的侵袭[1,2]。克隆耐旱相关基因、研究小麦耐旱机理,对提高小麦在干旱条件下的产量和保证粮食安全有重要意义。
目前,已在小麦中发现多种耐旱相关基因。Xue等[3]从面包小麦中克隆了一个与旱胁迫有关的基因TaNAC69,过表达该基因可以提高转基因小麦的作物生物量和根长,进而提高转基因小麦在干旱胁迫下的存活率。Mao等[4]从小麦中克隆了一个NAC家族的基因TaNAC67,超表达该基因提高了转基因拟南芥对旱、盐和低温等非生物胁迫的耐受性,并提高了叶绿素含量、水势、渗透势等耐旱相关生理指标。金秀锋等[5]利用SDS-PAGE方法检测了一个水分胁迫应答蛋白质(MW:66.2 kD)在128个耐旱等级不同的小麦品系中的表达情况,结果表明该蛋白质的表达量与小麦耐旱性等级呈正相关,说明这个水分胁迫应答蛋白质与小麦的耐旱性密切相关。TaWRKY10的表达量受PEG-6000、NaCl、低温或过氧化氢处理后上调,过表达可增强转基因烟草(Nicotiana tabacum L.)对干旱和盐胁迫的耐受性[6]。小麦TaODORANT1在PEG-6000处理时上调,TaODORANT1过表达转基因烟草在干旱胁迫下有较高的含水量和较低的失水率,这些结果表明TaODORANT1正调控植物的耐旱性[7]。 但是,这些基因功能还不足以全面了解小麦的耐旱机制,挖掘干旱耐受有关的新基因,有利于我们对小麦耐旱机制的研究。
[2] Fahad S, Bajwa A A, Nazir U, et al. Crop production under drought and heat stress: plant responses and management options[J]. Front. Plant Sci., 2017, 8: 1147.
[3] Xue G P, Way H M, Richardson T, et al. Overexpression of TaNAC69 leads to enhanced transcript levels of stress upregulated genes and dehydration tolerance in bread wheat[J]. Mol. Plant, 2011, 4: 697-712.
[4] Mao X, Chen S, Li A, et al. Novel NAC transcription factor TaNAC67 confers enhanced multi-abiotic stress tolerances in Arabidopsis[J]. PLoS ONE, 2014, 9: 1-15.
[5] 金秀鋒,王宪国,任万杰,等. 一个水分胁迫应答蛋白与小麦抗旱性的关系及其基因的定位[J]. 作物学报,2014, 40(2): 198-204.
[6] Wang C, Deng P, Chen L, et al. Wheat WRKY transcription actor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco[J]. PLoS ONE, 2013, 8: e65120.
[7] Wei Q, Luo Q, Wang R, et al. Wheat R2R3-type MYB transcription factor TaODORANT1 positively regulates drought and salt stress responses in transgenic tobacco plants[J]. Front. Plant Sci., 2017, 8: 1374.
[8] Caruso G, Cavaliere C, Foglia P, et al. Analysis of drought responsive proteins in wheat (Triticum durum) by 2D-PAGE and MALDI-TOF mass spectrometry[J]. Plant Sci., 2009, 177: 570-576.
[9] Ford K L, Cassin A, Bacic A. Quantitative proteomic analysis of wheat cultivars with differing drought stress tolerance[J]. Front. Plant Sci., 2011, 2: 44.
[10] Ge P, Ma C, Wang S, et al. Comparative proteomic analysis of grain development in two spring wheat varieties under drought stress[J]. Anal. Bioanal. Chem., 2012, 402: 1297-1313.
[11] Budak H, Akpinar B A, Unver T, et al.Proteome changes in wild and modern wheat leaves upon drought stress by two-dimensional electrophoresis and nanoLC-ESI-MS/MS[J]. Plant Mol. Biol., 2013, 83: 89-103.
[12] Peng Z, Wang M, Li F, et al. A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat[J]. Mol. Cell Proteomics, 2009, 8: 2676-2686.
[13] Damerval C, Vienne D D, Zivy M, et al. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat seedling proteins[J]. Electrophoresis, 1986, 7: 52-54.
[14] Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J]. Anal. Biochem., 1976, 72: 248-254.
[15] Gao L Y, Wang A L, Li X H, et al. Wheat quality related differential expressions of albumins and globulins revealed by two-dimensional difference gel electrophoresis(2-D DIGE)[J].J. Proteomics, 2009, 73: 279-296.
[16] Valliyodan B, Nguyen H T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants[J]. Plant Biol., 2006, 9: 189-195.
[17] 樊立強, 刘新月. 国审小麦新品种晋麦79号选育及应用研究[J]. 陕西农业科学, 2009(5): 8-9,26.
[18] 王镜岩, 朱圣庚, 徐长法. 生物化学[M]. 北京: 高等教育出版社, 2006.
[19] 马莉,陈丽梅. 植物丝氨酸羟甲基转移酶基因研究进展[J]. 生物技术通报, 2008(2): 15-19.
[20] 邓林,陈少良. ATPase与植物抗盐性[J]. 植物学通报, 2005, 22(S): 11-21.
[21] Csiszár J, Gallé A, Horváth E, et al. Different peroxidase activities and expression of abiotic stress-related peroxidases in apical root segments of wheat genotypes with different drought stress tolerance under osmotic stress[J]. Plant Physiol. Biochem., 2012, 52: 119-129.
[22] Leucci M R, Lenucci M S, Piro G, et al. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance[J]. J. Plant Physiol., 2008, 165: 1168-1180.
[23] Mittler R. Oxidative stress, antioxidants and stress tolerance[J]. Trends in Plant Science, 2002, 7: 405-410.
[24] Gill S S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiol. Biochem., 2010, 48: 909-930.
[25] Abdrabou A, Brandwein D, Liu C, et al. Rac1 S71 mediates the interaction between Rac1 and 14-3-3 proteins[J]. Cells, 2019, 8: 1006.
[26] Faghani E, Gharechahi J, Komatsu S, et al. Comparative physiology and proteomic analysis of two wheat genotypes contrasting in drought tolerance[J]. J. Proteomics, 2015, 114: 1-15.
[27] Mishra P, Mishra V, Takabe T, et al. Elucidation of salt-tolerance metabolic pathways in contrasting rice genotypes and their segregating progenies[J]. Plant Cell Rep., 2016, 35:1273-1286.
[28] Trivedi D K, Ansari M W, Tuteja N. Multiple abiotic stress responsive rice cyclophilin (OsCYP-25) mediates a wide range of cellular responses[J]. Commun. Integr. Biol., 2013, 6: e25260.
[29] Zhao Q, Zhao Y J, Zhao B C, et al. Cloning and functional analysis of wheat V-H+-ATPase subunit genes[J]. Plant Mol. Biol., 2009, 69: 33-46.

